Association between the Healthy Eating Index-2015 and Developmental Disabilities in Children: A Cross-Sectional Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of HEI-2015 Score
2.3. Assessment of DDs
2.4. Covariables
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, X.; Liu, B.; Yang, L.; Wang, H.; Wu, B.; Liu, R.; Chen, H.; Chen, X.; Yu, S.; Chen, B.; et al. Clinical exome sequencing as the first-tier test for diagnosing developmental disorders covering both CNV and SNV: A Chinese cohort. J. Med. Genet. 2020, 57, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Shevell, M.; Ashwal, S.; Donley, D.; Flint, J.; Gingold, M.; Hirtz, D.; Majnemer, A.; Noetzel, M.; Sheth, R.D. Practice parameter: Evaluation of the child with global developmental delay: Report of the Quality Standards Subcommittee of the American Academy of Neurology and The Practice Committee of the Child Neurology Society. Neurology 2003, 60, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Lipkin, P.H.; Macias, M.M.; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics; Norwood, K.W.; Brei, T.J.; Davidson, L.F.; Davis, B.E.; Ellerbeck, K.A.; Houtrow, A.J.; Hyman, S.L.; et al. Promoting Optimal Development: Identifying Infants and Young Children with Developmental Disorders Through Developmental Surveillance and Screening. Pediatrics 2020, 145, e20193449. [Google Scholar] [CrossRef] [PubMed]
- Zablotsky, B.; Black, L.I.; Maenner, M.J.; Schieve, L.A.; Danielson, M.L.; Bitsko, R.H.; Blumberg, S.J.; Kogan, M.D.; Boyle, C.A. Prevalence and Trends of Developmental Disabilities among Children in the United States: 2009–2017. Pediatrics 2019, 144, e20190811. [Google Scholar] [CrossRef] [PubMed]
- Zablotsky, B.; Ng, A.E.; Black, L.I.; Blumberg, S.J. Diagnosed Developmental Disabilities in Children Aged 3-17 Years: United States, 2019–2021. NCHS Data Brief 2023, 473, 1–8. [Google Scholar] [CrossRef]
- Benton, D.; ILSI Europe a.i.s.b.l. The influence of children’s diet on their cognition and behavior. Eur. J. Nutr. 2008, 47 (Suppl. S3), 25–37. [Google Scholar] [CrossRef] [PubMed]
- Leventakou, V.; Roumeliotaki, T.; Sarri, K.; Koutra, K.; Kampouri, M.; Kyriklaki, A.; Vassilaki, M.; Kogevinas, M.; Chatzi, L. Dietary patterns in early childhood and child cognitive and psychomotor development: The Rhea mother-child cohort study in Crete. Br. J. Nutr. 2016, 115, 1431–1437. [Google Scholar] [CrossRef]
- Marinoni, M.; Giordani, E.; Mosconi, C.; Rosolen, V.; Concina, F.; Fiori, F.; Carletti, C.; Knowles, A.; Pani, P.; Bin, M.; et al. Are Dietary Patterns Related to Cognitive Performance in 7-Year-Old Children? Evidence from a Birth Cohort in Friuli Venezia Giulia, Italy. Nutrients 2022, 14, 4168. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, Y.; Li, J.; Liu, Y.; Chang, H.; Jiang, Y.; Tuo, X.; Zhou, L.; Yu, Y. Association between healthy eating index-2015 and various cognitive domains in US adults aged 60 years or older: The National Health and Nutrition Examination Survey (NHANES) 2011–2014. BMC Public Health 2021, 21, 1862. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, Y.; Nie, J.; Xu, H.; Yu, C.; Wang, S. Higher HEI-2015 Score Is Associated with Reduced Risk of Depression: Result from NHANES 2005–2016. Nutrients 2021, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.G.; Nie, J.Q.; Li, Y.Y.; Yu, X.; Zhang, Z.J. Higher HEI-2015 Scores Are Associated with Lower Risk of Sleep Disorder: Results from a Nationally Representative Survey of United States Adults. Nutrients 2022, 14, 873. [Google Scholar] [CrossRef] [PubMed]
- Onvani, S.; Haghighatdoost, F.; Surkan, P.J.; Larijani, B.; Azadbakht, L. Adherence to the Healthy Eating Index and Alternative Healthy Eating Index dietary patterns and mortality from all causes, cardiovascular disease and cancer: A meta-analysis of observational studies. J. Hum. Nutr. Diet. 2017, 30, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Walhovd, K.B.; Krogsrud, S.K.; Amlien, I.K.; Bartsch, H.; Bjornerud, A.; Due-Tonnessen, P.; Grydeland, H.; Hagler, D.J., Jr.; Haberg, A.K.; Kremen, W.S.; et al. Neurodevelopmental origins of lifespan changes in brain and cognition. Proc. Natl. Acad. Sci. USA 2016, 113, 9357–9362. [Google Scholar] [CrossRef]
- Giedd, J.N.; Raznahan, A.; Alexander-Bloch, A.; Schmitt, E.; Gogtay, N.; Rapoport, J.L. Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology 2015, 40, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Del-Ponte, B.; Quinte, G.C.; Cruz, S.; Grellert, M.; Santos, I.S. Dietary patterns and attention deficit/hyperactivity disorder (ADHD): A systematic review and meta-analysis. J. Affect. Disord. 2019, 252, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Audhya, T.; Geis, E.; Gehn, E.; Fimbres, V.; Pollard, E.L.; Mitchell, J.; Ingram, J.; Hellmers, R.; Laake, D.; et al. Comprehensive Nutritional and Dietary Intervention for Autism Spectrum Disorder-A Randomized, Controlled 12-Month Trial. Nutrients 2018, 10, 369. [Google Scholar] [CrossRef] [PubMed]
- Lipkin, P.H.; Okamoto, J.; Council on Children with Disabilities and Council on School Health. The Individuals with Disabilities Education Act (IDEA) for Children with Special Educational Needs. Pediatrics 2015, 136, e1650–e1662. [Google Scholar] [CrossRef]
- Romo, M.L.; McVeigh, K.H.; Jordan, P.; Stingone, J.A.; Chan, P.Y.; Askew, G.L. Birth characteristics of children who used early intervention and special education services in New York City. J. Public Health 2020, 42, e401–e411. [Google Scholar] [CrossRef]
- Meng, F.; Qi, Y.; Wu, Y.; He, F. Association between acrylamide exposure and the odds of developmental disabilities in children: A cross-sectional study. Front. Public Health 2022, 10, 972368. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, H.; Peng, L.; Zhou, Y.; Wang, Y.; Jiang, F. Gender differences in the association between biomarkers of environmental smoke exposure and developmental disorders in children and adolescents. Environ. Sci. Pollut. Res. Int. 2022, 29, 84629–84639. [Google Scholar] [CrossRef] [PubMed]
- Abid, Z.; Roy, A.; Herbstman, J.B.; Ettinger, A.S. Urinary polycyclic aromatic hydrocarbon metabolites and attention/deficit hyperactivity disorder, learning disability, and special education in U.S. children aged 6 to 15. J. Environ. Public Health 2014, 2014, 628508. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Zhu, Y.; Vanage, V.; Jain, N.; Holschuh, N.; Hermetet Agler, A. Association between Ready-to-Eat Cereal Consumption and Nutrient Intake, Nutritional Adequacy, and Diet Quality among Infants, Toddlers, and Children in the National Health and Nutrition Examination Survey 2015–2016. Nutrients 2019, 11, 1989. [Google Scholar] [CrossRef] [PubMed]
- Cifelli, C.J.; Agarwal, S.; Fulgoni, V.L., 3rd. Association of Yogurt Consumption with Nutrient Intakes, Nutrient Adequacy, and Diet Quality in American Children and Adults. Nutrients 2020, 12, 3435. [Google Scholar] [CrossRef] [PubMed]
- Maillot, M.; Vieux, F.; Rehm, C.; Drewnowski, A. Consumption of 100% Orange Juice in Relation to Flavonoid Intakes and Diet Quality Among US Children and Adults: Analyses of NHANES 2013-16 Data. Front. Nutr. 2020, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Fulgoni, V.L., 3rd; Painter, J.; Carughi, A. Association of raisin and raisin-containing food consumption with nutrient intake and diet quality in US children: NHANES 2001–2012. Food Sci. Nutr. 2018, 6, 2162–2169. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Cowan, A.E.; Dodd, K.W.; Tooze, J.A.; Gahche, J.J.; Eicher-Miller, H.A.; Guenther, P.M.; Dwyer, J.T.; Potischman, N.; Bhadra, A.; et al. Association of food insecurity with dietary intakes and nutritional biomarkers among US children, National Health and Nutrition Examination Survey (NHANES) 2011–2016. Am. J. Clin. Nutr. 2021, 114, 1059–1069. [Google Scholar] [CrossRef]
- Mari-Bauset, S.; Llopis-Gonzalez, A.; Zazpe, I.; Mari-Sanchis, A.; Morales Suarez-Varela, M. Comparison of nutritional status between children with autism spectrum disorder and typically developing children in the Mediterranean Region (Valencia, Spain). Autism 2017, 21, 310–322. [Google Scholar] [CrossRef]
- Li, X.S.; Pinto-Martin, J.A.; Thompson, A.; Chittams, J.; Kral, T.V.E. Weight status, diet quality, perceived stress, and functional health of caregivers of children with autism spectrum disorder. J. Spec. Pediatr. Nurs. 2018, 23, e12205. [Google Scholar] [CrossRef]
- Curhan, S.G.; Halpin, C.; Wang, M.; Eavey, R.D.; Curhan, G.C. Prospective Study of Dietary Patterns and Hearing Threshold Elevation. Am. J. Epidemiol. 2020, 189, 204–214. [Google Scholar] [CrossRef]
- Pereira, A.R.; Oliveira, A. Dietary Interventions to Prevent Childhood Obesity: A Literature Review. Nutrients 2021, 13, 3447. [Google Scholar] [CrossRef] [PubMed]
- Mottalib, A.; Kasetty, M.; Mar, J.Y.; Elseaidy, T.; Ashrafzadeh, S.; Hamdy, O. Weight Management in Patients with Type 1 Diabetes and Obesity. Curr. Diab. Rep. 2017, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, F.; Asghari, G.; Yuzbashian, E.; Mahdavi, M.; Mirmiran, P.; Azizi, F. Modified Healthy Eating Index and Incidence of Metabolic Syndrome in Children and Adolescents: Tehran Lipid and Glucose Study. J. Pediatr. 2018, 197, 134–139.e2. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, R.; Odjidja, E.N.; Scott, D.; Shivappa, N.; Hebert, J.R.; Hodge, A.; de Courten, B. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes. Rev. 2022, 23, e13349. [Google Scholar] [CrossRef] [PubMed]
- Landau, Z.; Pinhas-Hamiel, O. Attention Deficit/Hyperactivity, the Metabolic Syndrome, and Type 2 Diabetes. Curr. Diab. Rep. 2019, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, K.K.; Orsso, C.E.; Richard, C.; Haqq, A.M.; Zwaigenbaum, L. Risk Factors for Unhealthy Weight Gain and Obesity among Children with Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 3285. [Google Scholar] [CrossRef] [PubMed]
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: A systematic review. Transl. Psychiatry 2021, 11, 71. [Google Scholar] [CrossRef]
- Rivell, A.; Mattson, M.P. Intergenerational Metabolic Syndrome and Neuronal Network Hyperexcitability in Autism. Trends Neurosci. 2019, 42, 709–726. [Google Scholar] [CrossRef]
- Kral, T.V.; Eriksen, W.T.; Souders, M.C.; Pinto-Martin, J.A. Eating behaviors, diet quality, and gastrointestinal symptoms in children with autism spectrum disorders: A brief review. J. Pediatr. Nurs. 2013, 28, 548–556. [Google Scholar] [CrossRef]
- Curtin, C.; Hubbard, K.; Anderson, S.E.; Mick, E.; Must, A.; Bandini, L.G. Food selectivity, mealtime behavior problems, spousal stress, and family food choices in children with and without autism spectrum disorder. J. Autism Dev. Disord. 2015, 45, 3308–3315. [Google Scholar] [CrossRef]
- Evans, E.W.; Must, A.; Anderson, S.E.; Curtin, C.; Scampini, R.; Maslin, M.; Bandini, L. Dietary Patterns and Body Mass Index in Children with Autism and Typically Developing Children. Res. Autism Spectr. Disord. 2012, 6, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Acosta, A.; Khokhlovich, E.; Reis, H.; Vyshedskiy, A. Dietary Factors Impact Developmental Trajectories in Young Autistic Children. J. Autism Dev. Disord. 2023. [Google Scholar] [CrossRef] [PubMed]
- Robinette, L.M.; Hatsu, I.E.; Johnstone, J.M.; Tost, G.; Bruton, A.M.; Leung, B.M.Y.; Odei, J.B.; Orchard, T.; Gracious, B.L.; Arnold, L.E. Fruit and vegetable intake is inversely associated with severity of inattention in a pediatric population with ADHD symptoms: The MADDY Study. Nutr. Neurosci. 2023, 26, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Bandini, L.G.; Curtin, C.; Phillips, S.M.; Rogers, G.T.; Eliasziw, M.; Perelli, J.; Jay, L.; Maslin, M.; Must, A. Nutrient adequacy, dietary patterns and diet quality among children with and without intellectual disabilities. J. Intellect. Disabil. Res. 2021, 65, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P. Flavonoids: Modulators of brain function? Br. J. Nutr. 2008, 99 (Suppl. 1), ES60–ES77. [Google Scholar] [CrossRef] [PubMed]
- Rutsch, A.; Kantsjo, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Tamura, M.; Ohnishi, Y.; Kotani, T.; Gato, N. Effects of new dietary fiber from Japanese Apricot (Prunus mume Sieb. et Zucc.) on gut function and intestinal microflora in adult mice. Int. J. Mol. Sci. 2011, 12, 2088–2099. [Google Scholar] [CrossRef]
- Sundermann, E.E.; Katz, M.J.; Lipton, R.B.; Lichtenstein, A.H.; Derby, C.A. A Brief Dietary Assessment Predicts Executive Dysfunction in an Elderly Cohort: Results from the Einstein Aging Study. J. Am. Geriatr. Soc. 2016, 64, e131–e136. [Google Scholar] [CrossRef]
- Gu, Y.; Guo, J.; Moshfegh, A.J. Race/ethnicity and gender modify the association between diet and cognition in U.S. older adults: National Health and Nutrition Examination Survey 2011–2014. Alzheimer’s Dement. 2021, 7, e12128. [Google Scholar] [CrossRef]

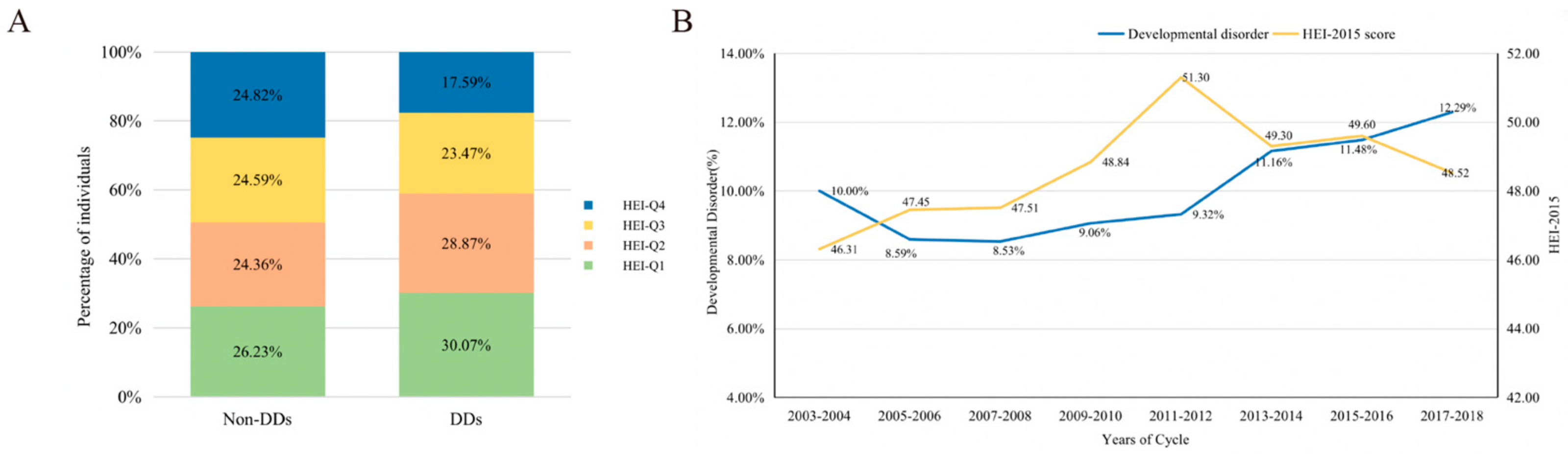
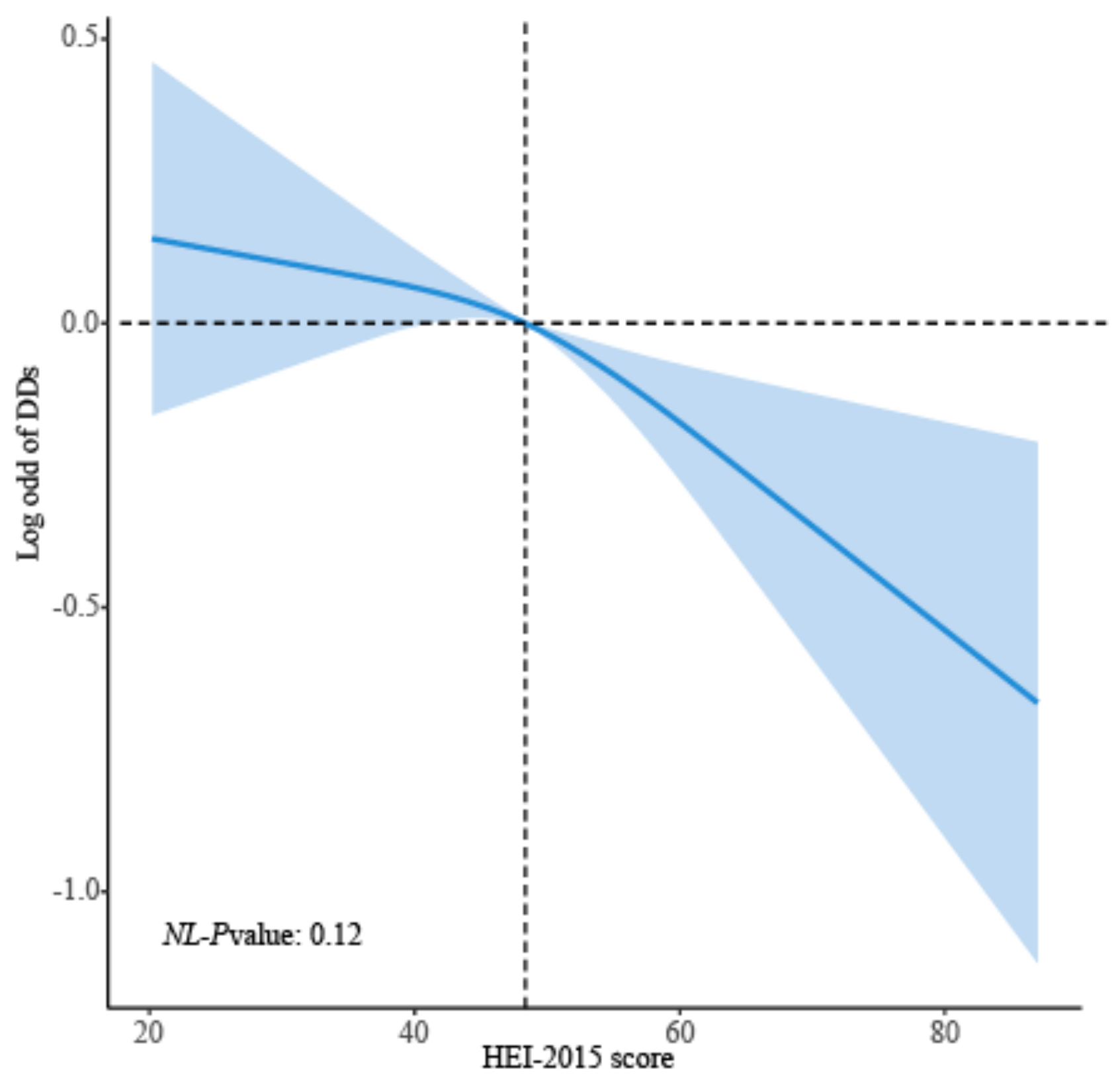

| Variable | All (n = 11,919) | Non-DDs (n = 10,727) | DDs (n = 1192) | p-Value b |
|---|---|---|---|---|
| Age | 10.04 (0.04) | 10.03 (0.05) | 10.05 (0.13) | 0.92 |
| Sex | <0.001 | |||
| Male | 5964 (50.04) | 5541 (50.53) | 414 (35.17) | |
| Female | 5955 (49.96) | 5186 (49.47) | 778 (64.83) | |
| Race/ethnicity | 0.01 | |||
| Mexican American | 3011 (25.26) | 2818 (14.76) | 193 (12.14) | |
| Non-Hispanic white | 3540 (29.7) | 3148 (58.04) | 392 (53.32) | |
| Non-Hispanic black | 3164 (26.55) | 2796 (13.37) | 368 (16.31) | |
| Other races | 2204 (18.49) | 1965 (13.84) | 239 (18.23) | |
| Poverty status | <0.001 | |||
| No | 8203 (68.82) | 7482 (78.40) | 721 (65.79) | |
| Yes | 3716 (31.18) | 3245 (21.60) | 471 (34.21) | |
| Birth weight | <0.001 | |||
| ≥5.5 lbs | 10,083 (84.6) | 9140 (86.38) | 943 (79.84) | |
| <5.5 lbs | 1836 (15.4) | 1587 (13.62) | 249 (20.16) | |
| Maternal smoking during pregnancy | <0.001 | |||
| No | 5697 (87.51) | 5157 (87.43) | 540 (77.89) | |
| Yes | 813 (12.49) | 661 (12.57) | 152 (22.11) | |
| Health insurance coverage | 0.04 | |||
| No | 1203 (10.09) | 1128 (8.09) | 75 (5.76) | |
| Yes | 10,716 (89.91) | 9599 (91.91) | 1117 (94.24) | |
| BMI | 19.88 (0.10) | 19.83 (0.11) | 20.30 (0.23) | 0.05 |
| HEI score | 48.79 (0.26) | 48.96 (0.26) | 47.24 (0.51) | <0.01 |
| Total vegetables | 2.26 (0.02) | 2.28 (0.02) | 2.14 (0.06) | 0.03 |
| Greens and beans | 1.13 (0.03) | 1.14 (0.03) | 1.06 (0.08) | 0.34 |
| Total fruit | 2.76 (0.04) | 2.80 (0.04) | 2.47 (0.10) | 0.001 |
| Whole fruits | 2.68 (0.05) | 2.72 (0.05) | 2.32 (0.10) | <0.001 |
| Whole grains | 2.57 (0.05) | 2.58 (0.05) | 2.49 (0.13) | 0.55 |
| Dairy | 7.35 (0.05) | 7.34 (0.05) | 7.38 (0.14) | 0.82 |
| Total protein foods | 3.89 (0.02) | 3.89 (0.02) | 3.90 (0.06) | 0.78 |
| Seafood and plant proteins | 2.01 (0.04) | 2.03 (0.04) | 1.83 (0.09) | 0.03 |
| Fatty acid | 3.55 (0.05) | 3.57 (0.05) | 3.38 (0.13) | 0.14 |
| Sodium | 4.75 (0.05) | 4.76 (0.05) | 4.68 (0.13) | 0.57 |
| Refined grains | 4.77 (0.06) | 4.76 (0.06) | 4.83 (0.12) | 0.62 |
| Saturated fats | 5.29 (0.06) | 5.33 (0.06) | 4.99 (0.13) | 0.02 |
| Added sugars | 5.77 (0.06) | 5.78 (0.06) | 5.77 (0.13) | 0.98 |
| Variable | Model 1 a | Model 2 b | ||
|---|---|---|---|---|
| OR (95%CI) | p-Value/ P-Trend c | OR (95%CI) | p-Value/ P-Trend c | |
| HEI-2015 score (continuous) | 0.99 (0.98, 0.99) | 0.001 | 0.99 (0.98, 1.00) | 0.01 |
| Quartile of HEI-2015 | <0.001 | <0.01 | ||
| Q1 [12.66, 40.91] | Ref | Ref | ||
| Q2 (40.91, 48.34] | 1.06 (0.84, 1.33) | 1.07 (0.84, 1.36) | ||
| Q3 (48.34, 56.39] | 0.91 (0.70, 1.18) | 0.95 (0.73, 1.25) | ||
| Q4 (56.39, 95.65] | 0.63 (0.49, 0.82) | 0.69 (0.53, 0.89) | ||
| HEI-2015 Components | Model 1 a | Model 2 b | ||
|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| Total vegetables | 0.93 (0.86, 0.99) | 0.03 | 0.94 (0.87, 1.00) | 0.05 |
| Greens and beans | 0.97 (0.92, 1.03) | 0.35 | 0.99 (0.94, 1.05) | 0.77 |
| Total fruit | 0.91 (0.87, 0.96) | 0.001 | 0.93 (0.88, 0.98) | <0.01 |
| Whole fruits | 0.92 (0.88, 0.96) | <0.001 | 0.94 (0.90, 0.98) | <0.01 |
| Whole grains | 0.99 (0.95, 1.03) | 0.55 | 1.00 (0.96, 1.04) | 0.82 |
| Dairy | 1.00 (0.97, 1.04) | 0.82 | 1.00 (0.97, 1.04) | 0.83 |
| Total protein foods | 1.01 (0.94, 1.09) | 0.78 | 0.99 (0.92, 1.06) | 0.76 |
| Seafood and plant proteins | 0.95 (0.91, 1.00) | 0.03 | 0.96 (0.92, 1.01) | 0.09 |
| Fatty acid | 0.98 (0.95, 1.01) | 0.15 | 0.98 (0.95, 1.01) | 0.26 |
| Sodium | 0.99 (0.96, 1.02) | 0.57 | 1.00 (0.97, 1.03) | 0.88 |
| Refined grains | 1.01 (0.98, 1.03) | 0.62 | 1.00 (0.97, 1.03) | 0.99 |
| Saturated fats | 0.96 (0.93, 0.99) | 0.02 | 0.96 (0.93, 1.00) | 0.03 |
| Added sugars | 1.00 (0.97, 1.03) | 0.98 | 1.01 (0.97, 1.04) | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gui, J.; Wang, L.; Han, Z.; Ding, R.; Yang, X.; Yang, J.; Luo, H.; Huang, D.; Liu, J.; Jiang, L. Association between the Healthy Eating Index-2015 and Developmental Disabilities in Children: A Cross-Sectional Analysis. Brain Sci. 2023, 13, 1353. https://doi.org/10.3390/brainsci13091353
Gui J, Wang L, Han Z, Ding R, Yang X, Yang J, Luo H, Huang D, Liu J, Jiang L. Association between the Healthy Eating Index-2015 and Developmental Disabilities in Children: A Cross-Sectional Analysis. Brain Sciences. 2023; 13(9):1353. https://doi.org/10.3390/brainsci13091353
Chicago/Turabian StyleGui, Jianxiong, Lingman Wang, Ziyao Han, Ran Ding, Xiaoyue Yang, Jiaxin Yang, Hanyu Luo, Dishu Huang, Jie Liu, and Li Jiang. 2023. "Association between the Healthy Eating Index-2015 and Developmental Disabilities in Children: A Cross-Sectional Analysis" Brain Sciences 13, no. 9: 1353. https://doi.org/10.3390/brainsci13091353





