Synchronous Muscle Synergy Evaluation of Jaw Muscle Activities during Chewing at Different Speeds, a Preliminary Study
Abstract
:1. Introduction
- By considering a comprehensive exploration of muscle synergies during chewing, the study acknowledges the significant individual differences in the masticatory system, such as variations in teeth health status, gum hardness, and an individual’s chewing side preference. This approach provides a better understanding of how muscle synergies adapt to such constraints and is paramount in comprehending the intricacies of the masticatory process.
- Examining muscle synergies across different chewing speeds aims to provide insights into the adaptability and flexibility of the masticatory system. This nuanced approach not only evaluates the stability of muscle synergies but also investigates how their activation patterns may change in response to variations in chewing speed. This dynamic perspective on muscle synergies during chewing is a unique aspect of the study and can provide a valuable contribution to the field.
2. Materials and Methods
2.1. Participants
2.2. Muscle Synergy Analysis
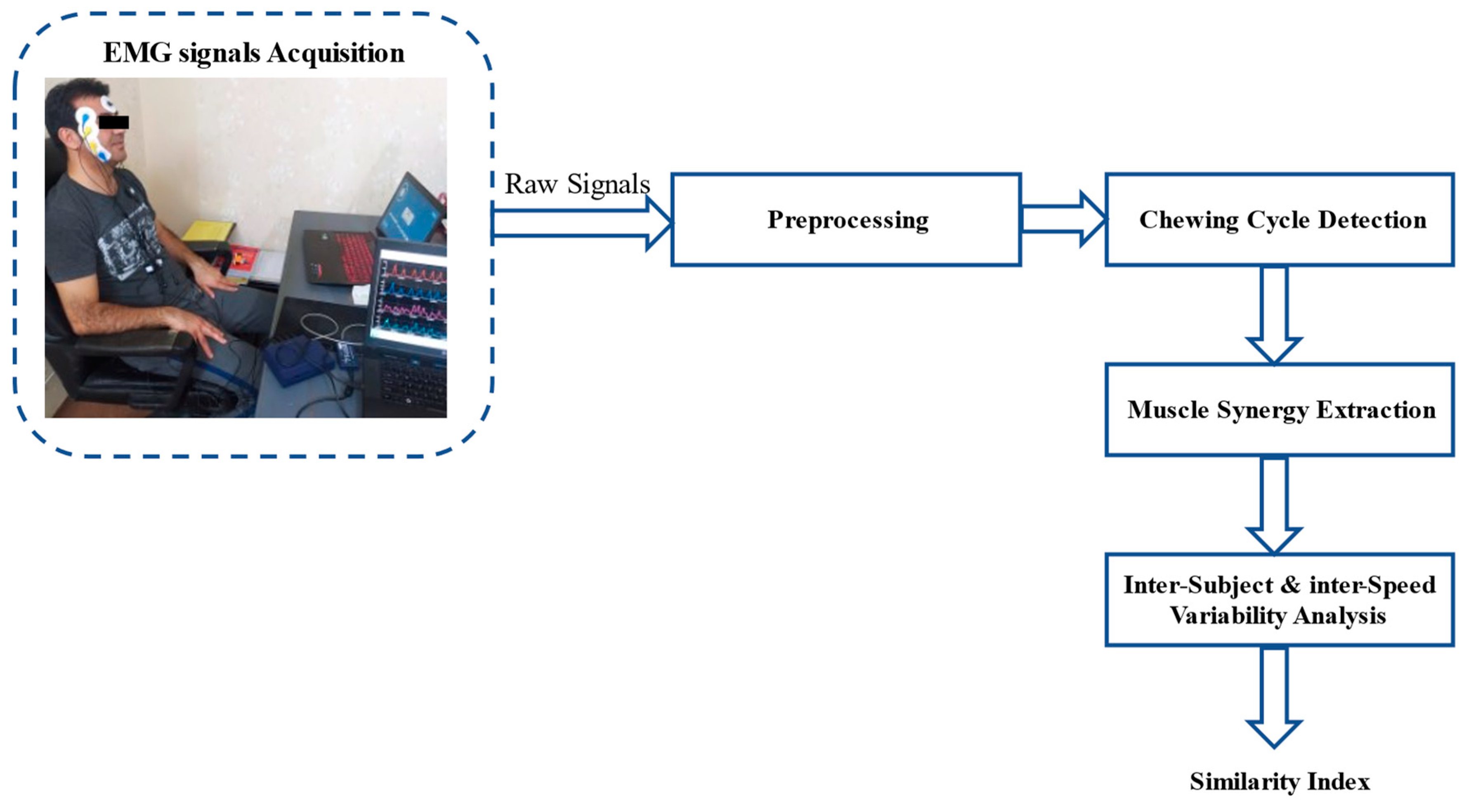
2.2.1. Experimental Protocol and Data Acquisition
2.2.2. Data Analysis
Preprocessing
Detection of Chewing Cycle Onset
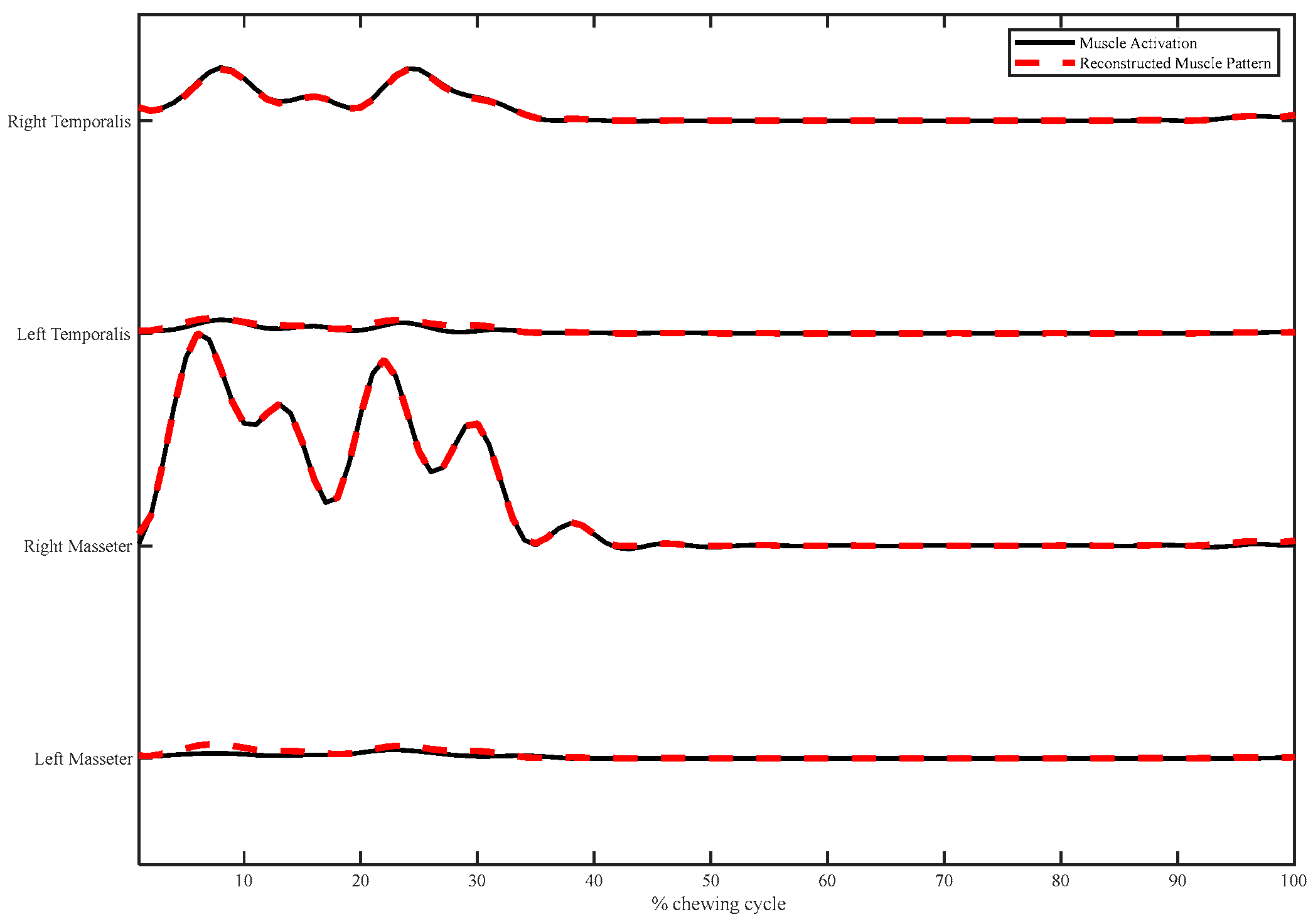
Muscle Synergy Extraction
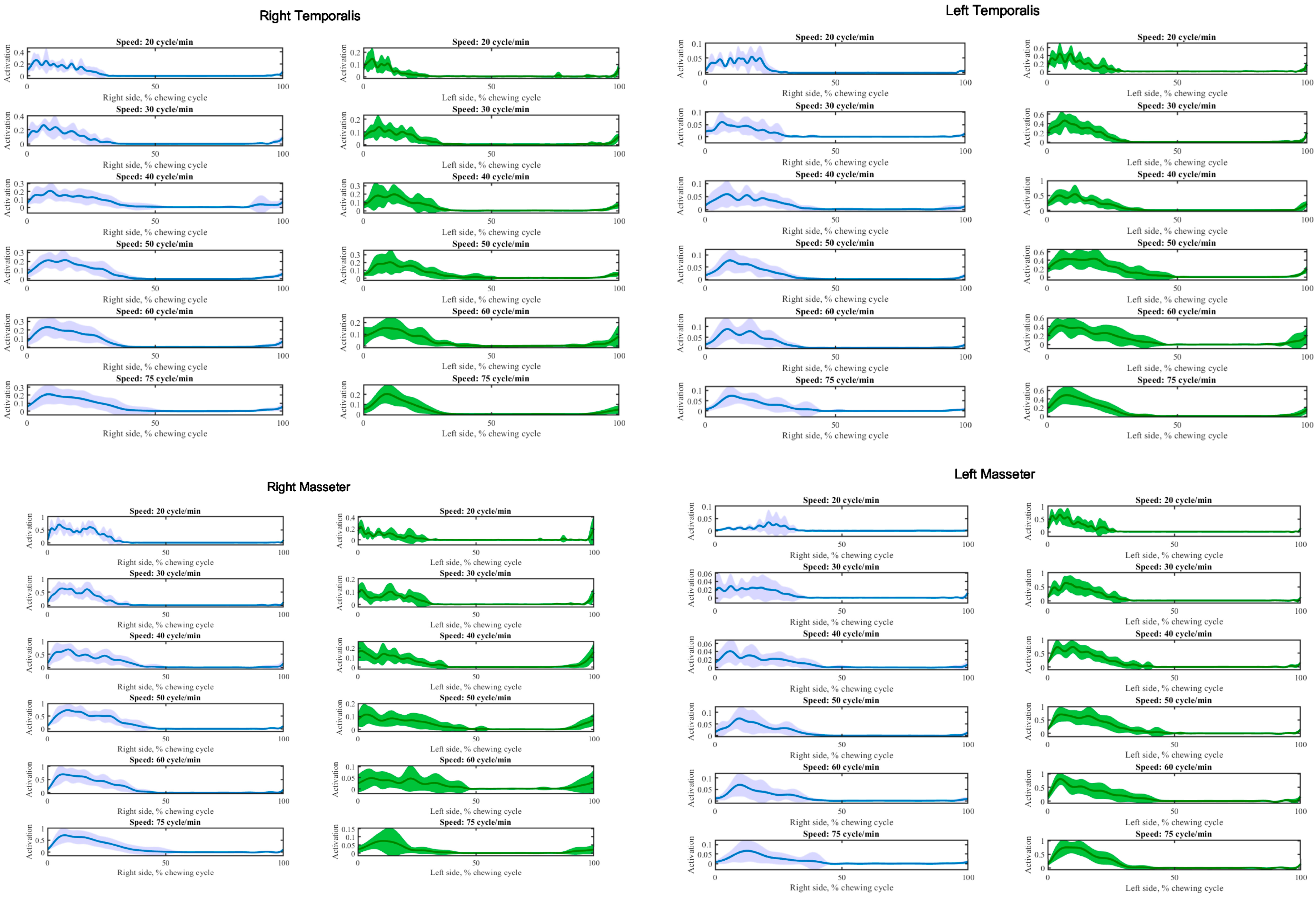
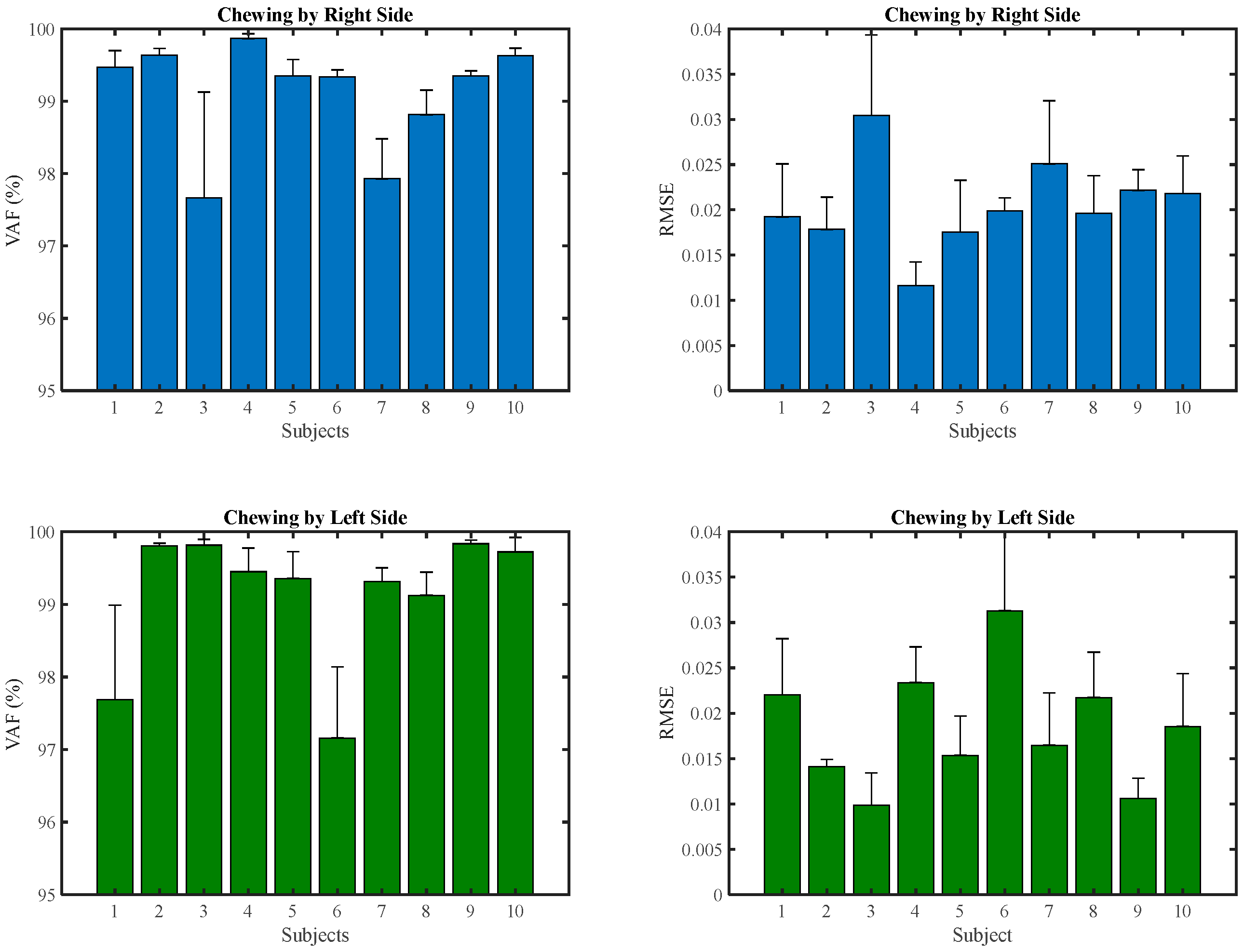
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yokoyama, H.; Ogawa, T.; Kawashima, N.; Shinya, M.; Nakazawa, K. Distinct sets of locomotor modules control the speed and modes of human locomotion. Sci. Rep. 2016, 6, 36275. [Google Scholar] [CrossRef]
- Fassicollo, C.E.; Garcia, D.M.; Machado, B.C.Z.; de Felício, C.M. Changes in jaw and neck muscle coactivation and coordination in patients with chronic painful TMD disk displacement with reduction during chewing. Physiol. Behav. 2021, 230, 113267. [Google Scholar] [CrossRef] [PubMed]
- Ow, R.K.K.; Carlsson, G.E.; Karlsson, S. Relationship of masticatory mandibular movements to masticatory performance of dentate adults: A method study. J. Oral Rehabil. 1998, 25, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Wieckiewicz, M.; Zietek, M.; Nowakowska, D.; Wieckiewicz, W. Comparison of selected kinematic facebows applied to mandibular tracing. BioMed Res. Int. 2014, 2014, 81869. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, A.; Caradonna, C. The relationship between the stomatognathic system and body posture. Clinics 2009, 64, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Almotairy, N.; Merzo, J.J.; Wendin, K.; Rothenberg, E.; Grigoriadis, A.; Sandborgh-Englund, G.; Trulsson, M. Chewing and its influence on swallowing, gastrointestinal and nutrition-related factors: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 1–31. [Google Scholar] [CrossRef]
- Nordio, S.; Arcara, G.; Berta, G.; Dellai, A.; Brisotto, C.; Koch, I.; Cazzador, D.; Aspidistria, M.; Ventura, L.; Turolla, A.; et al. Biofeedback as an Adjunctive Treatment for Post-stroke Dysphagia: A Pilot-Randomized Controlled Trial. Dysphagia 2022, 37, 1207–1216. [Google Scholar] [CrossRef]
- Ono, Y.; Yamamoto, T.; Kubo, K.Y.; Onozuka, M. Occlusion and brain function: Mastication as a prevention of cognitive dysfunction. J. Oral Rehabil. 2010, 37, 624–640. [Google Scholar] [CrossRef]
- Teixeira, F.B.; Fernandes, L.D.M.P.; Noronha, P.A.T.; dos Santos, M.A.R.; Gomes-Leal, W.; Maia, C.D.S.F.; Lima, R.R. Masticatory deficiency as a risk factor for cognitive dysfunction. Int. J. Med. Sci. 2014, 11, 209. [Google Scholar] [CrossRef]
- Ulloa, S.S.; Ordóñez, A.L.C.; Sardi, V.E.B. Relationship between dental occlusion and brain activity: A narrative review. Saudi Dent. J. 2022, 34, 538–543. [Google Scholar] [CrossRef]
- Ramazanoglu, E.; Turhan, B.; Usgu, S. Evaluation of the tone and viscoelastic properties of the masseter muscle in the supine position, and its relation to age and gender. Dent. Med. Probl. 2021, 58, 155–161. [Google Scholar] [CrossRef]
- Wu, Y.; Lan, Y.; Mao, J.; Shen, J.; Kang, T.; Xie, Z. The interaction between the nervous system and the stomatognathic system: From development to diseases. Int. J. Oral Sci. 2023, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Al-Ayyad, M.; Owida, H.A.; De Fazio, R.; Al-Naami, B.; Visconti, P. Electromyography Monitoring Systems in Rehabilitation: A Review of Clinical Applications, Wearable Devices and Signal Acquisition Methodologies. Electronics 2023, 12, 1520. [Google Scholar] [CrossRef]
- Ginszt, M.; Zieliński, G. Novel functional indices of masticatory muscle activity. J. Clin. Med. 2021, 10, 1440. [Google Scholar] [CrossRef]
- Manda, Y.; Kodama, N.; Maeda, N.; Minagi, S. Effect of food properties and chewing condition on the electromyographic activity of the posterior tongue. J. Oral Rehabil. 2019, 46, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Maezawa, H.; Koganemaru, S.; Matsuhashi, M.; Hirata, M.; Funahashi, M.; Mima, T. Entrainment of chewing rhythm by gait speed during treadmill walking in humans. Neurosci. Res. 2020, 156, 88–94. [Google Scholar] [CrossRef]
- Gadotti, I.; Hicks, K.; Koscs, E.; Lynn, B.; Estrazulas, J.; Civitella, F. Electromyography of the masticatory muscles during chewing in different head and neck postures-A pilot study. J. Oral Biol. Craniofacial Res. 2020, 10, 23–27. [Google Scholar] [CrossRef]
- Almotairy, N.; Kumar, A.; Grigoriadis, A. Effect of food hardness on chewing behavior in children. Clin. Oral Investig. 2021, 25, 1203–1216. [Google Scholar] [CrossRef]
- Yachida, W.; Arima, T.; Castrillon, E.E.; Baad-Hansen, L.; Ohata, N.; Svensson, P. Diagnostic validity of self-reported measures of sleep bruxism using an ambulatory single-channel EMG device. J. Prosthodont. Res. 2016, 60, 250–257. [Google Scholar] [CrossRef]
- Thymi, M.; Shimada, A.; Lobbezoo, F.; Svensson, P. Clinical jaw-muscle symptoms in a group of probable sleep bruxers. J. Dent. 2019, 85, 81–87. [Google Scholar] [CrossRef]
- Sonmezocak, T.; Kurt, S. Machine learning and regression analysis for diagnosis of bruxism by using EMG signals of jaw muscles. Biomed. Signal Process. Control 2021, 69, 102905. [Google Scholar] [CrossRef]
- Maeda, M.; Yamaguchi, T.; Mikami, S.; Yachida, W.; Saito, T.; Sakuma, T.; Nakamura, H.; Saito, M.; Mizuno, M.; Yamada, K.; et al. Validity of single-channel masseteric electromyography by using an ultraminiature wearable electromyographic device for diagnosis of sleep bruxism. J. Prosthodont. Res. 2019, 64, 90–97. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Domingos, N.; Júnior, R.B.; de Carvalho Gaspar, P.T.; Lizardo, F.B.; Amorim, C.F.; de Oliveira Silva, D.C. Electromyographic activity of the masseter muscle in individuals with group function and canine guidance. J. Dent. Res. Dent. Clin. Dent. Prospect. 2021, 15, 232. [Google Scholar] [CrossRef] [PubMed]
- Kalani, H.; Moghimi, S.; Akbarzadeh, A. Towards an SEMG-based tele-operated robot for masticatory rehabilitation. Comput. Biol. Med. 2016, 75, 243–256. [Google Scholar] [CrossRef]
- Kalani, H.; Moghimi, S.; Akbarzadeh, A. Toward a bio-inspired rehabilitation aid: sEMG-CPG approach for online generation of jaw trajectories for a chewing robot. Biomed. Signal Process. Control 2019, 51, 285–295. [Google Scholar] [CrossRef]
- Kameda, T.; Sakamoto, M.; Terada, K. Semi-powered exoskeleton that regulates the muscular activity of jaw movement for oral functional rehabilitation/training. Dent. Mater. J. 2021, 40, 101–109. [Google Scholar] [CrossRef]
- González, R.; Montoya, I.; Cárcel, J. The use of electromyography on food texture assessment. Food Sci. Technol. Int. 2001, 7, 461–471. [Google Scholar] [CrossRef]
- Brown, W.E. Method to investigate differences in chewing behaviour in humans: I. Use of electromyography in measuring chewing. J. Texture Stud. 1994, 25, 1–16. [Google Scholar] [CrossRef]
- Kohyama, K.; Sasaki, T.; Hayakawa, F. Characterization of food physical properties by the mastication parameters measured by electromyography of the jaw-closing muscles and mandibular kinematics in young adults. Biosci. Biotechnol. Biochem. 2008, 72, 1690–1695. [Google Scholar] [CrossRef]
- Ishihara, S.; Nakauma, M.; Funami, T.; Tanaka, T.; Nishinari, K.; Kohyama, K. Electromyography during oral processing in relation to mechanical and sensory properties of soft gels. J. Texture Stud. 2011, 42, 254–267. [Google Scholar] [CrossRef]
- Stepp, C.E. Surface Electromyography for Speech and Swallowing Systems: Measurement, Analysis, and Interpretation. JSLHR 2012, 55, 1232–1246. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.L.; Bronlund, J.E.; Potgieter, J.; Foster, K.D.; Röhrle, O.; Pullan, A.J.; Kieser, J.A. Review of the human masticatory system and masticatory robotics. Mech. Mach. Theory 2008, 43, 1353–1375. [Google Scholar] [CrossRef]
- Yokoyama, H.; Kato, T.; Kaneko, N.; Kobayashi, H.; Hoshino, M.; Kokubun, T.; Nakazawa, K. Basic locomotor muscle synergies used in land walking are finely tuned during underwater walking. Sci. Rep. 2021, 11, 18480. [Google Scholar] [CrossRef] [PubMed]
- Kibushi, B.; Hagio, S.; Moritani, T.; Kouzaki, M. Speed-dependent modulation of muscle activity based on muscle synergies during treadmill walking. Front. Hum. Neurosci. 2018, 12, 4. [Google Scholar] [CrossRef]
- Santuz, A.; Ekizos, A.; Kunimasa, Y.; Kijima, K.; Ishikawa, M.; Arampatzis, A. Lower complexity of motor primitives ensures robust control of high-speed human locomotion. Heliyon 2020, 6, e05377. [Google Scholar] [CrossRef]
- Esmaeili, J.; Maleki, A. Muscle coordination analysis by time-varying muscle synergy extraction during cycling across various mechanical conditions. Biocybern. Biomed. Eng. 2020, 40, 90–99. [Google Scholar] [CrossRef]
- Esmaeili, J.; Maleki, A. Comparison of muscle synergies extracted from both legs during cycling at different mechanical conditions. Australas. Phys. Eng. Sci. Med. 2019, 42, 827–838. [Google Scholar] [CrossRef]
- Hug, F.; Turpin, N.A.; Couturier, A.; Dorel, S. Consistency of muscle synergies during pedaling across different mechanical constraints. J. Neurophysiol. 2011, 106, 91–103. [Google Scholar] [CrossRef]
- Park, S.; Caldwell, G.E. Muscle synergies are modified with improved task performance in skill learning. Hum. Mov. Sci. 2022, 83, 102946. [Google Scholar] [CrossRef]
- Hertzog, M.A. Considerations in determining sample size for pilot studies. Res. Nurs. Health 2008, 31, 180–191. [Google Scholar] [CrossRef]
- Verhoeff, M.C.; Koutris, M.; Vries, R.D.; Berendse, H.W.; Dijk, K.D.; Lobbezoo, F. Salivation in Parkinson’s disease: A scoping review. Gerodontology 2023, 40, 26–38. [Google Scholar] [CrossRef]
- Oei, T.P.S.; Sawang, S.; Goh, Y.W.; Mukhtar, F. Using the depression anxiety stress scale 21 (DASS-21) across cultures. Int. J. Psychol. 2013, 48, 1018–1029. [Google Scholar] [CrossRef]
- Kubo, K.; Iinuma, M.; Chen, H. Mastication as a stress-coping behavior. BioMed Res. Int. 2015, 2015, 876409. [Google Scholar] [CrossRef]
- Tahara, Y.; Sakurai, K.; Ando, T. Influence of chewing and clenching on salivary cortisol levels as an indicator of stress. J. Prosthodont. 2007, 16, 129–135. [Google Scholar] [CrossRef]
- Zieliński, G.; Byś, A.; Ginszt, M.; Baszczowski, M.; Szkutnik, J.; Majcher, P.; Gawda, P. Depression and Resting Masticatory Muscle Activity. J. Clin. Med. 2020, 9, 1097. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Sabaneeff, A.; Caldas, L.D.; Garcia, M.A.C.; Nojima, M.D.C.G. Proposal of surface electromyography signal acquisition protocols for masseter and temporalis muscles. Res. Biomed. Eng. 2017, 33, 324–330. [Google Scholar] [CrossRef]
- Davarinia, F.; Maleki, A. SSVEP-gated EMG-based decoding of elbow angle during goal-directed reaching movement. Biomed. Signal Process. Control 2022, 71, 103222. [Google Scholar] [CrossRef]
- Li, X.; Zhou, P.; Aruin, A.S. Teager–Kaiser energy operation of surface EMG improves muscle activity onset detection. Ann. Biomed. Eng. 2007, 35, 1532–1538. [Google Scholar] [CrossRef]
- Solnik, S.; Rider, P.; Steinweg, K.; DeVita, P.; Hortobágyi, T. Teager–Kaiser energy operator signal conditioning improves EMG onset detection. Eur. J. Appl. Physiol. 2010, 110, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Dominici, N.; Ivanenko, Y.P.; Cappellini, G.; d’Avella, A.; Mondì, V.; Cicchese, M.; Fabiano, A.; Silei, T.; Di Paolo, A.; Giannini, C.; et al. Locomotor primitives in newborn babies and their development. Science 2011, 334, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Tresch, M.C.; Cheung, V.C.K.; d’Avella, A. Matrix factorization algorithms for the identification of muscle synergies: Evaluation on simulated and experimental data sets. J. Neurophysiol. 2006, 95, 2199–2212. [Google Scholar] [CrossRef] [PubMed]
- Hug, F.; Turpin, N.A.; Guével, A.; Dorel, S. Is interindividual variability of EMG patterns in trained cyclists related to different muscle synergies? J. Appl. Physiol. 2010, 108, 1727–1736. [Google Scholar] [CrossRef]
- Ishii, T.; Narita, N.; Endo, H. Evaluation of jaw and neck muscle activities while chewing using EMG-EMG transfer function and EMG-EMG coherence function analyses in healthy subjects. Physiol. Behav. 2016, 160, 35–42. [Google Scholar] [CrossRef]
- Nedeljković, Đ.; Lemić, A.M.; Pfićer, J.K.; Stančić, I.; Popovac, A.; Čelebić, A. Subjective and Objective Assessment of Chewing Performance in Older Adults with Different Dental Occlusion. Med. Princ. Pract. 2023, 32, 4–10. [Google Scholar] [CrossRef]
- Turcio, K.H.L.; Zuim, P.R.J.; Guiotti, A.M.; Santos, D.M.D.; Goiato, M.C.; Brandini, D.A. Does the habitual mastication side impact jaw muscle activity? Arch. Oral Biol. 2016, 67, 34–38. [Google Scholar] [CrossRef]
- Cheung, V.C.; Cheung, B.M.; Zhang, J.H.; Chan, Z.Y.; Ha, S.C.; Chen, C.Y.; Cheung, R.T. Plasticity of muscle synergies through fractionation and merging during development and training of human runners. Nat. Commun. 2020, 11, 4356. [Google Scholar] [CrossRef]
- Florjanski, W.; Malysa, A.; Orzeszek, S.; Smardz, J.; Olchowy, A.; Paradowska-Stolarz, A.; Wieckiewicz, M. Evaluation of biofeedback usefulness in masticatory muscle activity management—A systematic review. J. Clin. Med. 2019, 8, 766. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allami Sanjani, M.; Tahami, E.; Veisi, G. Synchronous Muscle Synergy Evaluation of Jaw Muscle Activities during Chewing at Different Speeds, a Preliminary Study. Brain Sci. 2023, 13, 1344. https://doi.org/10.3390/brainsci13091344
Allami Sanjani M, Tahami E, Veisi G. Synchronous Muscle Synergy Evaluation of Jaw Muscle Activities during Chewing at Different Speeds, a Preliminary Study. Brain Sciences. 2023; 13(9):1344. https://doi.org/10.3390/brainsci13091344
Chicago/Turabian StyleAllami Sanjani, Marzieh, Ehsan Tahami, and Gelareh Veisi. 2023. "Synchronous Muscle Synergy Evaluation of Jaw Muscle Activities during Chewing at Different Speeds, a Preliminary Study" Brain Sciences 13, no. 9: 1344. https://doi.org/10.3390/brainsci13091344





