Autism-Related Differences in Cortical Activation When Observing, Producing, and Imitating Communicative Gestures: An fNIRS Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
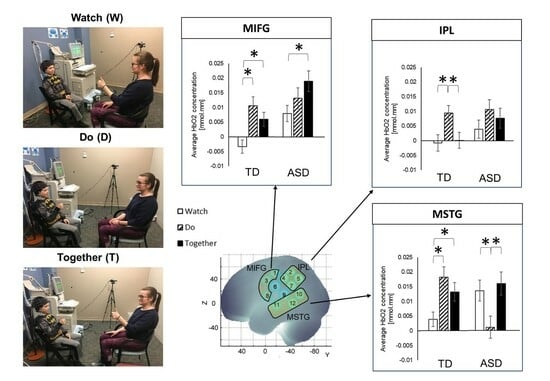
2.2. Experimental Procedures
2.3. fNIRS Data Collection
2.4. Spatial Registration Approach
2.5. Video Coding for Postural Praxis and Communicative Gestures Tasks
2.6. Processing Cortical Activation Data
2.7. Data Exclusion
2.8. Statistical Analyses
3. Results
3.1. Postural Praxis and Communicative Gesture Performance
3.2. Cortical Activation during the Communicative Gesture Task
3.2.1. Group Differences
3.2.2. Condition-Related and Regional Differences
3.2.3. Hemispheric Differences
3.3. Correlation between Praxis, Communicative Gestures, and Cortical Activation
3.4. Correlation between VABS, ADOS, SCQ, and Cortical Activation in Children with and without ASD
4. Discussion
4.1. Impaired Praxis and Communicative Gesture Performance in Children with ASD
4.2. ASD-Related Regional Hyperactivation in the MIFG and IPL during Gesture Observation and Imitation
4.3. ASD-Related Relative Regional Hypoactivation over STS during Gesture Production
4.4. Left-Lateralized Neural Networks for Communicative Gestures in Children with and without ASD
4.5. Neurobiomarkers and their Clinical Implications in Gesture-Based Interventions
4.6. Limitations and Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Ham, H.S.; Bartolo, A.; Corley, M.; Rajendran, G.; Szabo, A.; Swanson, S. Exploring the relationship between gestural recognition and imitation: Evidence of dyspraxia in autism spectrum disorders. J. Autism Dev. Disord. 2011, 41, 1–12. [Google Scholar]
- Bhat, A.N.; Srinivasan, S.M.; Woxholdt, C.; Shield, A. Differences in praxis performance and receptive language during fingerspelling between deaf children with and without autism spectrum disorder. Autism 2018, 22, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Shield, A.; Knapke, K.; Henry, M.; Srinivasan, S.; Bhat, A. Impaired praxis in gesture imitation by deaf children with autism spectrum disorder. Autism Dev. Lang. Impair. 2017, 2, 2396941517745674. [Google Scholar] [CrossRef]
- Mishra, A.; Ceballos, V.; Himmelwright, K.; McCabe, S.; Scott, L. Gesture production in toddlers with autism spectrum disorder. J. Autism Dev. Disord. 2021, 51, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- McAuliffe, D.; Zhao, Y.; Pillai, A.S.; Ament, K.; Adamek, J.; Caffo, B.S.; Mostofsky, S.H.; Ewen, J.B. Learning of skilled movements via imitation in ASD. Autism Res. 2020, 13, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Iverson, J.M. Early motor and communicative development in infants with an older sibling with autism spectrum disorder. J. Speech Lang. Hear. Res. 2018, 61, 2673–2684. [Google Scholar] [CrossRef] [PubMed]
- Iverson, J.M. Developmental variability and developmental cascades: Lessons from motor and language development in infancy. Curr. Dir. Psychol. Sci. 2021, 30, 228–235. [Google Scholar] [CrossRef]
- Fourie, E.; Palser, E.R.; Pokorny, J.J.; Neff, M.; Rivera, S.M. Neural processing and production of gesture in children and adolescents with autism spectrum disorder. Front. Psychol. 2020, 10, 3045. [Google Scholar] [CrossRef]
- Wadsworth, H.M.; Maximo, J.O.; Lemelman, A.R.; Clayton, K.; Sivaraman, S.; Deshpande, H.D.; Hoef, L.V.; Kana, R.K. The action imitation network and motor imitation in children and adolescents with autism. Neuroscience 2017, 343, 147–156. [Google Scholar] [CrossRef]
- Jola, C.; Grosbras, M.H. In the here and now: Enhanced motor corticospinal excitability in novices when watching live compared to video recorded dance. Cogn. Neurosci. 2013, 4, 90–98. [Google Scholar] [CrossRef]
- Afif, I.Y.; Farkhan, M.; Kurdi, O.; Maula, M.I.; Ammarullah, M.I.; Setiyana, B.; Jamari, J.; Winarni, T.I. Effect of short-term deep-pressure portable seat on behavioral and biological stress in children with Autism Spectrum Disorders: A pilot study. Bioengineering 2022, 9, 48. [Google Scholar] [CrossRef]
- Gevarter, C.; Najar, A.M.; Flake, J.; Tapia-Alvidrez, F.; Lucero, A. Naturalistic communication training for early intervention providers and Latinx parents of children with signs of autism. J. Dev. Phys. Disabil. 2022, 34, 147–169. [Google Scholar] [CrossRef]
- Manera, V.; Becchio, C.; Schouten, B.; Bara, B.G.; Verfaillie, K. Communicative interactions improve visual detection of biological motion. PLoS ONE 2011, 6, e14594. [Google Scholar] [CrossRef]
- Osiurak, F.; Claidière, N.; Bluet, A.; Brogniart, J.; Lasserre, S.; Bonhoure, T.; Di Rollo, L.; Gorry, N.; Polette, Y.; Saude, A.; et al. Technical reasoning bolsters cumulative technological culture through convergent transformations. Sci. Adv. 2022, 8, eabl7446. [Google Scholar] [CrossRef]
- Trettenbrein, P.C.; Papitto, G.; Friederici, A.D.; Zaccarella, E. Functional neuroanatomy of language without speech: An ALE meta-analysis of sign language. Hum. Brain Mapp. 2021, 42, 699–712. [Google Scholar] [CrossRef]
- Fischer, J.; Mikhael, J.G.; Tenenbaum, J.B.; Kanwisher, N. Functional neuroanatomy of intuitive physical inference. Proc. Natl. Acad. Sci. USA 2016, 113, E5072–E5081. [Google Scholar] [CrossRef]
- Federico, G.; Reynaud, E.; Navarro, J.; Lesourd, M.; Gaujoux, V.; Lamberton, F.; Ibarrola, D.; Cavaliere, C.; Alfano, V.; Aiello, M.; et al. The cortical thickness of the area PF of the left inferior parietal cortex mediates technical-reasoning skills. Sci. Rep. 2022, 12, 11840. [Google Scholar] [CrossRef]
- Grèzes, J.; Decety, J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta-analysis. Hum. Brain Mapp. 2011, 12, 1–19. [Google Scholar] [CrossRef]
- Iacoboni, M. Neural mechanisms of imitation. Curr. Opin. Neurobiol. 2005, 15, 632–637. [Google Scholar] [CrossRef]
- Iacoboni, M. Neurobiology of imitation. Curr. Opin. Neurobiol. 2009, 19, 661–665. [Google Scholar] [CrossRef]
- Yang, J.; Hofmann, J. Action observation and imitation in autism spectrum disorders: An ALE meta-analysis of fMRI studies. Brain Imaging. Behav. 2016, 10, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.; Heymans, U.; Birbaumer, N.; Veit, R.; Erb, M.; Flor, H.; Halsband, U. Differential cerebral activation during observation of expressive gestures and motor acts. Neuropsychologia 2006, 44, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Yuan, Y. Broca’s area is jointly activated during speech and gesture production. Neuroreport 2018, 29, 1214–1216. [Google Scholar] [CrossRef]
- Marstaller, L.; Burianová, H. A common functional neural network for overt production of speech and gesture. Neuroscience 2015, 284, 29–41. [Google Scholar] [CrossRef]
- Molenberghs, P.; Brander, C.; Mattingley, J.B.; Cunnington, R. The role of the superior temporal sulcus and the mirror neuron system in imitation. Hum. Brain Mapp. 2010, 31, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Frey, S.H.; Maloof, F.R.; Newman-Norlund, R.; Farrer, C.; Inati, S.; Grafton, S.T. Actions or hand-object interactions? Human inferior frontal cortex and action observation. Neuron 2003, 39, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Kilner, J.; Rodrigues, E.; Joffily, M.; Nighoghossian, N.; Vargas, C.; Sirigu, A. Role of the parietal cortex in predicting incoming actions. NeuroImage 2012, 59, 556–564. [Google Scholar] [CrossRef]
- Joue, G.; Boven, L.; Willmes, K.; Evola, V.; Demenescu, L.R.; Hassemer, J.; Mittelberg, I.; Mathiak, K.; Schneider, F.; Habel, U. Metaphor processing is supramodal semantic processing: The role of the bilateral lateral temporal regions in multimodal communication. Brain Lang. 2020, 205, 104772. [Google Scholar] [CrossRef]
- Aldaqre, I.; Schuwerk, T.; Daum, M.M.; Sodian, B.; Paulus, M. Sensitivity to communicative and non-communicative gestures in adolescents and adults with autism spectrum disorder: Saccadic and pupillary responses. Exp. Brain Res. 2016, 234, 2515–2527. [Google Scholar] [CrossRef]
- Koldewyn, K.; Whitney, D.; Rivera, S.M. The psychophysics of visual motion and global form processing in autism. Brain 2010, 133 Pt 2, 599–610. [Google Scholar] [CrossRef]
- Annaz, D.; Campbell, R.; Coleman, M.; Milne, E.; Swettenham, J. Young children with autism spectrum disorder do not preferentially attend to biological motion. J. Autism Dev. Disord. 2012, 42, 401–408. [Google Scholar] [CrossRef]
- Caron, M.J.; Mottron, L.; Berthiaume, C.; Dawson, M. Cognitive mechanisms, specificity and neural underpinnings of visuospatial peaks in autism. Brain 2006, 129 Pt 7, 1789–1802. [Google Scholar] [CrossRef]
- Muth, A.; Hönekopp, J.; Falter, C.M. Visuo-spatial performance in autism: A meta-analysis. J. Autism Dev. Disord. 2014, 44, 3245–3263. [Google Scholar] [CrossRef]
- Kaldy, Z.; Giserman, I.; Carter, A.S.; Blaser, E. The mechanisms underlying the ASD advantage in visual search. J. Autism Dev. Disord. 2016, 46, 1513–1527. [Google Scholar] [CrossRef]
- Pennisi, P.; Giallongo, L.; Milintenda, G.; Cannarozzo, M. Autism, autistic traits and creativity: A systematic review and meta-analysis. Cogn. Process. 2021, 22, 1–36. [Google Scholar] [CrossRef]
- Grèzes, J.; Wicker, B.; Berthoz, S.; de Gelder, B. A failure to grasp the affective meaning of actions in autism spectrum disorder subjects. Neuropsychologia 2009, 47, 1816–1825. [Google Scholar] [CrossRef]
- Somogyi, E.; Király, I.; Gergely, G.; Nadel, J. Understanding goals and intentions in low-functioning autism. Res. Dev. Disabil. 2013, 34, 3822–3832. [Google Scholar] [CrossRef]
- Perkins, T.J.; Bittar, R.G.; McGillivray, J.A.; Cox, I.I.; Stokes, M.A. Increased premotor cortex activation in high functioning autism during action observation. J. Clin. Neurosci. 2015, 22, 664–669. [Google Scholar] [CrossRef]
- Willems, R.M.; Ozyürek, A.; Hagoort, P. Differential roles for left inferior frontal and superior temporal cortex in multimodal integration of action and language. NeuroImage 2009, 47, 1992–2004. [Google Scholar] [CrossRef]
- Belyk, M.; Brown, S.; Lim, J.; Kotz, S.A. Convergence of semantics and emotional expression within the IFG pars orbitalis. NeuroImage 2017, 156, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Pelphrey, K.A.; Mitchell, T.V.; McKeown, M.J.; Goldstein, J.; Allison, T.; McCarthy, G. Brain activity evoked by the perception of human walking: Controlling for meaningful coherent motion. J. Neurosci. 2003, 23, 6819–6825. [Google Scholar] [CrossRef]
- Choi, B.; Shah, P.; Rowe, M.L.; Nelson, C.A.; Tager-Flusberg, H. Gesture development, caregiver responsiveness, and language and diagnostic outcomes in infants at high and low risk for autism. J. Autism Dev. Disord. 2020, 50, 2556–2572. [Google Scholar] [CrossRef] [PubMed]
- de Marchena, A.; Kim, E.S.; Bagdasarov, A.; Parish-Morris, J.; Maddox, B.B.; Brodkin, E.S.; Schultz, R.T. Atypicalities of gesture form and function in autistic adults. J. Autism Dev. Disord. 2019, 49, 1438–1454. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.N.; Landa, R.J.; Galloway, J.C. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Phys. Ther. 2011, 91, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.N. Motor impairment increases in children with autism spectrum disorder as a function of social communication, cognitive and functional impairment, repetitive behavior severity, and comorbid diagnoses: A SPARK study report. Autism Res. 2021, 14, 202–219. [Google Scholar] [CrossRef]
- Kaur, M.; Srinivasan, S.; Bhat, A. Comparing motor performance, praxis, coordination, and interpersonal synchrony between children with and without Autism Spectrum Disorder (ASD). Res. Dev. Disabil. 2018, 72, 79–95. [Google Scholar] [CrossRef]
- Bhat, A.N.; Boulton, A.J.; Tulsky, D.S. A further study of relations between motor impairment and social communication, cognitive, language, functional impairments, and repetitive behavior severity in children with ASD using the SPARK study dataset. Autism Res. 2022, 15, 1156–1178. [Google Scholar] [CrossRef]
- McPartland, J.C.; Lerner, M.D.; Bhat, A.; Clarkson, T.; Jack, A.; Koohsari, S.; Matuskey, D.; McQuaid, G.A.; Su, W.-C.; Trevisan, D.A. Looking back at the next 40 years of ASD neuroscience research. J. Autism Dev. Disord. 2021, 51, 4333–4353. [Google Scholar] [CrossRef]
- Su, W.-C.; Culotta, M.L.; Hoffman, M.D.; Trost, S.L.; Pelphrey, K.A.; Tsuzuki, D.; Bhat, A.N. Developmental differences in cortical activation during action observation, action execution and interpersonal synchrony: An fNIRS study. Front. Hum. Neurosci. 2020, 14, 57. [Google Scholar] [CrossRef]
- Su, W.C.; Culotta, M.; Mueller, J.; Tsuzuki, D.; Pelphrey, K.; Bhat, A. Differences in cortical activation patterns during action observation, action execution, and interpersonal synchrony between children with or without autism spectrum disorder (ASD): An fNIRS pilot study. PLoS ONE 2020, 15, e0240301. [Google Scholar] [CrossRef]
- Su, W.C.; Culotta, M.; Tsuzuki, D.; Bhat, A. Movement kinematics and cortical activation in children with and without autism spectrum disorder during sway synchrony tasks: An fNIRS study. Sci. Rep. 2021, 11, 15035. [Google Scholar] [CrossRef] [PubMed]
- Su, W.C.; Culotta, M.; Tsuzuki, D.; Bhat, A. Cortical activation during cooperative joint actions and competition in children with and without an autism spectrum condition (ASC): An fNIRS study. Sci. Rep. 2022, 12, 5177. [Google Scholar] [CrossRef] [PubMed]
- Su, W.C.; Culotta, M.; Mueller, J.; Tsuzuki, D.; Bhat, A. fNIRS-based differences in cortical activation during tool use, pantomimed actions, and meaningless actions between children with and without Autism Spectrum Disorder (ASD). Brain Sci. 2023, 13, 876. [Google Scholar] [CrossRef] [PubMed]
- Lord, C. Autism Diagnostic Observation Schedule, 2nd ed.; Western Psychological Services: Los Angeles, CA, USA, 2012. [Google Scholar]
- Roid, G.H. Stanford-Binet Intelligence Scales, 5th ed.; Riverside Publishing: Itasca, IL, USA, 2003. [Google Scholar]
- Rutter, M.; Bailey, A.; Lord, C. The social communication questionnaire: Manual. Los Angeles West. Psychol. Serv. 2003, 29, 30. [Google Scholar]
- Coren, S. The lateral preference inventory for measurement of handedness, footedness, eyedness, and earedness: Norms for young adults. Bull. Psychon. Soc. 1993, 31, 1–3. [Google Scholar] [CrossRef]
- Sparrow, S.S.; Cicchetti, D.V.; Balla, D.A. Vineland Adaptive Behavior Scales, 2nd ed.; NCS Pearson: San Antonio, TX, USA, 2005. [Google Scholar]
- Constantino, J.N.; Gruber, C.P. Social Responsiveness Scale; Western Psychological Services: Los Angeles, CA, USA, 2005. [Google Scholar]
- Cairns, R.B.; Leung, M.C.; Gest, S.D.; Cairns, B.D. Interpersonal Competence Scale-Teacher Form (ICS-T); APA PsycTests: Washington, DC, USA, 1995. [Google Scholar]
- Klem, G.H.; Lüders, H.O.; Jasper, H.H.; Elger, C. The ten-twenty electrode system of the international federation. The international federation of clinical neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 3–6. [Google Scholar] [PubMed]
- Lloyd-Fox, S.; Blasi, A.; Elwell, C.E. Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neurosci. Biobehav. Rev. 2010, 34, 269–284. [Google Scholar] [CrossRef]
- Tsuzuki, D.; Cai, D.S.; Dan, H. Stable and convenient spatial registration of stand-alone NIRS data through anchor-based probabilistic registration. Neurosci. Res. 2012, 72, 163–171. [Google Scholar] [CrossRef]
- Okamoto, M.; Dan, H.; Sakamoto, K.; Takeo, K.; Shimizu, K.; Kohno, S.; Oda, I.; Isobe, S.; Suzuki, T.; Kohyama, K.; et al. Threedimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. NeuroImage 2004, 21, 99–111. [Google Scholar] [CrossRef]
- Shattuck, D.W.; Mirza, M.; Adisetiyo, V.; Hojatkashani, C.; Salamon, G.; Narr, K.L.; Poldrack, R.A.; Bilder, R.M.; Toga, A.W. Construction of a 3D probabilistic atlas of human cortical structures. NeuroImage 2008, 39, 1064–1080. [Google Scholar] [CrossRef]
- Bhat, A.N.; Hoffman, M.D.; Trost, S.L.; Culotta, M.L.; Eilbott, J.; Tsuzuki, D.; Pelphrey, K.A. Cortical activation during action observation, action execution, and interpersonal synchrony in adults: A functional near-infrared spectroscopy (fNIRS) study. Front. Hum. Neurosci. 2017, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Sutoko, S.; Sato, H.; Maki, A.; Kiguchi, M.; Hirabayashi, Y.; Atsumori, H.; Obata, A.; Funane, T.; Katura, T. Tutorial on platform for optical topography analysis tools. Neurophotonics 2016, 3, 010801. [Google Scholar] [CrossRef]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, 280–298. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Fuchino, Y.; Kiguchi, M.; Katura, T.; Maki, A.; Yoro, T.; Koizumi, H. Intersubject variability of near-infrared spectroscopy signals during sensorimotor cortex activation. J. Biomed. Opt. 2005, 10, 44001. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Dan, I. Exploring the false discovery rate in multichannel NIRS. NeuroImage 2006, 33, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Mostofsky, S.H.; Ewen, J.B. Altered connectivity and action model formation in autism is autism. Neuroscientist 2011, 17, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Fournier, K.A.; Hass, C.J.; Naik, S.K.; Lodha, N.; Cauraugh, J.H. Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. J. Autism Dev. Disord. 2010, 40, 1227–1240. [Google Scholar] [CrossRef]
- Eigsti, I.M.; de Marchena, A. Perspectives on gesture from autism spectrum disorder: Alterations in timing and function. Behav. Brain Sci. 2017, 40, e53. [Google Scholar] [CrossRef]
- De Marchena, A.; Eigsti, I.M. Conversational gestures in autism spectrum disorders: Asynchrony but not decreased frequency. Autism Res. 2010, 3, 311–322. [Google Scholar] [CrossRef]
- Semin, G.; Cacioppo, J. From Embodied Representation to Coregulation in Mirror Neuron Systems; Springer Publishing: Cham, Switzerland, 2009. [Google Scholar]
- Vesper, C.; Butterfill, S.; Knoblich, G.; Sebanz, N. A minimal architecture for joint action. Neural. Netw. 2010, 23, 998–1003. [Google Scholar] [CrossRef]
- Franchini, M.; Glaser, B.; Wood de Wilde, H.; Gentaz, E.; Eliez, S.; Schaer, M. Social orienting and joint attention in preschoolers with autism spectrum disorders. PLoS ONE 2017, 12, e0178859. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.L. Evaluating the theory of executive dysfunction in autism. Dev. Rev. 2004, 24, 189–233. [Google Scholar] [CrossRef]
- Zheng, R.; Naiman, I.D.; Skultety, J.; Passmore, S.R.; Lyons, J.; Glazebrook, C.M. The impact of different movement types on motor planning and execution in individuals with autism spectrum disorder. Motor. Control 2019, 23, 398–417. [Google Scholar] [CrossRef] [PubMed]
- Hafri, A.; Trueswell, J.C.; Epstein, R.A. Neural Representations of Observed Actions Generalize across Static and Dynamic Visual Input. J. Neurosci. 2017, 37, 3056–3071. [Google Scholar] [CrossRef]
- Cunnington, R.; Windischberger, C.; Robinson, S.; Moser, E. The selection of intended actions and the observation of others’ actions: A time-resolved fMRI study. NeuroImage 2006, 29, 1294–1302. [Google Scholar] [CrossRef]
- Ocampo, B.; Kritikos, A.; Cunnington, R. How frontoparietal brain regions mediate imitative and complementary actions: An FMRI study. PLoS ONE 2011, 6, e26945. [Google Scholar] [CrossRef]
- Vingerhoets, G. Contribution of the posterior parietal cortex in reaching, grasping, and using objects and tools. Front. Psychol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Bhoyroo, R.; Hands, B.; Caeyenberghs, K.; de Luca, A.; Leemans, A.; Wigley, A.; Hyde, C. Association between Motor Planning and the Frontoparietal Network in Children: An Exploratory Multimodal Study. J. Int. Neuropsychol. Soc. 2022, 28, 926–936. [Google Scholar] [CrossRef]
- Limanowski, J.; Sarasso, P.; Blankenburg, F. Different responses of the right superior temporal sulcus to visual movement feedback during self-generated vs. externally generated hand movements. Eur. J. Neurosci. 2018, 47, 314–320. [Google Scholar] [CrossRef]
- Hakuno, Y.; Pirazzoli, L.; Blasi, A.; Johnson, M.H.; Lloyd-Fox, S. Optical imaging during toddlerhood: Brain responses during naturalistic social interactions. Neurophotonics 2018, 5, 011020. [Google Scholar] [CrossRef]
- Gianotti, L.; Lobmaier, J.S.; Calluso, C.; Dahinden, F.M.; Knoch, D. Theta resting EEG in TPJ/pSTS is associated with individual differences in the feeling of being looked at. Soc. Cogn. Affect. Neurosci. 2018, 13, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Pierce, K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Curr. Opin. Neurobiol. 2005, 15, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Su, W.C.; Srinivasan, S.; Cleffi, C.; Bhat, A. Short report on research trends during the COVID-19 pandemic and use of telehealth interventions and remote brain research in children with autism spectrum disorder. Autism 2021, 25, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Su, W.C.; Amonkar, N.; Cleffi, C.; Srinivasan, S.; Bhat, A. Neural effects of physical activity and movement interventions in individuals with developmental disabilities- a systematic review. Front. Psychiatry 2022, 13, 794652. [Google Scholar] [CrossRef]
- Su, W.C.; Dashtestani, H.; Miguel, H.O.; Condy, E.; Buckley, A.; Park, S.; Perreault, J.B.; Nguyen, T.; Zeytinoglu, S.; Millerhagen, J.; et al. Simultaneous multimodal fNIRS-EEG recordings reveal new insights in neural activity during motor execution, observation, and imagery. Sci. Rep. 2023, 13, 5151. [Google Scholar] [CrossRef]








| Characteristics | ASD Group (n = 15) Mean ± SE | TD Group (n = 17) Mean ± SE |
|---|---|---|
| Age | 11.1 ± 0.9 | 10.8 ± 0.7 |
| Sex | 11 M, 4 F | 11 M, 6 F |
| Race | 10 C, 3 A, 2 MR | 13 C; 1 A; 1 AI; 2 MR |
| Ethnicity | 15 NH, 0 H | 17 NH, 0 H |
| SES-Child | 65.9 ± 5.1 | 69.7 ± 4.4 |
| Handedness Coren’s score | Right-handed 33.5 ± 1.5 | Right-handed 33.4 ± 1.8 |
| SCQ | 24.5 ± 3.2 | - |
| ADOS Social affect Repetitive behavior | 18.9 ± 1.9 13.7 ± 1.4 5.2 ± 0.6 | - |
| Stanford-Binet IQ Full Scale IQ Verbal IQ Non-verbal IQ | 82.1 ± 7.6 * 87.2 ± 7.9 * 71.23± 6.4 * | 114.2 ± 1.7 114.6 ± 2.3 114.5 ± 1.7 |
| VABS-II (SS) Communication (SS) Daily living (SS) Socialization (SS) | 71.1 ± 3.2 * 73.5 ± 3.3 * 68.0 ± 3.8 * 76.6 ± 3.8 * | 110.3 ± 2.9 109.2 ± 2.6 108.1 ± 3.4 110.5 ± 3.2 |
| SRS (T scores) | 78.7 ± 2.2 * | 45.1 ± 1.3 |
| ICS | 4.2 ± 0.2 * | 5.1 ± 0.2 |
| r-Values | SIPT-PP Spatial Error | SIPT-PP Modulation Error | ||||
|---|---|---|---|---|---|---|
| W | D | T | W | D | T | |
| TD | ||||||
| Left hemisphere | ||||||
| MIFG MSTG IPL | 0.031 0.072 0.209* | 0.113 0.181 0.125 | −0.054 −0.014 −0.148 | −0.082 0.135 0.056 | 0.322 ** 0.270 ** 0.063 | 0.187 0.116 −0.167 |
| Right hemisphere | ||||||
| MIFG MSTG IPL | 0.329 ** 0.046 0.286 ** | 0.323 ** 0.123 0.341 ** | 0.159 0.066 0.132 | 0.249 ** −0.018 0.241 * | 0.418 ** 0.026 0.294 ** | 0.279 ** 0.035 0.009 |
| ASD | ||||||
| Left hemisphere | ||||||
| MIFG MSTG IPL | −0.083 −0.013 0.031 | −0.108 −0.100 −0.037 | −0.185 −0.047 0.023 | 0.000 −0.004 0.162 | −0.171 −0.153 −0.086 | −0.157 0.026 −0.108 |
| Right hemisphere | ||||||
| MIFG MSTG IPL | −0.177 0.054 0.169 | −0.041 0.082 0.085 | −0.294 ** −0.059 0.003 | −0.192 0.078 0.066 | −0.138 −0.137 −0.094 | −0.314 ** −0.088 −0.182 |
| r-Values | VABS-Communication | VABS-Socialization | ||||
|---|---|---|---|---|---|---|
| W | D | T | W | D | T | |
| TD | ||||||
| Left hemisphere | ||||||
| MIFG MSTG IPL | 0.024 0.009 0.087 | 0.107 0.134 0.152 | 0.162 0.237 * -0.274 | −0.094 −0.110 0.006 | −0.112 −0.103 0.063 | −0.247 ** 0.138 −0.133 |
| Right hemisphere | ||||||
| MIFG MSTG IPL | 0.047 0.049 0.045 | 0.035 0.334 ** 0.151 | −0.089 0.209 * −0.103 | −0.103 −0.113 −0.104 | −0.069 0.214 * 0.013 | −0.024 0.211 * −0.081 |
| ASD | ||||||
| Left hemisphere | ||||||
| MIFG MSTG IPL | −0.064 0.081 −0.026 | −0.064 0.120 −0.139 | −0.095 0.088 −0.172 | −0.177 0.034 −0.111 | 0.130 0.132 −0.111 | −0.266 ** 0.000 −0.170 |
| Right hemisphere | ||||||
| MIFG MSTG IPL | 0.057 0.273 ** −0.027 | 0.052 0.205 * 0.161 | −0.009 0.194 * −0.083 | 0.004 0.223 * −0.003 | 0.089 0.186 0.147 | −0.018 0.162 −0.062 |
| r-Values | ADOS-SA | ADOS-RRB | SCQ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| W | D | T | W | D | T | W | D | T | |
| ASD | |||||||||
| Left hemisphere | |||||||||
| MIFG MSTG IPL | 0.138 0.126 0.241 * | −0.087 −0.077 −0.052 | 0.011 0.188 0.086 | 0.033 0.013 0.190 | −0.299 * −0.305 * −0.011 | −0.130 0.003 0.054 | 0.093 0.019 0.142 | −0.243 * −0.388 ** −0.081 | 0.071 0.020 0.077 |
| Right hemisphere | |||||||||
| MIFG MSTG IPL | −0.019 −0.067 0.137 | −0.035 −0.161 −0.248 * | −0.048 −0.247 * −0.024 | −0.102 0.085 0.104 | −0.242 * −0.004 −0.051 | −0.186 −0.017 0.070 | −0.006 −0.113 0.165 | −0.250 * −0.109 −0.166 | −0.047 −0.106 0.220 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, W.-C.; Culotta, M.; Mueller, J.; Tsuzuki, D.; Bhat, A.N. Autism-Related Differences in Cortical Activation When Observing, Producing, and Imitating Communicative Gestures: An fNIRS Study. Brain Sci. 2023, 13, 1284. https://doi.org/10.3390/brainsci13091284
Su W-C, Culotta M, Mueller J, Tsuzuki D, Bhat AN. Autism-Related Differences in Cortical Activation When Observing, Producing, and Imitating Communicative Gestures: An fNIRS Study. Brain Sciences. 2023; 13(9):1284. https://doi.org/10.3390/brainsci13091284
Chicago/Turabian StyleSu, Wan-Chun, McKenzie Culotta, Jessica Mueller, Daisuke Tsuzuki, and Anjana N. Bhat. 2023. "Autism-Related Differences in Cortical Activation When Observing, Producing, and Imitating Communicative Gestures: An fNIRS Study" Brain Sciences 13, no. 9: 1284. https://doi.org/10.3390/brainsci13091284