An Evaluation of the Effectiveness of Repetitive Transcranial Magnetic Stimulation (rTMS) for the Management of Treatment-Resistant Depression with Somatic Attributes: A Hospital-Based Study in Oman
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Setting of the Study
2.2. Data Collection
2.3. Participant Inclusion and Exclusion Criteria
2.4. rTMS Protocol
2.5. Outcome Measures
2.5.1. Diagnosis of Major Depressive Disorders
2.5.2. Somatic Attributes
2.5.3. The Hamilton Rating Scale for Depression
2.6. Data Analysis
2.7. Ethical Approval
3. Results
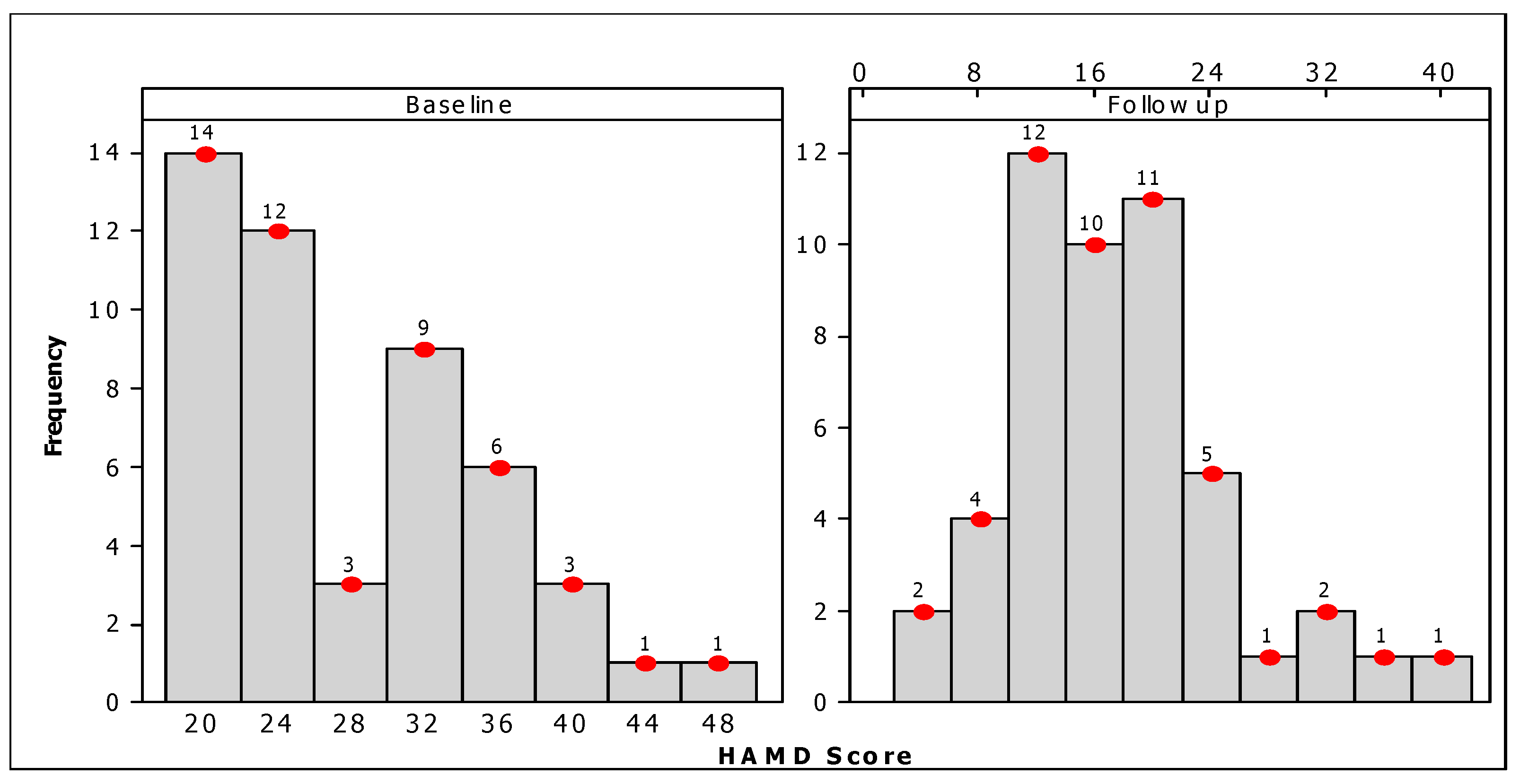
3.1. Profiles of the Participants
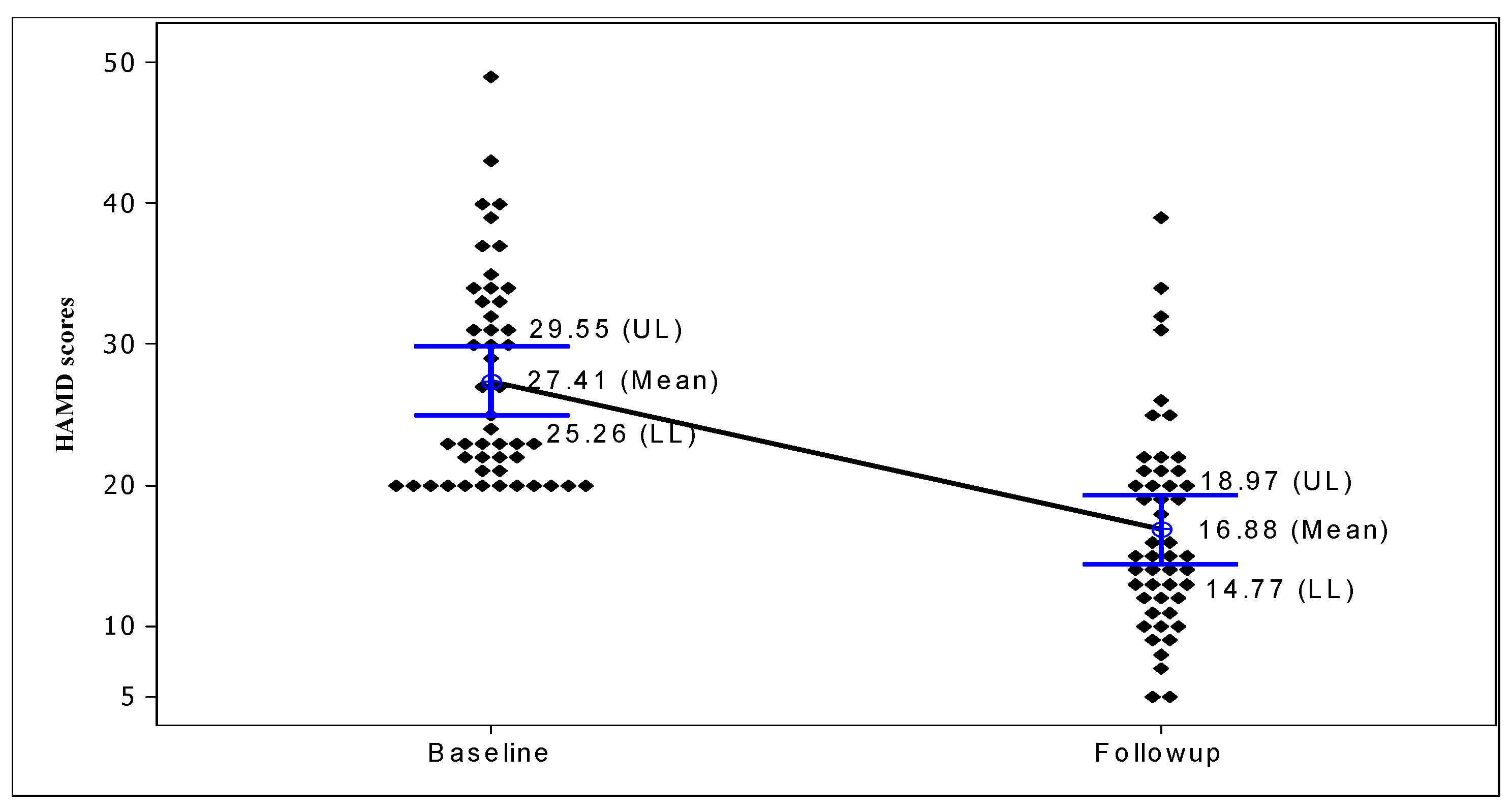
3.2. Evaluation of rTMS Treatment
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Assembly. Global Burden of Mental Disorders and the Need for a Comprehensive, Coordinated Response From Health and Social Sectors at the Country Level: Report by the Secretariat; World Health Organization: Geneva, Switzerland, 2012; 65; Available online: https://apps.who.int/iris/handle/10665/78898 (accessed on 8 July 2023).
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017; pp. 1–24.
- Draguns, J.G.; Tanaka-Matsumi, J. Assessment of psychopathology across and within cultures: Issues and findings. Behav. Res. Ther. 2003, 41, 755–776. [Google Scholar] [CrossRef] [PubMed]
- Carothers, J.C.; World Health Organization. The African Mind in Health and Disease: A Study in Ethnopsychiatry; World Health Organization: Geneva, Switzerland, 1953.
- Bhugra, D.; Mastrogianni, A. Globalisation and mental disorders. Overview with relation to depression. Br. J. Psychiatry 2004, 184, 10–20. [Google Scholar] [CrossRef]
- Kalibatseva, Z.; Leong, F.T.L. Cultural Factors, Depressive and Somatic Symptoms Among Chinese American and European American College Students. J. Cross-Cult. Psychol. 2018, 49, 1556–1572. [Google Scholar] [CrossRef]
- Ma-Kellams, C. Cross-cultural differences in somatic awareness and interoceptive accuracy: A review of the literature and directions for future research. Front. Psychol. 2014, 5, 1379. [Google Scholar] [CrossRef] [PubMed]
- Okulate, G.T.; Olayinka, M.O.; Jones, O.B. Somatic symptoms in depression: Evaluation of their diagnostic weight in an African setting. Br. J. Psychiatry 2004, 184, 422–427. [Google Scholar] [CrossRef]
- Deacon, B.J. The biomedical model of mental disorder: A critical analysis of its validity, utility, and effects on psychotherapy research. Clin. Psychol. Rev. 2013, 33, 846–861. [Google Scholar] [CrossRef] [PubMed]
- Gartlehner, G.; Dobrescu, A.; Chapman, A.; Toromanova, A.; Emprechtinger, R.; Persad, E.; Affengruber, L.; Pieh, C.; Klerings, I.; Wagner, G. Nonpharmacologic and Pharmacologic Treatments of Adult Patients with Major Depressive Disorder: A Systematic Review and Network Meta-Analysis for a Clinical Guideline by the American College of Physicians. Ann. Intern. Med. 2023, 176, 196–211. [Google Scholar] [CrossRef]
- Gadot, R.; Najera, R.; Hirani, S.; Anand, A.; Storch, E.; Goodman, W.K.; Shofty, B.; A Sheth, S.A. Efficacy of deep brain stimulation for treatment-resistant obsessive-compulsive disorder: Systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2022, 93, 1166–1173. [Google Scholar] [CrossRef]
- Karrouri, R.; Hammani, Z.; Benjelloun, R.; Otheman, Y. Major depressive disorder: Validated treatments and future challenges. World J. Clin. Cases. 2021, 9, 9350–9367. [Google Scholar] [CrossRef]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2022, 1–14. [Google Scholar] [CrossRef]
- Jaffe, D.H.; Rive, B.; Denee, T.R. The humanistic and economic burden of treatment-resistant depression in Europe: A cross-sectional study. BMC Psychiatry 2019, 19, 247. [Google Scholar] [CrossRef] [PubMed]
- Nemeroff, C.B. Prevalence and management of treatment-resistant depression. J. Clin. Psychiatry 2007, 68, 17. [Google Scholar] [PubMed]
- Zhang, Y.; Zhu, D.; Zhou, X.; Liu, Y.; Qin, B.; Ren, G.; Xie, P. Bilateral repetitive transcranial magnetic stimulation for treatment-resistant depression: A systematic review and meta-analysis of randomized controlled trials. Braz. J. Med. Biol. Res. 2015, 48, 198–206. [Google Scholar] [CrossRef]
- Avery, D.H.; Holtzheimer, P.E., III; Fawaz, W.; Russo, J.; Neumaier, J.; Dunner, D.L.; Haynor, D.R.; Claypoole, K.H.; Wajdik, C.; Roy-Byrne, P. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol. Psychiatry 2006, 59, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.B.; Brown, T.L.; Marston, N.A.U.; Daskalakis, Z.J.; de Castella, A.; Kulkarni, J. Transcranial magnetic stimulation in the treatment of depression: A double-blind, placebo-controlled trial. Arch. Gen. Psychiatry 2003, 60, 1002–1008. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Hoy, K.; Daskalakis, Z.J.; Kulkarni, J. A randomized trial of the anti-depressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depress. Anxiety 2009, 26, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Baeken, C.; De Raedt, R. Neurobiological mechanisms of repetitive transcranial magnetic stimulation on the underlying neurocircuitry in unipolar depression. Dialogues Clin. Neurosci. 2011, 13, 139–145. [Google Scholar] [CrossRef]
- Padberg, F.; Zwanzger, P.; E Keck, M.; Kathmann, N.; Mikhaiel, P.; Ella, R.; Rupprecht, P.; Thoma, H.; Hampel, H.; Toschi, N.; et al. Repetitive transcranial magnetic stimulation (rTMS) in major depression: Relation between efficacy and stimulation intensity. Neuropsychopharmacology 2002, 27, 638–645. [Google Scholar] [CrossRef]
- Drevets, W.C. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog. Brain Res. 2000, 126, 413–431. [Google Scholar] [CrossRef]
- George, M.S.; Wassermann, E.M.; Kimbrell, T.A.; Little, J.T.; Williams, W.E.; Danielson, A.L.; Greenberg, B.D.; Hallett, M.; Post, R.M. Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: A placebo-controlled crossover trial. Am. J. Psychiatry 1997, 154, 1752–1756. [Google Scholar] [CrossRef]
- Garcia-Toro, M.; Mayol, A.; Arnillas, H.; Capllonch, I.; Ibarra, O.; Crespí, M.; Micó, J.; Lafau, O.; Lafuente, L. Modest adjunctive benefit with transcranial magnetic stimulation in medication-resistant depression. J. Affect. Disord. 2001, 64, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.; Kreinin, I.; Chistyakov, A.; Koren, D.; Mecz, L.; Marmur, S.; Ben-Shachar, D.; Feinsod, M. Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: A double-blind controlled study. Arch. Gen. Psychiatry 1999, 56, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Al-Sibani, N.; Al-Maqbali, M.; Mahadevan, S.; Al-Huseini, S.; Al-Muzeni, M.; Al-Adawi, S. Psychiatric, cognitive functioning and socio-cultural views of menstrual psychosis in Oman: An idiographic approach. BMC Womens Health 2020, 20, 215. [Google Scholar] [CrossRef] [PubMed]
- Chand, S.P.; Al-Hussaini, A.A.; Martin, R.; Mustapha, S.; Zaidan, Z.; Viernes, N.; Al-Adawi, S. Dissociative disorders in the Sultanate of Oman. Acta Psychiatr. Scand. 2000, 102, 185–187. [Google Scholar] [CrossRef]
- Al-Sharbati, M.M.; Viernes, N.; Al-Hussaini, A.; Zaidan, Z.A.J.; Chand, P.; Al-Adawi, S. A case of bilateral ptosis with unsteady gait: Suggestibility and culture in conversion disorder. Int. J. Psychiatry Med. 2001, 31, 225–232. [Google Scholar] [CrossRef]
- Nielsen, G.; Stone, J.; Edwards, M.J. Physiotherapy for functional (psychogenic) motor symptoms: A systematic review. J. Psychosom. Res. 2013, 75, 93–102. [Google Scholar] [CrossRef]
- FitzGerald, T.L.; Southby, A.K.; Haines, T.P.; Hough, J.P.; Skinner, E.H. Is physiotherapy effective in the management of child and adolescent conversion disorder? A systematic review. J. Paediatr. Child Health 2015, 51, 159–167. [Google Scholar] [CrossRef]
- World Health Organization. Composite International Diagnostic Interview; WHO: Geneva, Switzerland, 1993.
- Eche, J.; Mondino, M.; Haesebaert, F.; Saoud, M.; Poulet, E.; Brunelin, J. Low- vs. High-Frequency Repetitive Transcranial Magnetic Stimulation as an Add-On Treatment for Refractory Depression. Front. Psychiatry 2012, 3, 13. [Google Scholar] [CrossRef]
- Goldberg, D.; Privett, M.; Ustun, B.; Simon, G.; Linden, M. The effects of detection and treatment on the outcome of major depression in primary care: A naturalistic study in 15 cities. Br. J. Gen. Pract. 1998, 48, 1840–1844. [Google Scholar]
- Viernes, N.; Zaidan, Z.A.; Dorvlo, A.S.; Kayano, M.; Yoishiuchi, K.; Kumano, H.; Kuboki, T.; Al-Adawi, S. Tendency toward deliberate food restriction, fear of fatness and somatic attribution in cross-cultural samples. Eat Behav. 2007, 8, 407–417. [Google Scholar] [CrossRef]
- Al-Lawati, J.; Al-Lawati, N.; Al-Siddiqui, M.; Antony, S.X.; Al-Naamani, A.; Martin, R.G.; Kolbe, R.; Theodorsson, T.; Osman, Y.; Al-Hussaini, A.A.; et al. Psychological morbidity in primary healthcare in Oman: A preliminary study. J. Sci. Res. Med. Sci. 2000, 2, 105–110. [Google Scholar]
- Zahid, M.A.; Motaal, M.A.A.; Razik, M.A. Psychiatric morbidity among medical out-patients in Kuwait: Evaluation of a somatic inventory to identify the psychiatric from the non-psychiatric patients. Med. Princ. Pract. 2001, 10, 23–28. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- von Glischinski, M.; von Brachel, R.; Thiele, C.; Hirschfeld, G. Not sad enough for a depression trial? A systematic review of depression measures and cut points in clinical trial registrations. J. Affect. Disord. 2021, 292, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Asghar, J.; Tabasam, M.; Althobaiti, M.M.; Ashour, A.A.; Aleid, M.A.; Khalaf, O.I.; Aldhyani, T.H.H. A Randomized Clinical Trial Comparing Two Treatment Strategies, Evaluating the Meaningfulness of HAM-D Rating Scale in Patients with Major Depressive Disorder. Front. Psychiatry 2022, 13, 873693. [Google Scholar] [CrossRef]
- Emanuel, E.J.; Wendler, D.; Grady, C. What makes clinical research ethical? JAMA 2000, 283, 2701–2711. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef]
- DiBernardo, A.; Lin, X.; Zhang, Q.; Xiang, J.; Lu, L.; Jamieson, C.; Benson, C.; Lee, K.; Bodén, R.; Brandt, L.; et al. Humanistic outcomes in treatment resistant depression: A secondary analysis of the STAR*D study. BMC Psychiatry 2018, 18, 352. [Google Scholar] [CrossRef]
- Arroll, B.; Elley, C.R.; Fishman, T.; A Goodyear-Smith, F.; Kenealy, T.; Blashki, G.; Kerse, N.; MacGillivray, S. Antidepressants versus placebo for depression in primary care. Cochrane Database Syst. Rev. 2009, 3, CD007954. [Google Scholar] [CrossRef]
- Mora, M.S.; Nestoriuc, Y.; Rief, W. Lessons learned from placebo groups in antidepressant trials. Philos. Trans. R Soc. B Biol. Sci. 2011, 366, 1879–1888. [Google Scholar] [CrossRef]
- Bailey, R.K.; Patel, M.; Barker, N.C.; Ali, S.; Jabeen, S. Major depressive disorder in the African American population. J. Natl. Med. Assoc. 2011, 103, 548–557. [Google Scholar] [CrossRef]
- Greden, J.F. Physical symptoms of depression: Unmet needs. J. Clin. Psychiatry 2003, 64 (Suppl. 7), 5–11. [Google Scholar]
- Siddiqi, S.H.; Taylor, S.F.; Cooke, D.; Pascual-Leone, A.; George, M.S.; Fox, M.D. Distinct Symptom-Specific Treatment Targets for Circuit-Based Neuromodulation. Am. J. Psychiatry 2020, 177, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Kaster, T.S.; Downar, J.; Vila-Rodriguez, F.; Baribeau, D.A.; Thorpe, K.E.; Daskalakis, Z.J.; Blumberger, D.M. Differential symptom cluster responses to repetitive transcranial magnetic stimulation treatment in depression. EClinicalMedicine 2022, 55, 101765. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.B. Targeting repetitive transcranial magnetic stimulation in depression: Do we really know what we are stimulating and how best to do it? Brain Stimul. 2021, 14, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Connolly, K.R.; Helmer, A.; Cristancho, M.A.; Cristancho, P.; O’reardon, J.P. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: Results observed with the first 100 consecutive cases of depression at an academic medical center. J. Clin. Psychiatry 2012, 73, e567–e573. [Google Scholar] [CrossRef]
- Berlim, M.T.; Van den Eynde, F.; Jeff Daskalakis, Z. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: A meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology 2013, 38, 543–551. [Google Scholar] [CrossRef]
- Carpenter, L.L.; Janicak, P.G.; Aaronson, S.T.; Boyadjis, T.; Brock, D.G.; Cook, I.A.; Dunner, D.L.; Lanocha, K.; Solvason, H.B.; Demitrack, M.A. Transcranial magnetic stimulation (TMS) for major depression: A multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress. Anxiety 2012, 29, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, H.; Bukhari, F.; Nazir, M.; Anwar, M.N.; Shahzad, A. Therapeutic Efficacy of Neurostimulation for Depression: Techniques, Current Modalities, and Future Challenges. Neurosci. Bull. 2016, 32, 115–126. [Google Scholar] [CrossRef]
- Padberg, F.; Zwanzger, P.; Thoma, H.; Kathmann, N.; Haag, C.; Greenberg, B.D.; Hampel, H.; Möller, H.-J. Repetitive transcranial magnetic stimulation (rTMS) in pharmacotherapy-refractory major depression: Comparative study of fast, slow and sham rTMS. Psychiatry Res. 1999, 88, 163–171. [Google Scholar] [CrossRef]
- Leggett, L.E.; Soril, L.J.J.; Coward, S.; Lorenzetti, D.L.; MacKean, G.; Clement, F.M. Repetitive Transcranial Magnetic Stimulation for Treatment-Resistant Depression in Adult and Youth Populations: A Systematic Literature Review and Meta-Analysis. Prim Care Companion CNS Disord. 2015, 17, 17476. [Google Scholar] [CrossRef] [PubMed]
- Kedzior, K.K.; Reitz, S.K.; Azorina, V.; Loo, C. Durability of the antidepressant effect of the high-frequency repetitive transcranial magnetic stimulation (rTMS) in the absence of maintenance treatment in major depression: A systematic review and meta-analysis of 16 double-blind, randomized, sham-controlled trials. Depress. Anxiety 2015, 32, 193–203. [Google Scholar] [PubMed]
- Razza, L.B.; Moffa, A.H.; Moreno, M.L.; Carvalho, A.F.; Padberg, F.; Fregni, F.; Brunoni, A.R. A systematic review and meta-analysis on placebo response to repetitive transcranial magnetic stimulation for depression trials. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 81, 105–113. [Google Scholar] [CrossRef] [PubMed]

| Number of Courses of Antidepressant Treatment | n | % |
|---|---|---|
| Patients with 2 antidepressants as monotherapy | 19 | 38.8 |
| Patients with 3 antidepressants as monotherapy | 20 | 40.8 |
| Patients with 4 antidepressants | 10 | 20.4 |
| Variable | Total Sample n (%) | Reduction in the HAMD Score | ||
|---|---|---|---|---|
| No Response | Response | p-Value 𝔛 | ||
| Total | 49 (100) | 31 (63.3) | 18 (36.7) | |
| Gender | 0.332 | |||
| Male | 34 (69.4) | 20 (58.8) | 14 (41.2) | |
| Female | 15 (30.6) | 11 (73.3) | 4 (26.7) | |
| Age | 0.519 | |||
| <40 | 22 (44.9) | 15 (68.2) | 7 (31.8) | |
| 40+ | 27 (55.1) | 21 (57.1) | 11 (42.9) | |
| Age, mean ± SD | 42.53 ± 13.31 | 40.77 ± 12.89 | 45.56 ± 13.84 | 0.229 |
| Place of Living | ||||
| Urban | 35 (71.4) | 21 (60.0) | 14 (40.0) | 0.453 |
| Rural | 14 (28.6) | 10 (71.4) | 4 (28.6) | |
| Marital status | ||||
| Single | 15 (30.6) | 10 (66.7) | 5 (33.3) | 0.743 |
| Married | 34 (69.4) | 21 (61.8) | 13 (38.2) | |
| Job | ||||
| Working | 26 (53.1) | 16 (61.5) | 10 (38.5) | 0.790 |
| Not working | 23 (46.9) | 15 (65.2) | 8 (34.8) | |
| History of alcohol | ||||
| Yes | 6 (12.2) | 3 (50.0) | 3 (50.0) | 0.259 |
| No | 24 (49.0) | 17 (70.8) | 7 (29.2) | |
| Unknown | 19 (38.8) | 11 (57.9) | 8 (42.1) | |
| Previous rTMS | ||||
| Yes | 9 (18.4) | 5 (55.6) | 4 (44.4) | 0.595 |
| No | 40 (81.6) | 26 (65.0) | 14 (35.0) | |
| Target area | ||||
| Left DLPFC | 27 (55.1) | 14 (51.9) | 13 (48.1) | 0.066 |
| Bilateral | 22 (44.9) | 17 (77.3) | 5 (22.7) | |
| rTMS protocol used | ||||
| FDA standard 10 HZ | 27 (55.1) | 14 (51.9) | 13 (48.1) | 0.066 |
| Theta bursts | 22 (44.9) | 17 (77.3) | 5 (22.7) | |
| Baseline Mean ± SD | Follow-Up Mean ± SD | t | p-Value | |
|---|---|---|---|---|
| Overall | 27.41 ± 7.46 | 16.88 ± 7.34 | 10.82 | <0.001 |
| Group | ||||
| Response | 27.72 ± 7.57 | 11.17 ± 3.92 | 8.71 | <0.001 |
| Non-response | 27.23 ± 7.46 | 20.19 ± 6.81 | 3.25 | 0.002 |
| rTMS protocol used | ||||
| Standard 10 HZ | 26.30 ± 6.02 | 14.04 ± 4.68 | 8.36 | <0.001 |
| Theta bursts | 28.77 ± 8.87 | 20.36 ± 8.53 | 3.21 | 0.003 |
| B | S.E. of B | Odds Ratio (OR) | 95% CI for OR | p-Value | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age | 0.020 | 0.034 | 1.020 | 0.955 | 1.090 | 0.554 |
| Gender | ||||||
| Male | 0.113 | 0.943 | 1.120 | 0.176 | 7.109 | 0.904 |
| Female (ref.) | 0 | 1.000 | ||||
| Place of Living | ||||||
| Urban | 0.741 | 0.836 | 2.098 | 0.407 | 10.803 | 0.376 |
| Rural (ref.) | 0 | 1.000 | ||||
| Marital status | ||||||
| Married | 0.700 | 1.048 | 2.014 | 0.258 | 15.711 | 0.504 |
| Single (ref.) | 0 | 1.000 | ||||
| Work Status | ||||||
| Working | 0.509 | 0.749 | 1.663 | 0.383 | 7.218 | 0.497 |
| Not working (ref.) | 1.000 | |||||
| Previous rTMS | ||||||
| Yes | 0.362 | 0.934 | 1.436 | 0.230 | 8.957 | 0.699 |
| No | 1.000 | |||||
| rTMS protocol used | ||||||
| FDA standard 10 Hz | 1.727 | 0.870 | 5.623 | 1.022 | 30.936 | 0.037 |
| Theta-burst stimulation | 1.000 | 1.000 | ||||
| History of alcohol | ||||||
| Yes | 1.575 | 1.333 | 4.830 | 0.354 | 65.820 | 0.237 |
| No | 1.000 | |||||
| Constant | −3.784 | 1.972 | .023 | 0.055 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ruhaili, I.; Al-Huseini, S.; Al-Kaabi, S.; Mahadevan, S.; Al-Sibani, N.; Al Balushi, N.; Islam, M.M.; Jose, S.; Mehr, G.K.; Al-Adawi, S. An Evaluation of the Effectiveness of Repetitive Transcranial Magnetic Stimulation (rTMS) for the Management of Treatment-Resistant Depression with Somatic Attributes: A Hospital-Based Study in Oman. Brain Sci. 2023, 13, 1289. https://doi.org/10.3390/brainsci13091289
Al-Ruhaili I, Al-Huseini S, Al-Kaabi S, Mahadevan S, Al-Sibani N, Al Balushi N, Islam MM, Jose S, Mehr GK, Al-Adawi S. An Evaluation of the Effectiveness of Repetitive Transcranial Magnetic Stimulation (rTMS) for the Management of Treatment-Resistant Depression with Somatic Attributes: A Hospital-Based Study in Oman. Brain Sciences. 2023; 13(9):1289. https://doi.org/10.3390/brainsci13091289
Chicago/Turabian StyleAl-Ruhaili, Intisar, Salim Al-Huseini, Said Al-Kaabi, Sangeetha Mahadevan, Nasser Al-Sibani, Naser Al Balushi, M. Mazharul Islam, Sachin Jose, Gilda Kiani Mehr, and Samir Al-Adawi. 2023. "An Evaluation of the Effectiveness of Repetitive Transcranial Magnetic Stimulation (rTMS) for the Management of Treatment-Resistant Depression with Somatic Attributes: A Hospital-Based Study in Oman" Brain Sciences 13, no. 9: 1289. https://doi.org/10.3390/brainsci13091289
APA StyleAl-Ruhaili, I., Al-Huseini, S., Al-Kaabi, S., Mahadevan, S., Al-Sibani, N., Al Balushi, N., Islam, M. M., Jose, S., Mehr, G. K., & Al-Adawi, S. (2023). An Evaluation of the Effectiveness of Repetitive Transcranial Magnetic Stimulation (rTMS) for the Management of Treatment-Resistant Depression with Somatic Attributes: A Hospital-Based Study in Oman. Brain Sciences, 13(9), 1289. https://doi.org/10.3390/brainsci13091289







