What General Neurologists Should Know about Autoinflammatory Syndromes?
Abstract
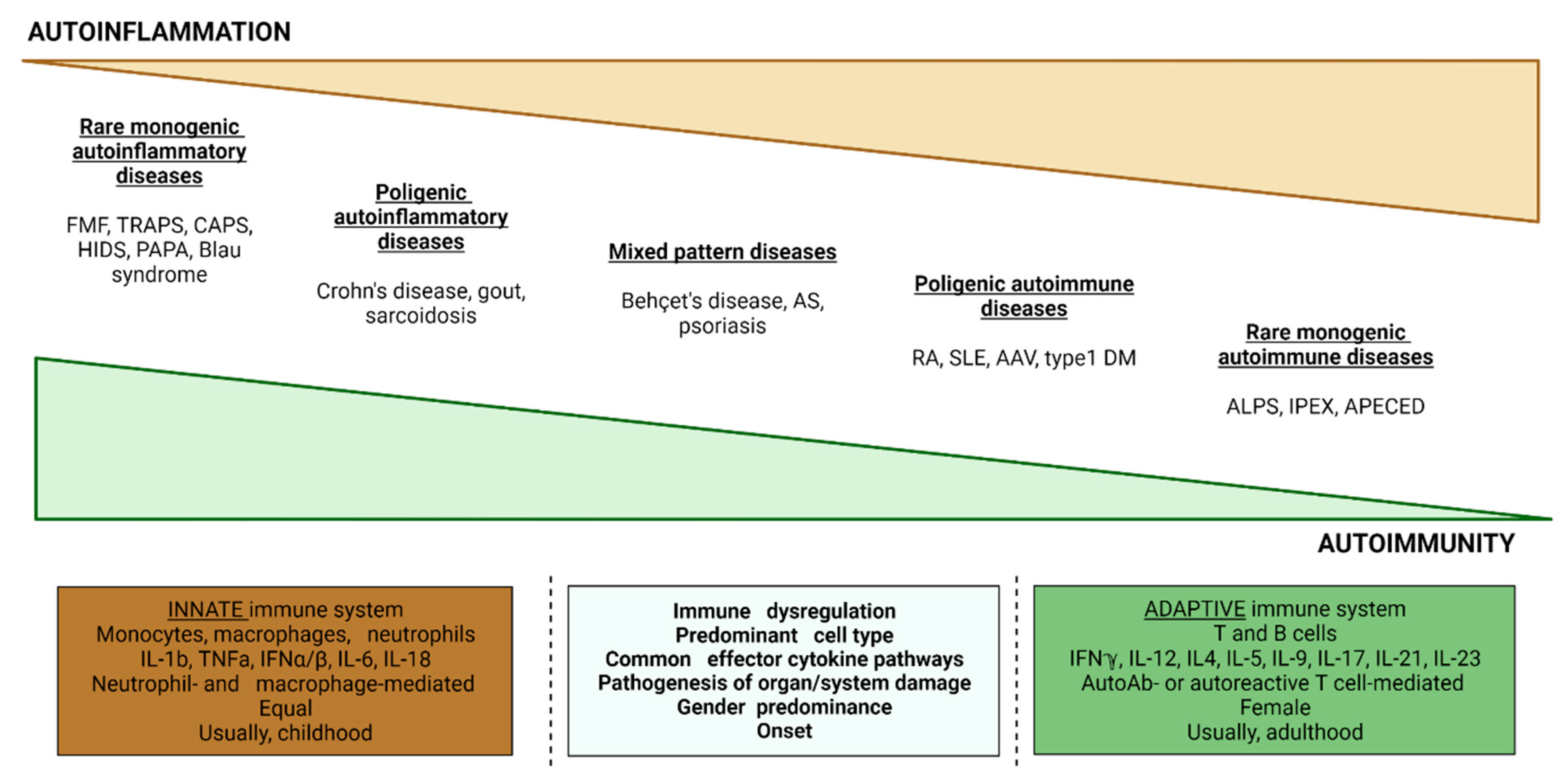
:1. Introduction
2. Pathophysiological Mechanisms
3. Classification of SAIDs
3.1. Cryopirin-Associated Periodic Syndrome (CAPS)
3.2. Familial Mediterranean Fever (FMF)
3.3. Mevalonate Kinase Deficiency (MKD) and Mevalonic Aciduria (MVA)
3.4. Type I Interferonopathies
3.5. Tumor Necrosis Factor Associated Periodic Syndrome (TRAPS)
3.6. A20 Haploinsufficiency (HA20)
3.7. Blau Syndrome (BS)
3.8. Deficiency of Adenosine Deaminase 2 (DADA2)
4. General Approach to SAIDs
General Approach
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozkurede, V.U.; Franchi, L. Immunology in clinic review series; focus on autoinflammatory diseases: Role of inflammasomes in autoinflammatory syndromes. Clin. Exp. Immunol. 2012, 167, 382–390. [Google Scholar] [CrossRef]
- McDermott, M.F.; Aksentijevich, I. The autoinflammatory syndromes. Curr. Opin. Allergy Clin. Immunol. 2002, 2, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Bousfiha, A.; Jeddane, L.; Picard, C.; Al-Herz, W.; Ailal, F.; Chatila, T.; Cunningham-Rundles, C.; Etzioni, A.; Franco, J.L.; Holland, S.M.; et al. Human Inborn Errors of Immunity: 2019 Update of the IUIS Phenotypical Classification. J. Clin. Immunol. 2020, 40, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Gaggiano, C.; Rigante, D.; Vitale, A.; Lucherini, O.M.; Fabbiani, A.; Capozio, G.; Marzo, C.; Gelardi, V.; Grosso, S.; Frediani, B.; et al. Hints for genetic and clinical differentiation of adult-onset monogenic autoinflammatory diseases. Mediat. Inflamm. 2019, 2019, 3293145. [Google Scholar] [CrossRef] [PubMed]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- De Jesus, A.A.; Canna, S.W.; Liu, Y.; Goldbach-Mansky, R. Molecular mechanisms in genetically defined autoinflammatory diseases: Disorders of amplified danger signaling. Annu. Rev. Immunol. 2015, 33, 823–874. [Google Scholar] [CrossRef]
- Diprose, W.K.; Jordan, A.; Anderson, N.E. Autoinflammatory syndromes in neurology: When our first line of defense misbehaves. Pract. Neurol. 2022, 22, 145–153. [Google Scholar] [CrossRef]
- Caso, F.; Costa, L.; Nucera, V.; Barilaro, G.; Masala, I.F.; Talotta, R.; Caso, P.; Scarpa, R.; Sarzi-Puttini, P.; Atzeni, F. From autoinflammation to autoimmunity: Old and recent findings. Clin. Rheumatol. 2018, 37, 2305–2321. [Google Scholar] [CrossRef]
- Van Kempen, T.S.; Wenink, M.H.; Leijten, E.F.; Radstake, T.R.; Boes, M. Perception of self: Distinguishing autoimmunity from autoinflammation. Nat. Rev. Rheumatol. 2015, 11, 483–492. [Google Scholar] [CrossRef]
- Berg, S.; Wekell, P.; Fasth, A.; Hawkins, P.N.; Lachmann, H. Autoinflammatory Disorders. In Primary Immunodeficiency Diseases; Rezaei, N., Aghamohammadi, A., Notarangelo, L.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 393–435. [Google Scholar]
- McGonagle, D.; McDermott, M.F. A proposed classification of the immunological diseases. PLoS Med. 2006, 3, e297. [Google Scholar] [CrossRef]
- Hausmann, J.; Dedeoglu, F. Autoinflammatory diseases. In Neurorheumatology: A Comprehensive Guide to Immune-Mediated Disorders of the Nervous System; Cho, T.A., Bhattacharyya, S., Helfgott, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 123–133. [Google Scholar]
- Martinon, F.; Tschopp, J. Inflammatory caspases and inflammasomes: Master switches of inflammation. Cell Death Differ. 2007, 14, 10–22. [Google Scholar] [CrossRef]
- Tartey, S.; Kanneganti, T.-D. Inflammasomes in the pathophysiology of autoinflammatory syndromes. J. Leukoc. Biol. 2020, 107, 379–391. [Google Scholar] [CrossRef]
- Di Donato, G.; d’Angelo, D.M.; Breda, L.; Chiarelli, F. Monogenic Autoinflammatory Diseases: State of the Art and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 6360. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Man, S.M.; Kanneganti, T.D. Regulation of inflammasome activation. Immunol. Rev. 2015, 265, 6–21. [Google Scholar] [CrossRef]
- Manna, R.; Rigante, D. The everchanging framework of autoinflammation. Intern. Emerg. Med. 2021, 16, 1759–1770. [Google Scholar] [CrossRef]
- Aksentijevich, I.; Schnappauf, O. Molecular mechanisms of phenotypic variability in monogenic autoinflammatory diseases. Nat. Rev. Rheumatol. 2021, 17, 405–425. [Google Scholar] [CrossRef]
- Kone-Paut, I. Cryopyrine-associated periodic syndrome: CAPS seen from adulthood. Rev. Med. Interne 2015, 36, 277–282. [Google Scholar] [PubMed]
- Kitley, J.L.; Lachmann, H.J.; Pinto, A.; Ginsberg, L. Neurologic manifestations of the cryopyrin-associated periodic syndrome. Neurology 2010, 74, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Mamoudjy, N.; Maurey, H.; Marie, I.; Koné-Paut, I.; Deiva, K. Neurological outcome of patients with cryopyrin-associated periodic syndrome (CAPS). Orphanet J. Rare Dis. 2017, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, K.; McCalmont, T.H.; Leslie, K.S. Cryopyrin-associated periodic syndromes and autoinflammation. Clin. Exp. Dermatol. 2007, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Gérard, L.; Kuemmerle-Deschner, J.; Lachmann, H.J.; Koné-Paut, I.; Cantarini, L.; Woo, P.; Naselli, A.; Bader-Meunier, B.; Insalaco, A.; et al. Phenotypic and genotypic characteristics of cryopyrin-associated periodic syndrome: A series of 136 patients from the Eurofever Registry. Ann. Rheum. Dis. 2015, 74, 2043–2049. [Google Scholar] [CrossRef]
- Parker, T.; Keddie, S.; Kidd, D.; Lane, T.; Maviki, M.; Hawkins, P.N.; Lachmann, H.J.; Ginsberg, L. Neurology of the cryopyrin-associated periodic fever syndrome. Eur. J. Neurol. 2016, 23, 1145–1151. [Google Scholar] [CrossRef]
- Kuemmerle-Deschner, J.B.; Ozen, S.; Tyrrell, P.N.; Kone-Paut, I.; Goldbach-Mansky, R.; Lachmann, H.; Blank, N.; Hoffman, H.M.; Weissbarth-Riedel, E.; Hugle, B.; et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann. Rheum. Dis. 2017, 76, 942–947. [Google Scholar] [CrossRef]
- Schnappauf, O.; Chae, J.J.; Kastner, D.L.; Aksentijevich, I. The Pyrin Inflammasome in Health and Disease. Front. Immunol. 2019, 10, 1745. [Google Scholar] [CrossRef] [PubMed]
- Heilig, R.; Broz, P. Function and mechanism of the pyrin inflammasome. Eur. J. Immunol. 2018, 48, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Boursier, G.; Hentgen, V.; Sarrabay, G.; Koné-Paut, I.; Touitou, I. The Changing Concepts Regarding the Mediterranean Fever Gene: Toward a Spectrum of Pyrin-Associated Autoinflammatory Diseases with Variable Heredity. J. Pediatr. 2019, 209, 12–16.e1. [Google Scholar] [CrossRef]
- Saatci, U.; Ozen, S.; Ozdemir, S.; Bakkaloglu, A.; Besbas, N.; Topaloglu, R.; Arslan, S. Familial Mediterranean fever in children: Report of a large series and discussion of the risk and prognostic factors of amyloidosis. Eur. J. Pediatr. 1997, 156, 619–623. [Google Scholar] [CrossRef]
- Padeh, S. Periodic fever syndromes. Pediatr. Clin. N. Am. 2005, 52, 577–609. [Google Scholar] [CrossRef]
- Turkish FMF Study Group. Familial Mediterranean fever (FMF) in Turkey: Results of a nationwide multicenter study. Medicine 2005, 84, 1–11. [Google Scholar] [CrossRef]
- Salehzadeh, F.; Azami, A.; Motezarre, M.; Nematdoust Haghi, R.; Ahmadabadi, F. Neurological Manifestations in Familial Mediterranean Fever: A Genotype-Phenotype Correlation Study. Open Access Rheumatol. 2020, 12, 15–19. [Google Scholar] [CrossRef]
- Kalyoncu, U.; Eker, A.; Oguz, K.K.; Kurne, A.; Kalan, I.; Topcuoglu, A.M.; Anlar, B.; Bilginer, Y.; Arici, M.; Yilmaz, E.; et al. Familial Mediterranean fever and central nervous system involvement: A case series. Medicine 2010, 89, 75–84. [Google Scholar] [CrossRef]
- Elhani, I.; Dumont, A.; Vergneault, H.; Ardois, S.; Le Besnerais, M.; Levesque, H.; Ouallet, J.C.; Savey, L.; Aouba, A.; Amselem, S.; et al. Association between familial Mediterranean fever and multiple sclerosis: A case series from the JIR cohort and systematic literature review. Mult. Scler. Relat. Disord. 2021, 50, 102834. [Google Scholar] [CrossRef]
- Livneh, A.; Langevitz, P.; Zemer, D.; Zaks, N.; Kees, S.; Lidar, T.; Migdal, A.; Padeh, S.; Pras, M. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum. 1997, 40, 1879–1885. [Google Scholar] [CrossRef]
- Gattorno, M.; Hofer, M.; Federici, S.; Vanoni, F.; Bovis, F.; Aksentijevich, I.; Anton, J.; Arostegui, J.I.; Barron, K.; Ben-Cherit, E.; et al. Classification criteria for autoinflammatory recurrent fevers. Ann. Rheum. Dis. 2019, 78, 1025–1032. [Google Scholar] [CrossRef]
- Kallinich, T.; Haffner, D.; Niehues, T.; Huss, K.; Lainka, E.; Neudorf, U.; Schaefer, C.; Stojanov, S.; Timmann, C.; Keitzer, R.; et al. Colchicine use in children and adolescents with familial Mediterranean fever: Literature review and consensus statement. Pediatrics 2007, 119, e474–e483. [Google Scholar] [CrossRef]
- Hashkes, P.J.; Spalding, S.J.; Giannini, E.H.; Huang, B.; Johnson, A.; Park, G.; Barron, K.S.; Weisman, M.H.; Pashinian, N.; Reiff, A.O.; et al. Rilonacept for colchicine resistant or intolerant familial Mediterranean fever: A randomized trial. Ann. Intern. Med. 2012, 157, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Pillinger, M.H.; Kishimoto, M.; Abeles, A.M.; Livneh, A. Familial Mediterranean fever successfully treated with etanercept. J. Clin. Rheumatol. 2007, 13, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Mulders-Manders, C.M.; Simon, A. Hyper-IgD syndrome/mevalonate kinase deficiency: What is new? Semin. Immunopathol. 2015, 37, 371–376. [Google Scholar] [CrossRef]
- Van der Burgh, R.; ter Haar, N.M.; Boes, M.L.; Frenkel, J. Mevalonate kinase deficiency, a metabolic autoinflammatory disease. Clin. Immunol. 2013, 147, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.; Hoffmann, G.F. Mevalonate kinase deficiencies: From mevalonic aciduria to hyperimmunoglobulinemia D syndrome. Orphanet J. Rare Dis. 2006, 1, 13. [Google Scholar] [CrossRef]
- Simon, A.; Kremer, H.P.; Wevers, R.A.; Scheffer, H.; De Jong, J.G.; Van Der Meer, J.W.; Drenth, J.P. Mevalonate kinase deficiency: Evidence for a phenotypic continuum. Neurology 2004, 62, 994–997. [Google Scholar] [CrossRef]
- Brennenstuhl, H.; Nashawi, M.; Schröter, J.; Baronio, F.; Beedgen, L.; Gleich, F.; Jeltsch, K.; von Landenberg, C.; Martini, S.; Simon, A.; et al. Unified Registry for Inherited Metabolic Disorders (U-IMD) Consortium and the European Registry for Hereditary Metabolic Disorders (MetabERN). Phenotypic diversity, disease progression, and pathogenicity of MVK missense variants in mevalonic aciduria. J. Inherit. Metab. Dis. 2021, 44, 1272–1287. [Google Scholar] [CrossRef]
- Hoffmann, G.F.; Charpentier, C.; Mayatepek, E.; Mancini, J.; Leichsenring, M.; Gibson, K.M.; Divry, P.; Hrebicek, M.; Lehnert, W.; Sartor, K.; et al. Clinical and biochemical phenotype in 11 patients with mevalonic aciduria. Pediatrics 1993, 91, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Prietsch, V.; Mayatepek, E.; Krastel, H.; Haas, D.; Zundel, D.; Waterham, H.R.; Wanders, R.J.; Gibson, K.M.; Hoffmann, G.F. Mevalonate kinase deficiency: Enlarging the clinical and biochemical spectrum. Pediatrics 2003, 111, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Arkwright, P.D.; Abinun, M.; Cant, A.J. Mevalonic aciduria cured by bone marrow transplantation. N. Engl. J. Med. 2007, 357, 1350. [Google Scholar] [CrossRef]
- Ter Haar, N.M.; Jeyaratnam, J.; Lachmann, H.J.; Simon, A.; Brogan, P.A.; Doglio, M.; Cattalini, M.; Anton, J.; Modesto, C.; Quartier, P.; et al. Paediatric Rheumatology International Trials Organisation and Eurofever Project. The Phenotype and Genotype of Mevalonate Kinase Deficiency: A Series of 114 Cases from the Eurofever Registry. Arthritis Rheumatol. 2016, 68, 2795–2805. [Google Scholar] [CrossRef] [PubMed]
- Crow, Y.J.; Black, D.N.; Ali, M.; Bond, J.; Jackson, A.P.; Lefson, M.; Michaud, J.; Roberts, E.; Stephenson, J.B.; Woods, C.G.; et al. Cree encephalitis is allelic with Aicardi-Goutières syndrome: Implications for the pathogenesis of disorders of interferon-alpha metabolism. J. Med. Genet. 2003, 40, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Rodero, M.P.; Crow, Y.J. Type I interferon-mediated monogenic autoinflammation: The type I interferonopathies, a conceptual overview. J. Exp. Med. 2016, 213, 2527–2538. [Google Scholar] [CrossRef]
- Crow, Y.J. Type I interferonopathies: A novel set of inborn errors of immunity. Ann. N. Y. Acad. Sci. 2011, 1238, 91–98. [Google Scholar] [CrossRef]
- Davidson, S.; Steiner, A.; Harapas, C.R.; Masters, S.L. An update on autoinflammatory diseases: Interferonopathies. Curr. Rheumatol. Rep. 2018, 20, 38. [Google Scholar] [CrossRef]
- Negishi, H.; Taniguchi, T.; Yanai, H. The interferon (IFN) class of cytokines and the IFN regulatory factor (IRF) transcription factor family. Cold Spring Harb. Perspect. Biol. 2018, 10, a028423. [Google Scholar] [CrossRef]
- Trinchieri, G. Type I interferon: Friend or foe? J. Exp. Med. 2010, 207, 2053–2063. [Google Scholar] [CrossRef]
- Lee-Kirsch, M.A.; Wolf, C.; Kretschmer, S.; Roers, A. Type I interferonopathies—An expanding disease spectrum of immune dysregulation. Semin. Immunopathol. 2015, 37, 349–357. [Google Scholar] [CrossRef]
- Crow, Y.J.; Stetson, D.B. The type I interferonopathies: 10 years on. Nat. Rev. Immunol. 2021, 22, 471–483. [Google Scholar] [CrossRef]
- Anderson, S.R.; Vetter, M.L. Developmental roles of microglia: A window into mechanisms of disease. Dev. Dyn. 2019, 248, 98–117. [Google Scholar] [CrossRef] [PubMed]
- McDonough, A.; Lee, R.V.; Weinstein, J.R. Microglial interferon signaling and white matter. Neurochem. Res. 2017, 42, 2625–2638. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, T.; Blank, T.; Prinz, M. Fine-tuning of type I IFN signaling in microglia-implications for homeostasis, CNS autoimmunity, and interferonopathies. Curr. Opin. Neurobiol. 2016, 36, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Crow, Y.J.; Rehwinkel, J. Aicardi-Goutières syndrome, and related phenotypes: Linking nucleic acid metabolism with autoimmunity. Hum. Mol. Genet. 2009, 18, R130–R136. [Google Scholar] [CrossRef]
- Benjamin, P.; Sudhakar, S.; D’Arco, F.; Löbel, U.; Carney, O.; Roux, C.J.; Boddaert, N.; Hemingway, C.; Eleftheriou, D.; Mankad, K. Spectrum of Neuroradiologic Findings Associated with Monogenic Interferonopathies. AJNR Am. J. Neuroradiol. 2022, 43, 2–10. [Google Scholar] [CrossRef]
- Wilms, A.E.; de Boer, I.; Terwindt, G.M. Retinal Vasculopathy with Cerebral Leukoencephalopathy and Systemic manifestations (RVCL-S): An update on basic science and clinical perspectives. Cereb. Circ. Cogn. Behav. 2022, 3, 100046. [Google Scholar] [CrossRef]
- Stam, A.H.; Kothari, P.H.; Shaikh, A.; Gschwendter, A.; Jen, J.C.; Hodgkinson, S.; Hardy, T.A.; Hayes, M.; Kempster, P.A.; Kotschet, K.E.; et al. Retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations. Brain 2016, 139, 2909–2922. [Google Scholar] [CrossRef]
- Pelzer, N.; Hoogeveen, E.S.; Haan, J.; Bunnik, R.; Poot, C.C.; van Zwet, E.W.; Inderson, A.; Fogteloo, A.J.; Reinders, M.E.J.; Middelkoop, H.A.M.; et al. Systemic features of retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations: A monogenic small vessel disease. J. Intern. Med. 2019, 285, 317–333. [Google Scholar] [CrossRef]
- Lindahl, H.; Bryceson, Y.T. Neuroinflammation Associated with Inborn Errors of Immunity. Front. Immunol. 2022, 12, 827815. [Google Scholar] [CrossRef] [PubMed]
- Cetin Gedik, K.; Lamot, L.; Romano, M.; Demirkaya, E.; Piskin, D.; Torreggiani, S.; Adang, L.A.; Armangue, T.; Barchus, K.; Cordova, D.R.; et al. The 2021 European Alliance of Associations for Rheumatology/American College of Rheumatology Points to Consider for Diagnosis and Management of Autoinflammatory Type I Interferonopathies: CANDLE/PRAAS, SAVI, and AGS. Arthritis Rheumatol. 2022, 74, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Magnotti, F.; Vitale, A.; Rigante, D.; Lucherini, O.M.; Cimaz, R.; Muscari, I.; Granados Afonso de Faria, A.; Frediani, B.; Galeazzi, M.; Cantarini, L. The most recent advances in pathophysiology and management of tumor necrosis factor receptor-associated periodic syndrome (TRAPS): Personal experience and literature review. Clin. Exp. Rheumatol. 2013, 31 (Suppl. S77), S141–S149. [Google Scholar]
- Rigante, D.; Lopalco, G.; Vitale, A.; Lucherini, O.M.; De Clemente, C.; Caso, F.; Emmi, G.; Costa, L.; Silvestri, E.; Andreozzi, L.; et al. Key facts and hot spots on tumor necrosis factor receptor-associated periodic syndrome. Clin. Rheumatol. 2014, 33, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, H.J.; Papa, R.; Gerhold, K.; Obici, L.; Touitou, I.; Cantarini, L.; Frenkel, J.; Anton, J.; Kone-Paut, I.; Cattalini, M.; et al. The phenotype of TNF receptor-associated autoinflammatory syndrome (TRAPS) at presentation: A series of 158 cases from the Eurofever/EUROTRAPS international registry. Ann. Rheum. Dis. 2014, 73, 2160–2167. [Google Scholar] [CrossRef]
- Caminero, A.; Comabella, M.; Montalban, X. Role of tumor necrosis factor (TNF)-α and TNFRSF1A R92Q mutation in the pathogenesis of TNF receptor-associated periodic syndrome and multiple sclerosis. Clin. Exp. Immunol. 2011, 166, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Arici, Z.S.; Piskin, D.; Alehashemi, S.; Aletaha, D.; Barron, K.; Benseler, S.; Berard, R.A.; Broderick, L.; Dedeoglu, F.; et al. The 2021 EULAR/American College of Rheumatology Points to Consider for Diagnosis, Management and Monitoring of the Interleukin-1 Mediated Autoinflammatory Diseases: Cryopyrin-Associated Periodic Syndromes, Tumor Necrosis Factor Receptor-Associated Periodic Syndrome, Mevalonate Kinase Deficiency, and Deficiency of the Interleukin-1 Receptor Antagonist. Arthritis Rheumatol. 2022, 81, 907–921. [Google Scholar]
- Kirresh, A.; Everitt, A.; Kon, O.M.; DasGupta, R.; Pickering, M.C.; Lachmann, H.J. Trapped without a diagnosis: Tumour necrosis factor receptor-associated periodic syndrome (TRAPS). Pract. Neurol. 2016, 16, 304–307. [Google Scholar] [CrossRef]
- Catrysse, L.; Vereecke, L.; Beyaert, R.; van Loo, G. A20 in inflammation and autoimmunity. Trends Immunol. 2014, 35, 22–31. [Google Scholar] [CrossRef]
- Aeschlimann, F.A.; Batu, E.D.; Canna, S.W.; Go, E.; Gül, A.; Hoffmann, P.; Leavis, H.L.; Ozen, S.; Schwartz, D.M.; Stone, D.L.; et al. A20 haploinsufficiency (HA20): Clinical phenotypes and disease course of patients with a newly recognized NF-kB-mediated autoinflammatory disease. Ann. Rheum. Dis. 2018, 77, 728–735. [Google Scholar] [CrossRef]
- Wouters, C.H.; Maes, A.; Foley, K.P.; Bertin, J.; Rose, C.D. Blau Syndrome is the prototypic auto-inflammatory granulomatous disease. Pediatr. Rheumatol. 2014, 12, 33. [Google Scholar] [CrossRef]
- Sfriso, P.; Caso, F.; Tognon, S.; Galozzi, P.; Gava, A.; Punzi, L. Blau syndrome, clinical and genetic aspects. Autoimmun. Rev. 2012, 12, 44–51. [Google Scholar] [CrossRef]
- Negroni, A.; Pierdomenico, M.; Cucchiara, S.; Stronati, L. NOD2, and inflammation: Current insights. J. Inflamm. Res. 2018, 11, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Rosé, C.D.; Aróstegui, J.I.; Martin, T.M.; Espada, G.; Scalzi, L.; Yagüe, J.; Rosenbaum, J.T.; Modesto, C.; Cristina Arnal, M.; Merino, R.; et al. NOD2-associated pediatric granulomatous arthritis, an expanding phenotype: A study of an international registry and a national cohort in Spain. Arthritis Rheum. 2009, 60, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Emaminia, A.; Nabavi, M.; Mousavi Nasab, M.; Kashef, S. Central nervous system involvement in Blau syndrome: A new feature of the syndrome? J. Rheumatol. 2007, 34, 2504–2505, Erratum in J. Rheumatol. 2008, 35, 943. [Google Scholar]
- Caso, F.; Costa, L.; Rigante, D.; Vitale, A.; Cimaz, R.; Lucherini, O.M.; Sfriso, P.; Verrecchia, E.; Tognon, S.; Bascherini, V.; et al. Caveats and truths in genetic, clinical, autoimmune and autoinflammatory issues in Blau syndrome and early onset sarcoidosis. Autoimmun. Rev. 2014, 13, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Barron, K.S.; Aksentijevich, I.; Deuitch, N.T.; Stone, D.L.; Hoffmann, P.; Videgar-Laird, R.; Soldatos, A.; Bergerson, J.; Toro, C.; Cudrici, C.; et al. The Spectrum of the Deficiency of Adenosine Deaminase 2: An Observational Analysis of a 60 Patient Cohort. Front. Immunol. 2022, 12, 811473. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, D.; Ombrello, A.K.; Zavialov, A.V.; Toro, C.; Zavialov, A.V.; Stone, D.L.; Chae, J.J.; Rosenzweig, S.D.; Bishop, K.; et al. Early-onset stroke and Vasculopathy Associated with Mutations in ADA2. N. Engl. J. Med. 2014, 370, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Davidson, B.A.; Abraham, R.S.; Alter, B.; Arostegui, J.I.; Bell, K.; Belot, A.; Bergerson, J.R.E.; Bernard, T.J.; Brogan, P.A.; et al. Evaluation and Management of Deficiency of Adenosine Deaminase 2: An International Consensus Statement. JAMA Netw. Open 2023, 6, e2315894. [Google Scholar] [CrossRef] [PubMed]
- Piram, M.; Koné-Paut, I.; Lachmann, H.J.; Frenkel, J.; Ozen, S.; Kuemmerle-Deschner, J.; Stojanov, S.; Simon, A.; Finetti, M.; Sormani, M.P.; et al. Validation of the Auto-Inflammatory Diseases Activity Index (AIDAI) for hereditary recurrent fever syndromes. Ann. Rheum. Dis. 2014, 73, 2168–2173. [Google Scholar] [CrossRef] [PubMed]
- Ter Haar, N.M.; Annink, K.V.; Al-Mayouf, S.M.; Amaryan, G.; Anton, J.; Barron, K.S.; Benseler, S.M.; Brogan, P.A.; Cantarini, L.; Cattalini, M.; et al. Development of the autoinflammatory disease damage index (ADDI). Ann. Rheum. Dis. 2017, 76, 821–830. [Google Scholar] [CrossRef] [PubMed]




| Treatments | ||
|---|---|---|
| IL-1β-Mediated Autoinflammatory Disorders | Cryopyrin-Associated Periodic Syndrome (CAPS) | IL-1 antagonists, steroids |
| Familial Mediterranean Fever (FMF) | Colchicine, steroids, TNF antagonists, IL-6 antagonists, and IL-1 antagonists | |
| Mevalonate kinase deficiency (MKD) and mevalonic aciduria (MVA) | IL-1 antagonists, steroids, colchicine, IL-6 antagonists, and TNF antagonists | |
| Relopathies | A20 Haploinsufficiency | anti-TNF, anti-IL-1, and hematopoietic stem cell transplant (severe and refractory disease) |
| Dysregulation of TNF activity | Blau syndrome | Steroids, TNF antagonist |
| Deficiency of adenosine deaminase 2 (DADA2) | anti-TNF, and hematopoietic stem cell transplant | |
| Type I interferonopathies | Aicardi-Goutières syndrome Proteasome-associated autoinflammatory syndromes (PRAAS) ISG15 (interferon-stimulated gene 15) deficiency Singleton–Merten syndrome (SMS) COPA (coatomer protein subunit alpha) syndrome STING-associated vasculopathy with onset in infancy (SAVI) | JAK inhibitors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Moraes, M.P.M.; do Nascimento, R.R.N.R.; Abrantes, F.F.; Pedroso, J.L.; Perazzio, S.F.; Barsottini, O.G.P. What General Neurologists Should Know about Autoinflammatory Syndromes? Brain Sci. 2023, 13, 1351. https://doi.org/10.3390/brainsci13091351
de Moraes MPM, do Nascimento RRNR, Abrantes FF, Pedroso JL, Perazzio SF, Barsottini OGP. What General Neurologists Should Know about Autoinflammatory Syndromes? Brain Sciences. 2023; 13(9):1351. https://doi.org/10.3390/brainsci13091351
Chicago/Turabian Stylede Moraes, Marianna Pinheiro Moraes, Renan Rodrigues Neves Ribeiro do Nascimento, Fabiano Ferreira Abrantes, José Luiz Pedroso, Sandro Félix Perazzio, and Orlando Graziani Povoas Barsottini. 2023. "What General Neurologists Should Know about Autoinflammatory Syndromes?" Brain Sciences 13, no. 9: 1351. https://doi.org/10.3390/brainsci13091351
APA Stylede Moraes, M. P. M., do Nascimento, R. R. N. R., Abrantes, F. F., Pedroso, J. L., Perazzio, S. F., & Barsottini, O. G. P. (2023). What General Neurologists Should Know about Autoinflammatory Syndromes? Brain Sciences, 13(9), 1351. https://doi.org/10.3390/brainsci13091351






