Sensory Gating during Voluntary Finger Movement in Amyotrophic Lateral Sclerosis with Sensory Cortex Hyperexcitability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
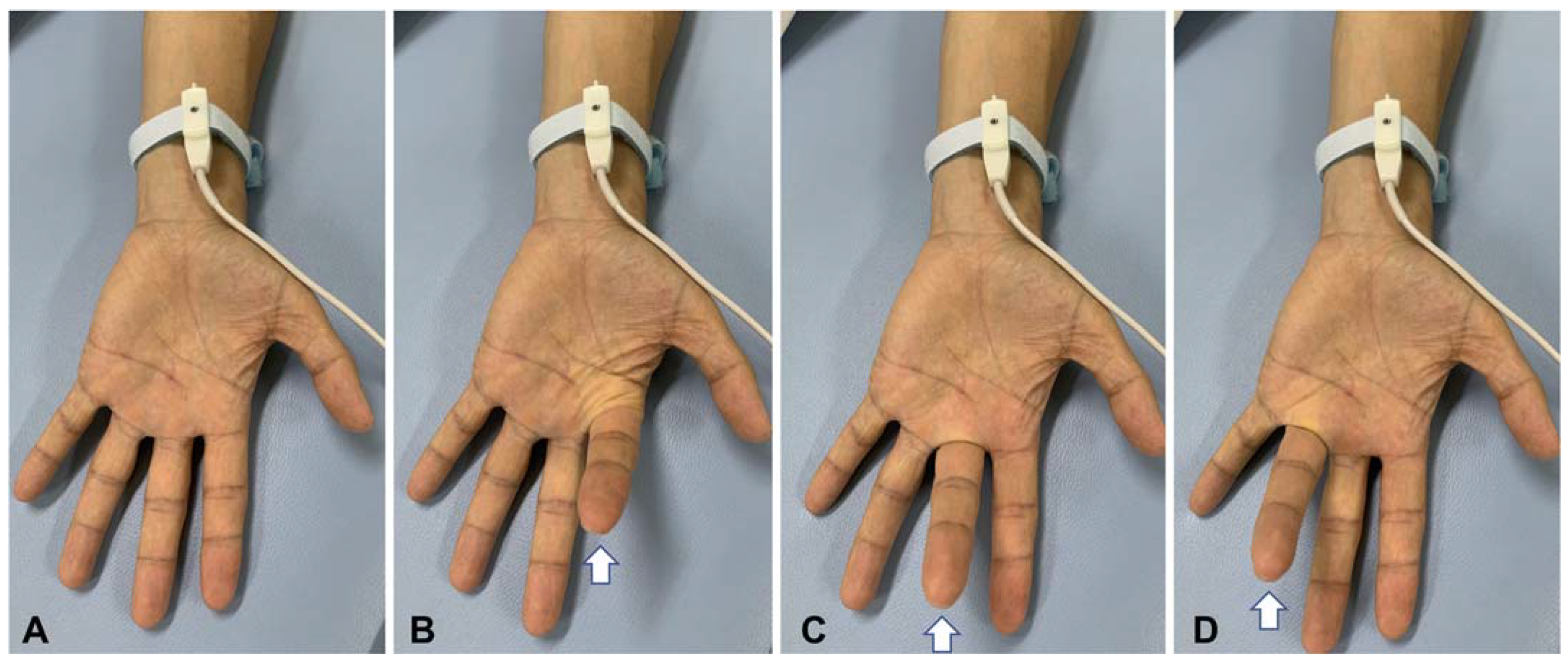
2.2. Nerve Conduction Study
2.3. Somatosensory Evoked Potential
2.4. Statistical Analysis
3. Results
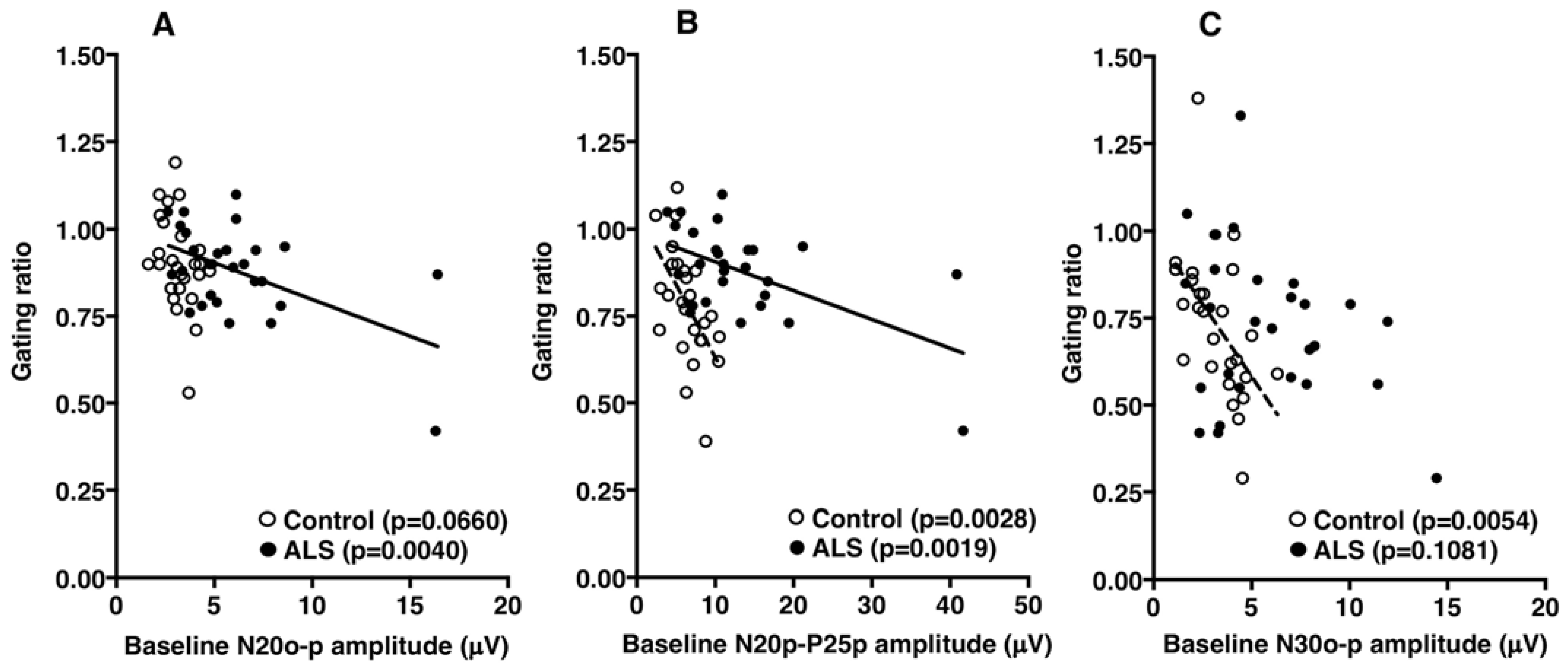
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bombaci, A.; Lupica, A.; Pozzi, F.E.; Remoli, G.; Manera, U.; Di Stefano, V. Sensory neuropathy in amyotrophic lateral sclerosis: A systematic review. J. Neurol. 2023. early online. [Google Scholar] [CrossRef] [PubMed]
- Brettschneider, J.; Del Tredici, K.; Toledo, J.B.; Robinson, J.L.; Irwin, D.J.; Grossman, M.; Suh, E.; Van Deerlin, V.M.; Wood, E.M.; Baek, Y.; et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 2013, 74, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.A.; Herrando-Grabulosa, M.; Navarro, X. Sensory Involvement in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2022, 23, 15521. [Google Scholar] [CrossRef]
- Hamada, M.; Hanajima, R.; Terao, Y.; Sato, F.; Okano, T.; Yuasa, K.; Furubayashi, T.; Okabe, S.; Arai, N.; Ugawa, Y. Median nerve somatosensory evoked potentials and their high-frequency oscillations in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2007, 118, 877–886. [Google Scholar] [CrossRef]
- Norioka, R.; Shimizu, T.; Bokuda, K.; Morishima, R.; Kawazoe, T.; Kimura, H.; Asano, Y.; Nakayama, Y.; Takahashi, K. Enlarged high frequency oscillations of the median nerve somatosensory evoked potential and survival in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2021, 132, 2003–2011. [Google Scholar] [CrossRef]
- Shimizu, T.; Bokuda, K.; Kimura, H.; Kamiyama, T.; Nakayama, Y.; Kawata, A.; Isozaki, E.; Ugawa, Y. Sensory cortex hyperexcitability predicts short survival in amyotrophic lateral sclerosis. Neurology 2018, 90, e1578–e1587. [Google Scholar] [CrossRef]
- Menon, P.; Geevasinga, N.; Yiannikas, C.; Howells, J.; Kiernan, M.C.; Vucic, S. Sensitivity and specificity of threshold tracking transcranial magnetic stimulation for diagnosis of amyotrophic lateral sclerosis: A prospective study. Lancet Neurol. 2015, 14, 478–484. [Google Scholar] [CrossRef]
- Shibuya, K.; Park, S.B.; Geevasinga, N.; Menon, P.; Howells, J.; Simon, N.G.; Huynh, W.; Noto, Y.; Gotz, J.; Kril, J.J.; et al. Motor cortical function determines prognosis in sporadic ALS. Neurology 2016, 87, 513–520. [Google Scholar] [CrossRef]
- Aguilar, J.R.; Castro-Alamancos, M.A. Spatiotemporal gating of sensory inputs in thalamus during quiescent and activated states. J. Neurosci. 2005, 25, 10990–11002. [Google Scholar] [CrossRef]
- McCormick, D.A.; Bal, T. Sensory gating mechanisms of the thalamus. Curr. Opin. Neurobiol. 1994, 4, 550–556. [Google Scholar] [CrossRef]
- Seki, K.; Fetz, E.E. Gating of sensory input at spinal and cortical levels during preparation and execution of voluntary movement. J. Neurosci. 2012, 32, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Seki, K.; Perlmutter, S.I.; Fetz, E.E. Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nat. Neurosci. 2003, 6, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, M.; Dengler, R.; Eisen, A.; England, J.D.; Kaji, R.; Kimura, J.; Mills, K.; Mitsumoto, H.; Nodera, H.; Shefner, J.; et al. Electrodiagnostic criteria for diagnosis of ALS. Clin. Neurophysiol. 2008, 119, 497–503. [Google Scholar] [CrossRef]
- Kim, W.K.; Liu, X.; Sandner, J.; Pasmantier, M.; Andrews, J.; Rowland, L.P.; Mitsumoto, H. Study of 962 patients indicates progressive muscular atrophy is a form of ALS. Neurology 2009, 73, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Ince, P.G.; Evans, J.; Knopp, M.; Forster, G.; Hamdalla, H.H.; Wharton, S.B.; Shaw, P.J. Corticospinal tract degeneration in the progressive muscular atrophy variant of ALS. Neurology 2003, 60, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Riku, Y.; Atsuta, N.; Yoshida, M.; Tatsumi, S.; Iwasaki, Y.; Mimuro, M.; Watanabe, H.; Ito, M.; Senda, J.; Nakamura, R.; et al. Differential motor neuron involvement in progressive muscular atrophy: A comparative study with amyotrophic lateral sclerosis. BMJ Open 2014, 4, e005213. [Google Scholar] [CrossRef]
- Shefner, J.M.; Al-Chalabi, A.; Baker, M.R.; Cui, L.Y.; de Carvalho, M.; Eisen, A.; Grosskreutz, J.; Hardiman, O.; Henderson, R.; Matamala, J.M.; et al. A proposal for new diagnostic criteria for ALS. Clin. Neurophysiol. 2020, 131, 1975–1978. [Google Scholar] [CrossRef]
- Cedarbaum, J.M.; Stambler, N.; Malta, E.; Fuller, C.; Hilt, D.; Thurmond, B.; Nakanishi, A. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J. Neurol. Sci. 1999, 169, 13–21. [Google Scholar] [CrossRef]
- Rossini, P.M.; Babiloni, F.; Babiloni, C.; Ambrosini, A.; Onorati, P.; Urbano, A. Topography of spatially enhanced human short-latency somatosensory evoked potentials. Neuroreport 1997, 8, 991–994. [Google Scholar] [CrossRef]
- Rossini, P.M.; Caramia, D.; Bassetti, M.A.; Pasqualetti, P.; Tecchio, F.; Bernardi, G. Somatosensory evoked potentials during the ideation and execution of individual finger movements. Muscle Nerve 1996, 19, 191–202. [Google Scholar] [CrossRef]
- Allison, T.; Wood, C.C.; McCarthy, G.; Spencer, D.D. Cortical somatosensory evoked potentials. II. Effects of excision of somatosensory or motor cortex in humans and monkeys. J. Neurophysiol. 1991, 66, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Rocco-Donovan, M.; Ramos, R.L.; Giraldo, S.; Brumberg, J.C. Characteristics of synaptic connections between rodent primary somatosensory and motor cortices. Somatosens. Mot. Res. 2011, 28, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Xu, R.; Dowd, E.; Zang, Y.; Gong, H.; Wang, Z. Alterations in regional functional coherence within the sensory-motor network in amyotrophic lateral sclerosis. Neurosci. Lett. 2014, 558, 192–196. [Google Scholar] [CrossRef]
- Shimizu, T.; Nakayama, Y.; Funai, A.; Morishima, R.; Hayashi, K.; Bokuda, K.; Nakata, Y.; Isozaki, E. Progressive deterioration of sensory cortex excitability in advanced amyotrophic lateral sclerosis with invasive ventilation. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 147–149. [Google Scholar] [CrossRef]
- Cebolla, A.M.; De Saedeleer, C.; Bengoetxea, A.; Leurs, F.; Balestra, C.; d’Alcantara, P.; Palmero-Soler, E.; Dan, B.; Cheron, G. Movement gating of beta/gamma oscillations involved in the N30 somatosensory evoked potential. Hum. Brain Mapp. 2009, 30, 1568–1579. [Google Scholar] [CrossRef] [PubMed]
- Cheron, G.; Cebolla, A.M.; De Saedeleer, C.; Bengoetxea, A.; Leurs, F.; Leroy, A.; Dan, B. Pure phase-locking of beta/gamma oscillation contributes to the N30 frontal component of somatosensory evoked potentials. BMC Neurosci. 2007, 8, 75. [Google Scholar] [CrossRef]
- Barba, C.; Valeriani, M.; Colicchio, G.; Mauguiere, F. Short and middle-latency Median Nerve (MN) SEPs recorded by depth electrodes in human pre-SMA and SMA-proper. Clin. Neurophysiol. 2005, 116, 2664–2674. [Google Scholar] [CrossRef]
- Kanovsky, P.; Bares, M.; Rektor, I. The selective gating of the N30 cortical component of the somatosensory evoked potentials of median nerve is different in the mesial and dorsolateral frontal cortex: Evidence from intracerebral recordings. Clin. Neurophysiol. 2003, 114, 981–991. [Google Scholar] [CrossRef]


| Patients | Controls | p-Value | |
|---|---|---|---|
| Number | 14 | 13 | |
| Sex (male:female) | 9:5 | 7:6 | |
| Age at onset (years) | 65.4 ± 8.9 | – | |
| Age at examination (years) | 67.2 ± 8.4 | 66.2 ± 6.1 | 0.7631 |
| Onset region (bulbar:spinal) | 3:11 | – | |
| Disease duration (years) | 1.8 ± 1.0 | – | |
| ALSFRS-R | 35.8 ± 6.0 | – | |
| Body mass index (kg/m2) | 19.6 ± 3.9 | – | |
| Forced vital capacity (%) | 67.7 ± 22.3 | – | |
| CMAP amplitude (mV) * | 3.0 ± 3.3 | 7.3 ± 1.6 | <0.0001 |
| SNAP amplitude (µV) * | 27.6 ± 12.6 | 26.0 ± 9.7 | 0.2643 |
| All Patients | Controls | p-Value | PMA Patients | p-Value | |
|---|---|---|---|---|---|
| Amplitude at rest (µV) | |||||
| N20o–p | 6.12 ± 0.63 | 3.19 ± 0.16 | <0.0001 | 9.22 ± 5.61 | 0.0464 |
| N20p–P25p | 13.18 ± 1.71 | 6.41 ± 0.43 | 0.0006 | 22.90 ± 14.37 | 0.0373 |
| N30o–p | 5.76 ± 0.63 | 3.25 ± 0.26 | 0.0007 | 8.33 ± 4.26 | 0.0327 |
| Amplitude ratio | |||||
| N20o–p | 0.88 ± 0.14 | 0.90 ± 0.14 | 0.4994 | 0.82 ± 0.20 | 0.3482 |
| N20p–P25p | 0.77 ± 0.14 | 0.78 ± 0.16 | 0.7686 | 0.68 ± 0.18 | 0.2358 |
| N30o–p | 0.73 ± 0.23 | 0.73 ± 0.21 | 0.9566 | 0.54 ± 0.16 | 0.0362 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, T.; Nakayama, Y.; Bokuda, K.; Takahashi, K. Sensory Gating during Voluntary Finger Movement in Amyotrophic Lateral Sclerosis with Sensory Cortex Hyperexcitability. Brain Sci. 2023, 13, 1325. https://doi.org/10.3390/brainsci13091325
Shimizu T, Nakayama Y, Bokuda K, Takahashi K. Sensory Gating during Voluntary Finger Movement in Amyotrophic Lateral Sclerosis with Sensory Cortex Hyperexcitability. Brain Sciences. 2023; 13(9):1325. https://doi.org/10.3390/brainsci13091325
Chicago/Turabian StyleShimizu, Toshio, Yuki Nakayama, Kota Bokuda, and Kazushi Takahashi. 2023. "Sensory Gating during Voluntary Finger Movement in Amyotrophic Lateral Sclerosis with Sensory Cortex Hyperexcitability" Brain Sciences 13, no. 9: 1325. https://doi.org/10.3390/brainsci13091325
APA StyleShimizu, T., Nakayama, Y., Bokuda, K., & Takahashi, K. (2023). Sensory Gating during Voluntary Finger Movement in Amyotrophic Lateral Sclerosis with Sensory Cortex Hyperexcitability. Brain Sciences, 13(9), 1325. https://doi.org/10.3390/brainsci13091325






