Brain Response to Interferential Current Compared with Alternating Current Stimulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Stereotaxic Surgery
2.3. fMRI Experiments
2.4. fMRI Data Processing
3. Results
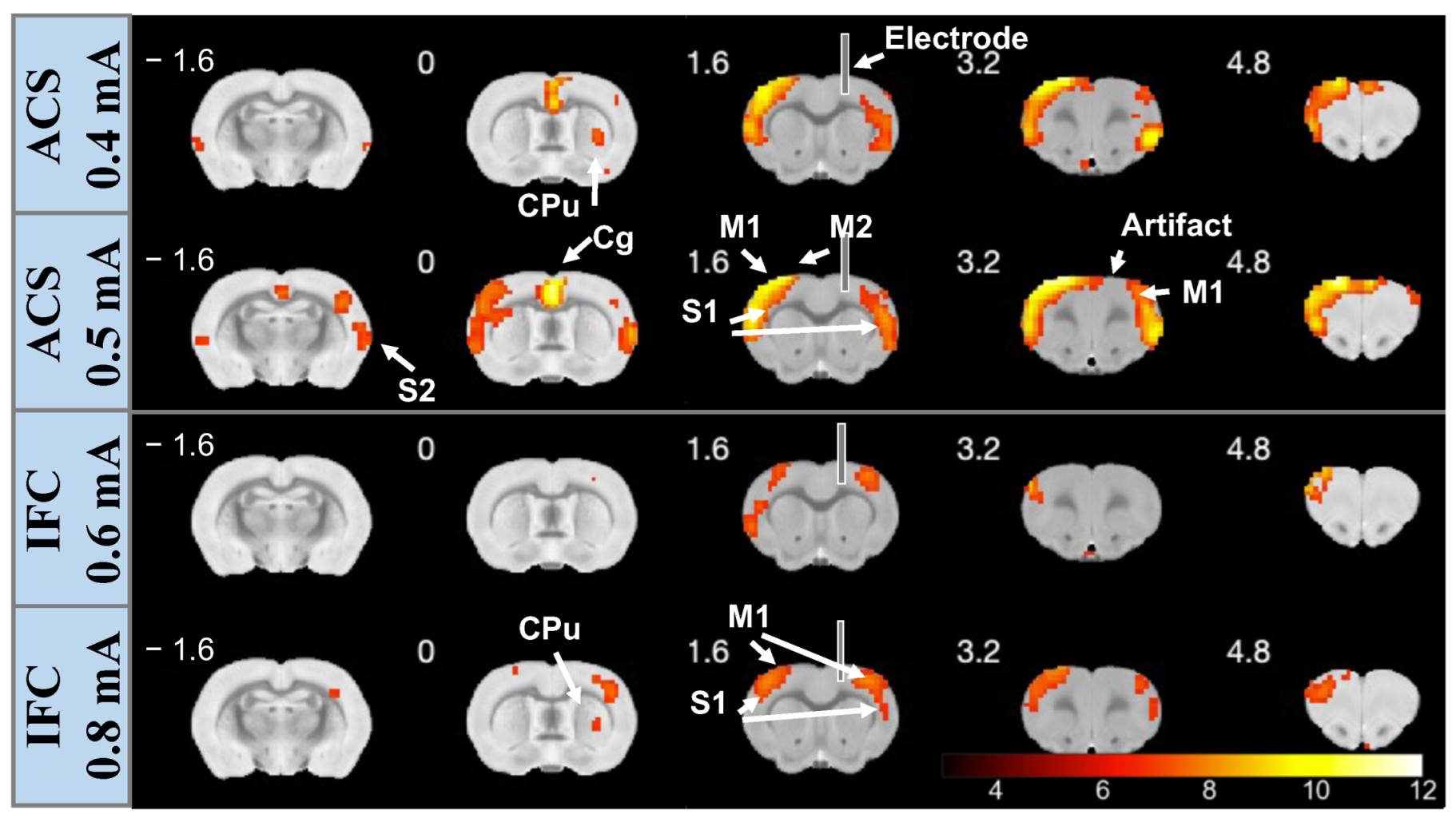
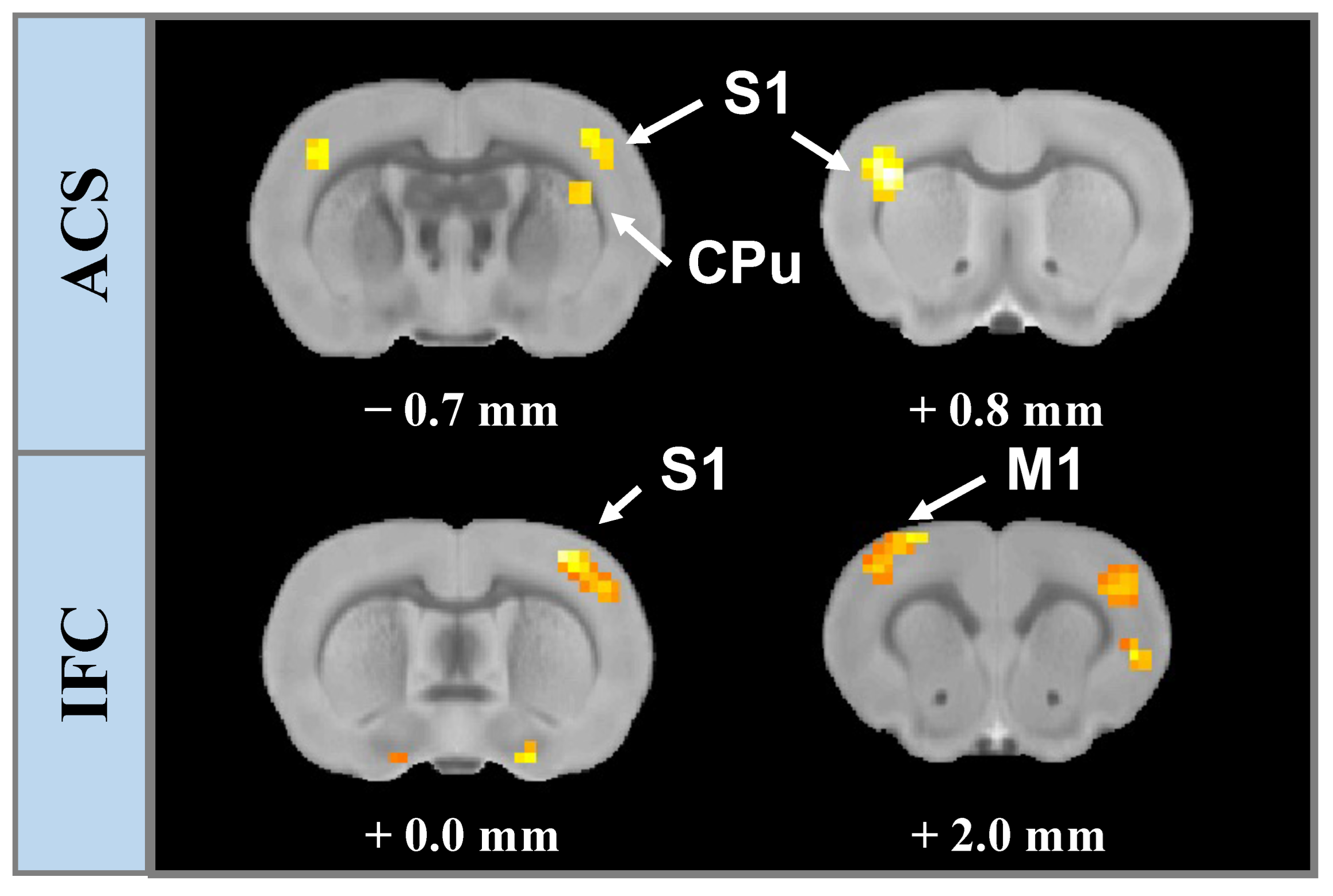
3.1. Spatial Contribution of Whole-Brain BOLD Activation Evoked by Stimulation
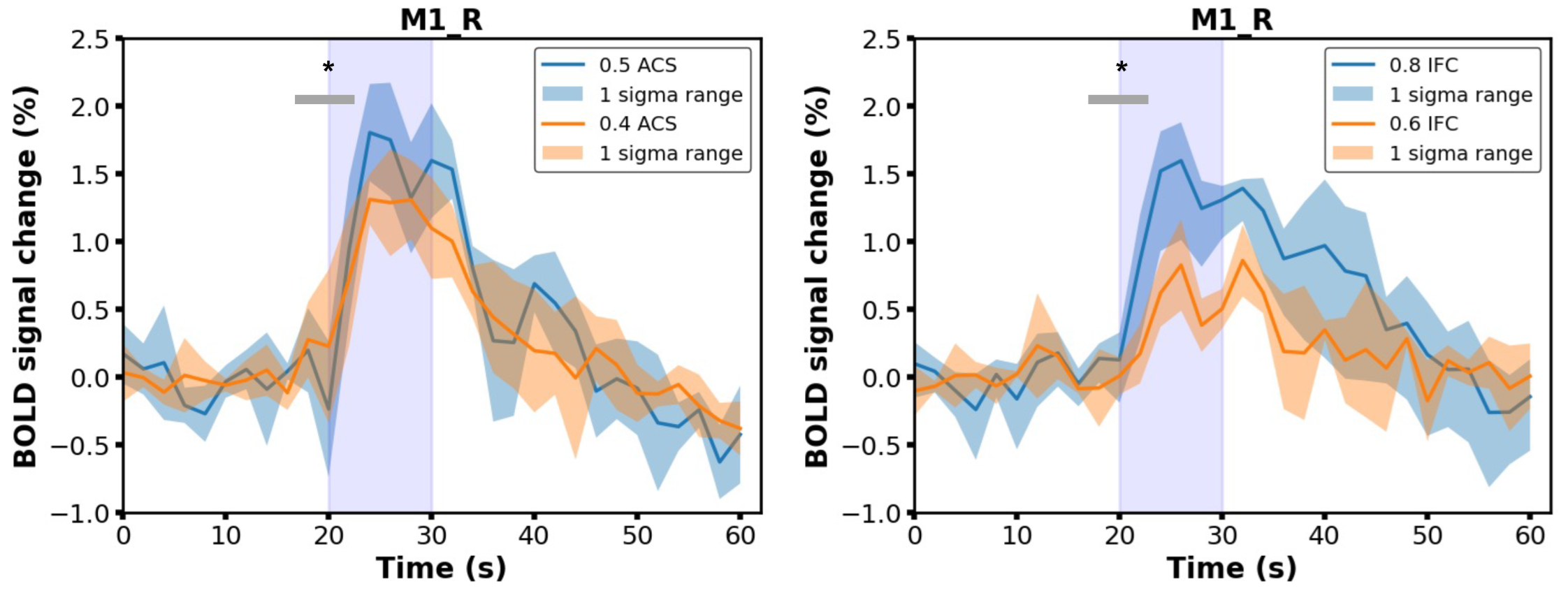
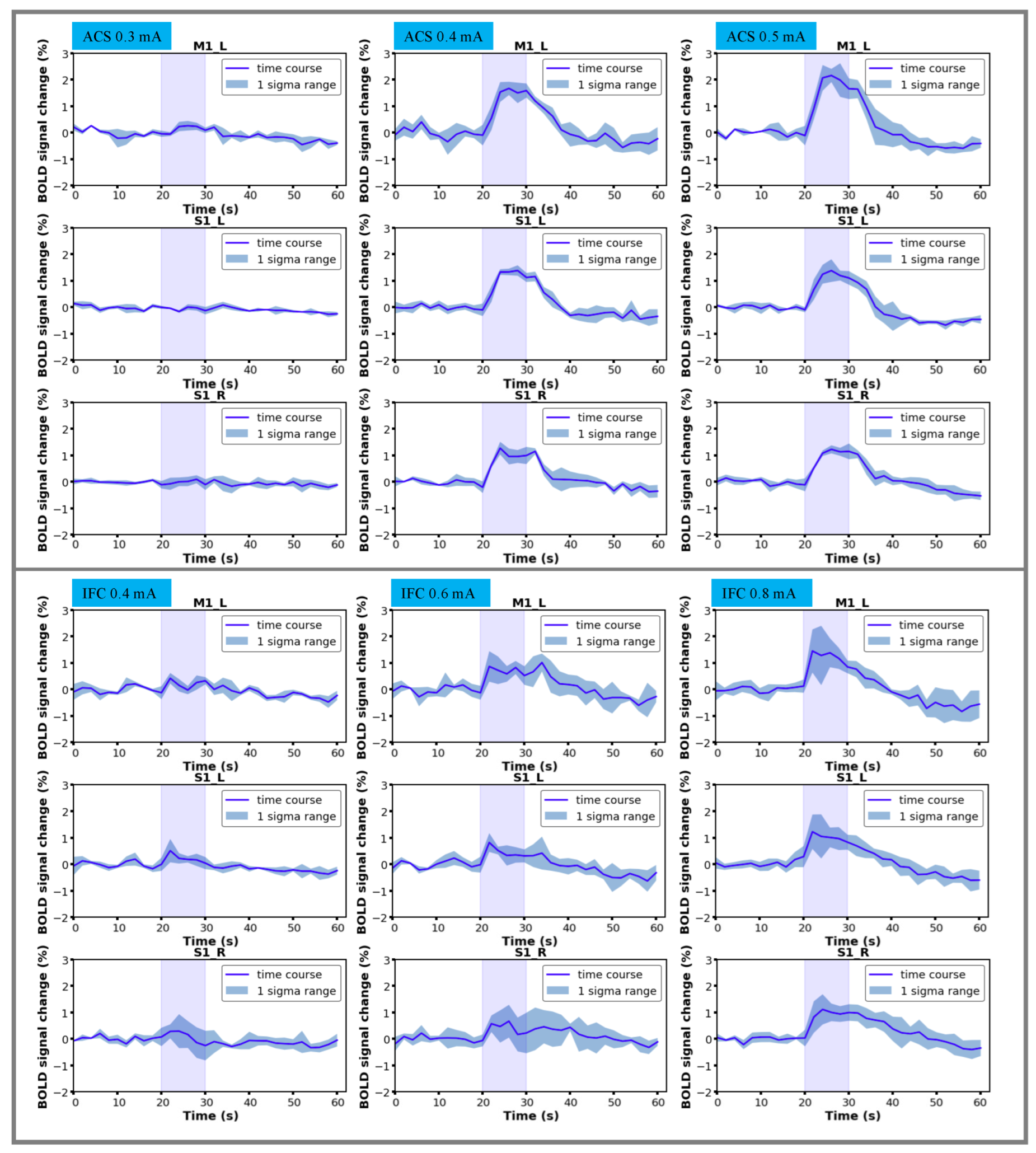
3.2. Characterization of BOLD Time Courses
3.3. Stimulation Effects Modulated by Stimulus Intensity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Grossman, N.; Bono, D.; Dedic, N.; Kodandaramaiah, S.B.; Rudenko, A.; Suk, H.J.; Cassara, A.M.; Neufeld, E.; Kuster, N.; Tsai, L.H.; et al. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 2017, 169, 1029–1041.e16. [Google Scholar] [CrossRef] [PubMed]
- Hutcheon, B.; Yarom, Y. Resonance, oscillation and the intrinsic frequency preferences of neurons. Trends Neurosci. 2000, 23, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, S.; Roig-Solvas, B.; Yarossi, M.; Kulkarni, P.P.; Santarnecchi, E.; Dorval, A.D.; Brooks, D.H. Prospects for transcranial temporal interference stimulation in humans: A computational study. NeuroImage 2019, 202, 116124. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, Y.; Datta, A.; Parra, L.C. Optimization of interferential stimulation of the human brain with electrode arrays. J. Neural Eng. 2020, 17, 36023. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.; Lee, C.; Im, C.H. Multipair transcranial temporal interference stimulation for improved focalized stimulation of deep brain regions: A simulation study. Comput. Biol. Med. 2022, 143, 105337. [Google Scholar] [CrossRef]
- Huang, Y.; Parra, L.C. Can transcranial electric stimulation with multiple electrodes reach deep targets? Brain Stimul. 2019, 12, 30–40. [Google Scholar] [CrossRef]
- Lee, S.; Lee, C.; Park, J.; Im, C.H. Individually customized transcranial temporal interference stimulation for focused modulation of deep brain structures: A simulation study with different head models. Sci. Rep. 2020, 10, 11730. [Google Scholar] [CrossRef]
- Song, X.; Zhao, X.; Li, X.; Liu, S.; Ming, D. Multi-channel transcranial temporally interfering stimulation (tTIS): Application to living mice brain. J. Neural Eng. 2021, 18, 036003. [Google Scholar] [CrossRef]
- Missey, F.; Rusina, E.; Acerbo, E.; Botzanowski, B.; Trébuchon, A.; Bartolomei, F.; Jirsa, V.; Carron, R.; Williamson, A. Orientation of Temporal Interference for Non-Invasive Deep Brain Stimulation in Epilepsy. Front. Neurosci. 2021, 15, 656. [Google Scholar] [CrossRef]
- Cao, J.; Grover, P. Do single neuron models exhibit temporal interference stimulation? In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OH, USA, 17–19 October 2018; pp. 1–4. [Google Scholar]
- Mirzakhalili, E.; Barra, B.; Capogrosso, M.; Lempka, S.F. Biophysics of Temporal Interference Stimulation. Cell Syst. 2020, 11, 557–572.e5. [Google Scholar] [CrossRef]
- Huang, W.A.; Stitt, I.M.; Negahbani, E.; Passey, D.; Ahn, S.; Davey, M.; Dannhauer, M.; Doan, T.T.; Hoover, A.C.; Peterchev, A.V.; et al. Transcranial alternating current stimulation entrains alpha oscillations by preferential phase synchronization of fast-spiking cortical neurons to stimulation waveform. Nat. Commun. 2021, 12, 3151. [Google Scholar] [CrossRef] [PubMed]
- Vöröslakos, M.; Takeuchi, Y.; Brinyiczki, K.; Zombori, T.; Oliva, A.; Fernández-Ruiz, A.; Kozák, G.; Kincses, Z.T.; Iványi, B.; Buzsáki, G.; et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat. Commun. 2018, 9, 483. [Google Scholar] [CrossRef] [PubMed]
- Negahbani, E.; Kasten, F.H.; Herrmann, C.S.; Fröhlich, F. Targeting alpha-band oscillations in a cortical model with amplitude-modulated high-frequency transcranial electric stimulation. Neuroimage 2018, 173, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilpour, Z.; Kronberg, G.; Reato, D.; Parra, L.C.; Bikson, M. Temporal interference stimulation targets deep brain regions by modulating neural oscillations. Brain Stimul. 2021, 14, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Abe, Y.; Liu, S.; Tanaka, K.F.; Hosomi, K.; Saitoh, Y.; Sekino, M. Direct Impact of Motor Cortical Stimulation on the Blood Oxygen-Level Dependent Response in Rats. Magn. Reson. Med. Sci. 2021, 20, 83. [Google Scholar] [CrossRef]
- Abe, Y.; Tsurugizawa, T.; Le Bihan, D.; Ciobanu, L. Spatial contribution of hippocampal BOLD activation in high-resolution fMRI. Sci. Rep. 2019, 9, 3152. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Paasonen, J.; Stenroos, P.; Salo, R.A.; Kiviniemi, V.; Gröhn, O. Functional connectivity under six anesthesia protocols and the awake condition in rat brain. Neuroimage 2018, 172, 9–20. [Google Scholar] [CrossRef]
- Fonoff, E.T.; Pereira, J.F., Jr.; Camargo, L.V.; Dale, C.S.; Pagano, R.L.; Ballester, G.; Teixeira, M.J. Functional mapping of the motor cortex of the rat using transdural electrical stimulation. Behav. Brain Res. 2009, 202, 138–141. [Google Scholar] [CrossRef]
- Barrière, D.; Magalhães, R.; Novais, A.; Marques, P.; Selingue, E.; Geffroy, F.; Marques, F.; Cerqueira, J.; Sousa, J.; Boumezbeur, F.; et al. The SIGMA rat brain templates and atlases for multimodal MRI data analysis and visualization. Nat. Commun. 2019, 10, 5699. [Google Scholar] [CrossRef]
- Valdes Hernandez, P.A.; Sumiyoshi, A.; Nonaka, H.; Haga, R.; Aubert Vasquez, E.; Ogawa, T.; Iturria Medina, Y.; Riera, J.J.; Kawashima, R. An in vivo MRI template set for morphometry, tissue segmentation, and fMRI localization in rats. Front. Neuroinformatics 2011, 5, 26. [Google Scholar]
- Poline, J.B.; Brett, M. The general linear model and fMRI: Does love last forever? Neuroimage 2012, 62, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Howell, B.; McIntyre, C.C. Feasibility of Interferential and Pulsed Transcranial Electrical Stimulation for Neuromodulation at the Human Scale. Neuromodul. Technol. Neural Interface 2021, 24, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Iszak, K.; Gronemann, S.M.; Meyer, S.; Hunold, A.; Zschüntzsch, J.; Bähr, M.; Paulus, W.; Antal, A. Why Temporal Inference Stimulation May Fail in the Human Brain: A Pilot Research Study. Biomedicines 2023, 11, 1813. [Google Scholar] [CrossRef]
- Goats, G. Interferential current therapy. Br. J. Sport. Med. 1990, 24, 87. [Google Scholar] [CrossRef]
- Fuentes, J.P.; Armijo Olivo, S.; Magee, D.J.; Gross, D.P. Effectiveness of interferential current therapy in the management of musculoskeletal pain: A systematic review and meta-analysis. Phys. Ther. 2010, 90, 1219–1238. [Google Scholar] [CrossRef]
- Austin, V.; Blamire, A.; Grieve, S.; O’Neill, M.; Styles, P.; Matthews, P.; Sibson, N. Differences in the BOLD fMRI response to direct and indirect cortical stimulation in the rat. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2003, 49, 838–847. [Google Scholar] [CrossRef]
- Polanía, R.; Paulus, W.; Nitsche, M.A. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum. Brain Mapp. 2012, 33, 2499–2508. [Google Scholar] [CrossRef]
- von Conta, J.; Kasten, F.H.; Schellhorn, K.; Ćurčić-Blake, B.; Aleman, A.; Herrmann, C.S. Benchmarking the effects of transcranial temporal interference stimulation (tTIS) in humans. Cortex 2022, 154, 299–310. [Google Scholar] [CrossRef]
- Vosskuhl, J.; Huster, R.J.; Herrmann, C.S. BOLD signal effects of transcranial alternating current stimulation (tACS) in the alpha range: A concurrent tACS–fMRI study. Neuroimage 2016, 140, 118–125. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, A.A.; Lafon, B.; Friedman, D.; Dayan, M.; Wang, X.; Bikson, M.; Doyle, W.K.; Devinsky, O.; Parra, L.C. Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation. eLife 2017, 6, e18834. [Google Scholar] [CrossRef]
- Dmochowski, J.P.; Datta, A.; Bikson, M.; Su, Y.; Parra, L.C. Optimized multi-electrode stimulation increases focality and intensity at target. J. Neural Eng. 2011, 8, 046011. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; He, Y.; Zhang, W.; Sun, Y.; Wang, J.; Liu, S.; Ming, D. A novel non-invasive brain stimulation technique: “Temporally interfering electrical stimulation”. Front. Neurosci. 2023, 17, 1092539. [Google Scholar] [CrossRef] [PubMed]
- Howarth, C.; Mishra, A.; Hall, C.N. More than just summed neuronal activity: How multiple cell types shape the BOLD response. Philos. Trans. R. Soc. B 2021, 376, 20190630. [Google Scholar] [CrossRef]
- Aksenov, D.P.; Li, L.; Miller, M.J.; Wyrwicz, A.M. Role of the inhibitory system in shaping the BOLD fMRI response. Neuroimage 2019, 201, 116034. [Google Scholar] [CrossRef]
- Moon, H.S.; Jiang, H.; Vo, T.T.; Jung, W.B.; Vazquez, A.L.; Kim, S.G. Contribution of excitatory and inhibitory neuronal activity to BOLD fMRI. Cereb. Cortex 2021, 31, 4053–4067. [Google Scholar] [CrossRef]
- Bhatia, A.; Moza, S.; Bhalla, U.S. Precise excitation-inhibition balance controls gain and timing in the hippocampus. eLife 2019, 8, e43415. [Google Scholar] [CrossRef]
- Lee, K.Y.; Bae, C.; Lee, D.; Kagan, Z.; Bradley, K.; Chung, J.M.; La, J.H. Low-intensity, kilohertz frequency spinal cord stimulation differently affects excitatory and inhibitory neurons in the rodent superficial dorsal horn. Neuroscience 2020, 428, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Shechter, R.; Yang, F.; Xu, Q.; Cheong, Y.K.; He, S.Q.; Sdrulla, A.; Carteret, A.F.; Wacnik, P.W.; Dong, X.; Meyer, R.A.; et al. Conventional and kilohertz-frequency spinal cord stimulation produces intensity-and frequency-dependent inhibition of mechanical hypersensitivity in a rat model of neuropathic pain. Anesthesiology 2013, 119, 422–432. [Google Scholar] [CrossRef]
- Sirotin, Y.B.; Das, A. Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature 2009, 457, 475–479. [Google Scholar] [CrossRef]
- Tavakoli, A.V.; Yun, K. Transcranial alternating current stimulation (tACS) mechanisms and protocols. Front. Cell. Neurosci. 2017, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Xia, X.; Zhang, W.; Lu, Z.; Wu, Q.; Cui, J.; Song, H.; Fan, C.; Chen, X.; Wei, J.; et al. High Gamma and Beta Temporal Interference Stimulation in the Human Motor 4 Cortex Improves Motor Functions. Front. Neurosci. 2022, 15, 800436. [Google Scholar] [CrossRef]
- Pariz, A.; Trotter, D.; Hutt, A.; Lefebvre, J. Selective control of synaptic plasticity in heterogeneous networks through transcranial alternating current stimulation (tACS). PLoS Comput. Biol. 2023, 19, e1010736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Yang, L.; Zhao, H. Regulation of local alternating electric fields on synaptic plasticity in brain tissue. Biomed. Eng. Lett. 2023, 13, 391–396. [Google Scholar] [CrossRef]
- Zhu, Z.; Xiong, Y.; Chen, Y.; Jiang, Y.; Qian, Z.; Lu, J.; Liu, Y.; Zhuang, J. Temporal interference (ti) stimulation boosts functional connectivity in human motor cortex: A comparison study with transcranial direct current stimulation (tdcs). Neural Plast. 2022, 2022, 7605046. [Google Scholar] [CrossRef]
- Xin, Z.; Kuwahata, A.; Liu, S.; Sekino, M. Magnetically induced temporal interference for focal and deep-brain stimulation. Front. Hum. Neurosci. 2021, 15, 693207. [Google Scholar] [CrossRef] [PubMed]
- Kilgore, K.L.; Bhadra, N. Reversible nerve conduction block using kilohertz frequency alternating current. Neuromodul. Technol. Neural Interface 2014, 17, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Neudorfer, C.; Chow, C.T.; Boutet, A.; Loh, A.; Germann, J.; Elias, G.J.; Hutchison, W.D.; Lozano, A.M. Kilohertz-frequency stimulation of the nervous system: A review of underlying mechanisms. Brain Stimul. 2021, 14, 513–530. [Google Scholar] [CrossRef]
- Nguyen, J.P.; Nizard, J.; Keravel, Y.; Lefaucheur, J.P. Invasive brain stimulation for the treatment of neuropathic pain. Nat. Rev. Neurol. 2011, 7, 699–709. [Google Scholar] [CrossRef]
- Liu, X.; Qiu, F.; Hou, L.; Wang, X. Review of noninvasive or minimally invasive deep brain stimulation. Front. Behav. Neurosci. 2022, 15, 820017. [Google Scholar] [CrossRef]
- Lai, H.Y.; Albaugh, D.L.; Kao, Y.C.J.; Younce, J.R.; Shih, Y.Y.I. Robust deep brain stimulation functional MRI procedures in rats and mice using an MR-compatible tungsten microwire electrode. Magn. Reson. Med. 2015, 73, 1246–1251. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, Z.; Abe, Y.; Kuwahata, A.; Tanaka, K.F.; Sekino, M. Brain Response to Interferential Current Compared with Alternating Current Stimulation. Brain Sci. 2023, 13, 1317. https://doi.org/10.3390/brainsci13091317
Xin Z, Abe Y, Kuwahata A, Tanaka KF, Sekino M. Brain Response to Interferential Current Compared with Alternating Current Stimulation. Brain Sciences. 2023; 13(9):1317. https://doi.org/10.3390/brainsci13091317
Chicago/Turabian StyleXin, Zonghao, Yoshifumi Abe, Akihiro Kuwahata, Kenji F. Tanaka, and Masaki Sekino. 2023. "Brain Response to Interferential Current Compared with Alternating Current Stimulation" Brain Sciences 13, no. 9: 1317. https://doi.org/10.3390/brainsci13091317
APA StyleXin, Z., Abe, Y., Kuwahata, A., Tanaka, K. F., & Sekino, M. (2023). Brain Response to Interferential Current Compared with Alternating Current Stimulation. Brain Sciences, 13(9), 1317. https://doi.org/10.3390/brainsci13091317




