The Distant Molecular Effects on the Brain by Cancer Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Animal Treatment
2.3. Tissue Preparation
2.4. NanoString Gene Expression Profiling
2.5. Data Analysis
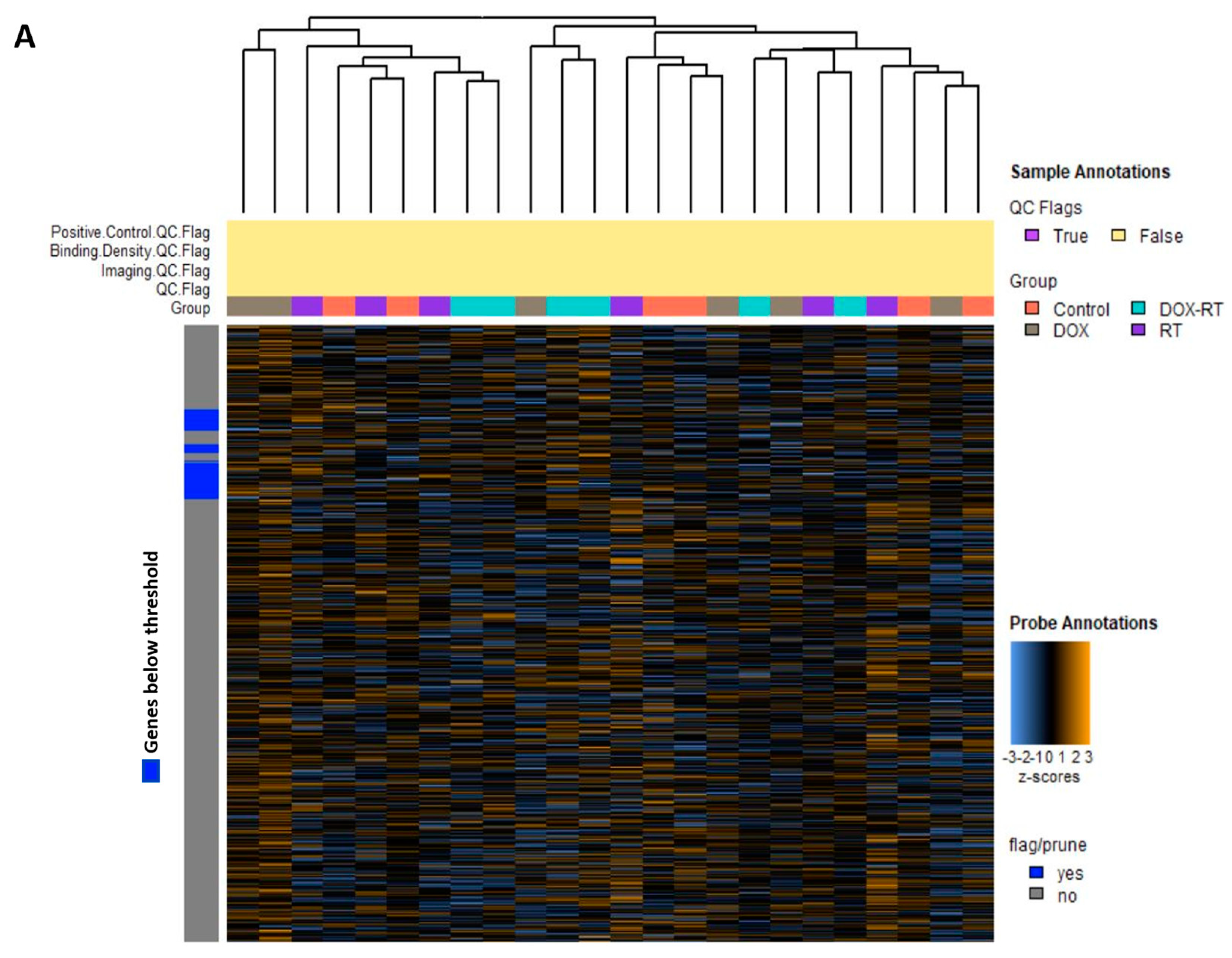
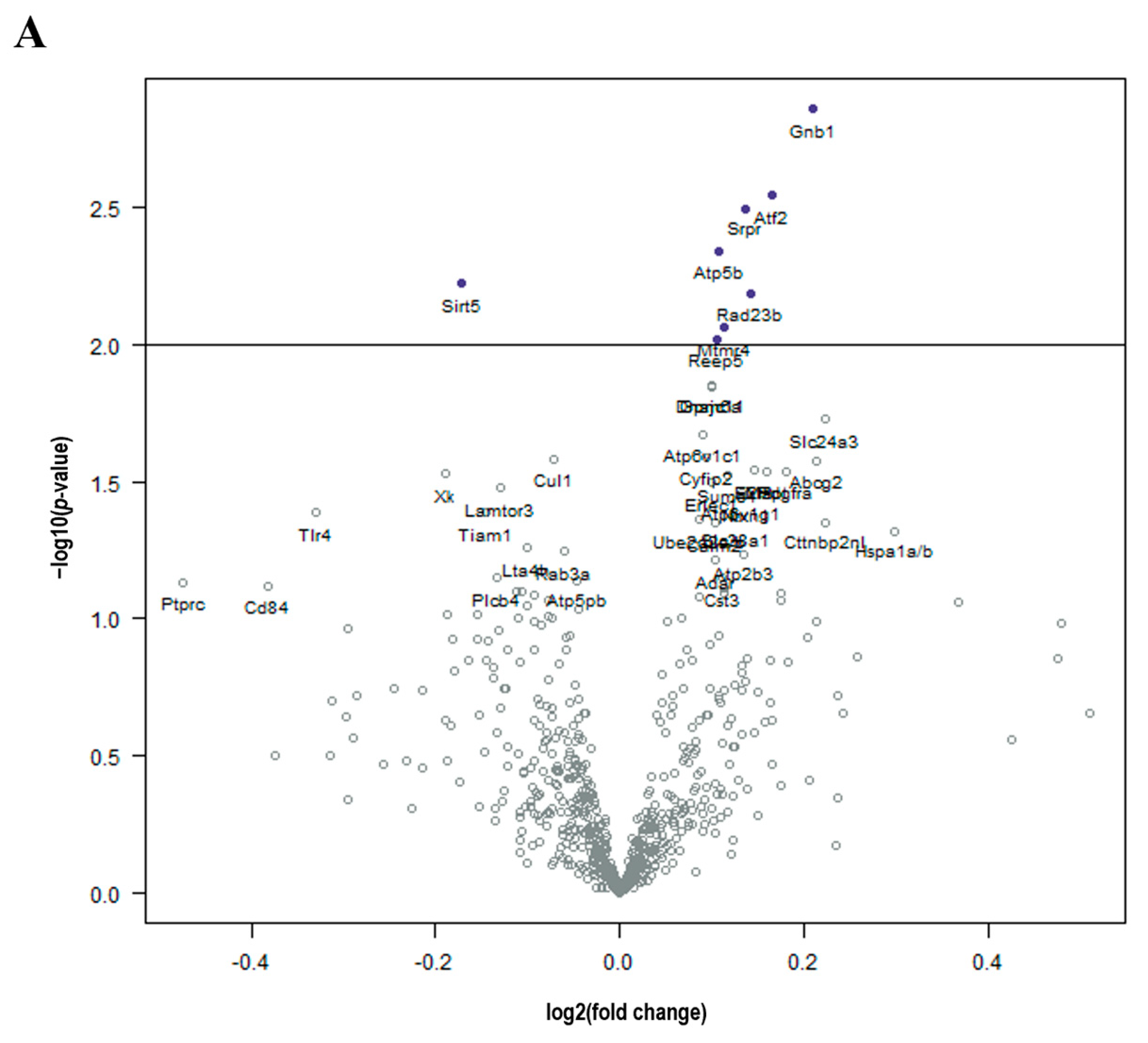
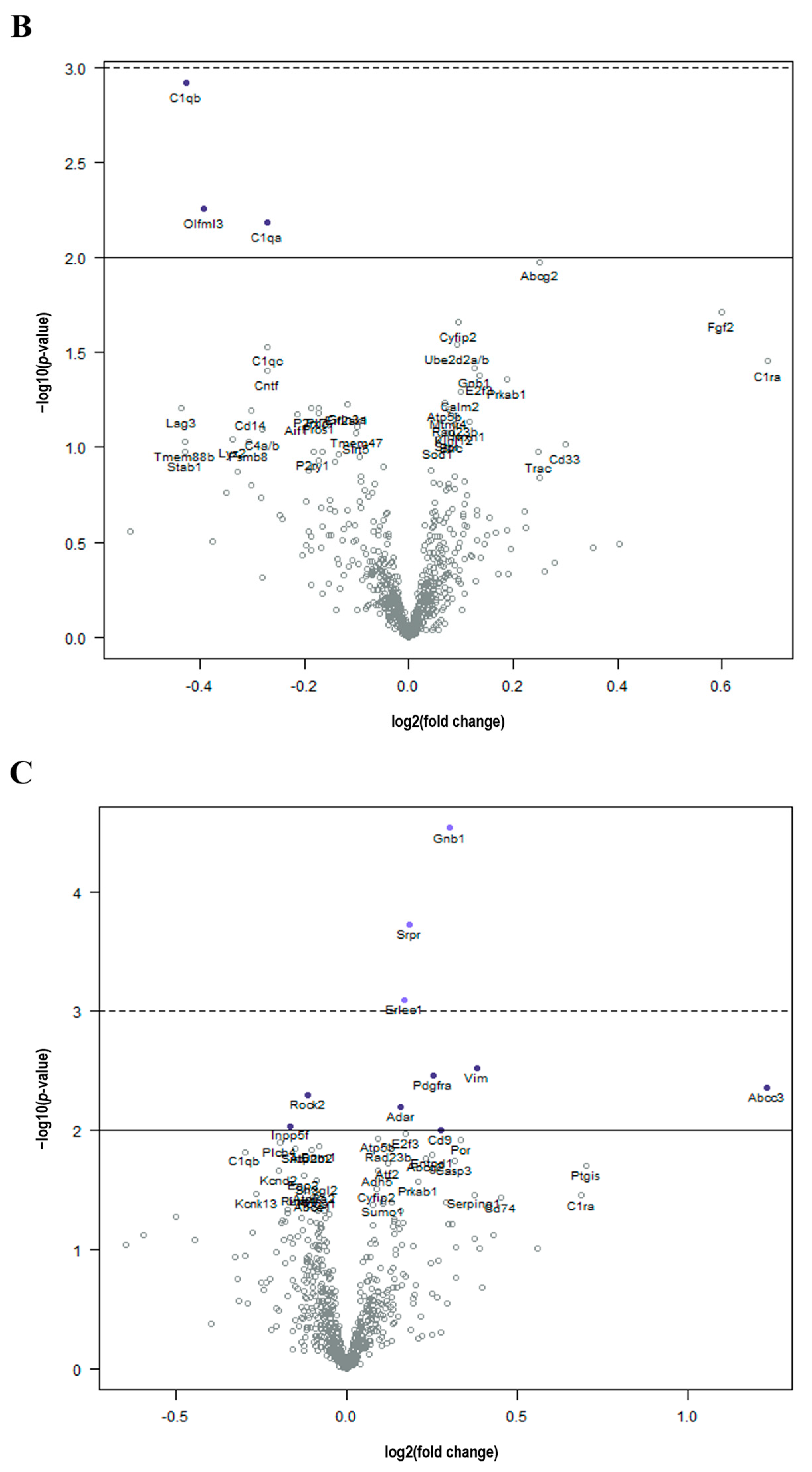
3. Results
Cancer Treatment Is Associated with Unintended Molecular Changes in the Normal Brain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demos-Davies, K.; Lawrence, J.; Rogich, A.; Lind, E.; Seelig, D. Cancer treatment induces neuroinflammation and behavioral deficits in mice. Front. Behav. Neurosci. 2022, 16, 1067298. [Google Scholar] [CrossRef] [PubMed]
- Feiock, C.; Yagi, M.; Maidman, A.; Rendahl, A.; Hui, S.; Seelig, D. Central Nervous System Injury—A Newly Observed Bystander Effect of Radiation. PLoS ONE 2016, 11, e0163233. [Google Scholar] [CrossRef] [PubMed]
- Kerstens, C.; Wildiers, H.P.M.W.; Schroyen, G.; Almela, M.; Mark, R.E.; Lambrecht, M.; Deprez, S.; Sleurs, C. A Systematic Review on the Potential Acceleration of Neurocognitive Aging in Older Cancer Survivors. Cancers 2023, 15, 1215. [Google Scholar] [CrossRef] [PubMed]
- Schroyen, G.; Blommaert, J.; van Weehaeghe, D.; Sleurs, C.; Vandenbulcke, M.; Dedoncker, N.; Hatse, S.; Goris, A.; Koole, M.; Smeets, A.; et al. Neuroinflammation and Its Association with Cognition, Neuronal Markers and Peripheral Inflammation after Chemotherapy for Breast Cancer. Cancers 2021, 13, 4198. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Kohli, S.; Mohile, S.G.; Usuki, K.; Ahles, T.A.; Morrow, G.R. An update on cancer- and chemotherapy-related cognitive dysfunction: Current status. Semin. Oncol. 2011, 38, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Seigers, R.; Loos, M.; Van Tellingen, O.; Boogerd, W.; Smit, A.B.; Schagen, S.B. Cognitive impact of cytotoxic agents in mice. Psychopharmacology 2015, 232, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Oppegaard, K.R.; Armstrong, T.S.; Anguera, J.A.; Kober, K.M.; Kelly, D.L.; Laister, R.C.; Saligan, L.N.; Ayala, A.P.; Kuruvilla, J.; Alm, M.W.; et al. Blood-based biomarkers of cancer-related cognitive impairment in non-central nervous system cancer: A scoping review. Crit. Rev. Oncol. Hematol. 2022, 180, 103822. [Google Scholar] [CrossRef]
- Brown, T.; McElroy, T.; Simmons, P.; Walters, H.; Ntagwabira, F.; Wang, J.; Byrum, S.D.; Allen, A.R. Cognitive impairment resulting from treatment with docetaxel, doxorubicin, and cyclophosphamide. Brain Res. 2021, 1760, 147397. [Google Scholar] [CrossRef]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.; Castel, H. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef]
- McDonald, B.C.; Conroy, S.K.; Ahles, T.A.; West, J.D.; Saykin, A.J. Gray matter reduction associated with systemic chemotherapy for breast cancer: A prospective MRI study. Breast Cancer Res. Treat. 2010, 123, 819–828. [Google Scholar] [CrossRef]
- Fleming, B.; Edison, P.; Kenny, L. Cognitive impairment after cancer treatment: Mechanisms, clinical characterization, and management. BMJ 2023, 380, e071726. [Google Scholar] [CrossRef] [PubMed]
- Matsos, A.; Johnston, I.N. Chemotherapy-induced cognitive impairments: A systematic review of the animal literature. Neurosci. Biobehav. Rev. 2019, 102, 382–399. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.; Pyter, L.M. Neuroimmunology of Behavioral Comorbidities Associated with Cancer and Cancer Treatments. Front. Immunol. 2018, 9, 1195. [Google Scholar] [CrossRef] [PubMed]
- Cuccurullo, V.; Di Stasio, G.D.; Cascini, G.L.; Gatta, G.; Bianco, C. The Molecular Effects of Ionizing Radiations on Brain Cells: Radiation Necrosis vs. Tumor Recurrence. Diagnostics 2019, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Lumniczky, K.; Szatmári, T.; Sáfrány, G. Ionizing Radiation-Induced Immune and Inflammatory Reactions in the Brain. Front. Immunol. 2017, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bryant, A.K.; Banegas, M.P.; Martinez, M.E.; Mell, L.K.; Murphy, J.D. Trends in Radiation Therapy among Cancer Survivors in the United States, 2000–2030. Cancer Epidemiol. Biomark. Prev. 2017, 26, 963–970. [Google Scholar] [CrossRef]
- Wilson, B.E.; Jacob, S.; Yap, M.L.; Ferlay, J.; Bray, F.; Barton, M.B. Estimates of global chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040: A population-based study. Lancet Oncol. 2019, 20, 769–780. [Google Scholar] [CrossRef]
- Wefel, J.S.; Kesler, S.R.; Noll, K.R.; Schagen, S.B. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J. Clin. 2015, 65, 123–138. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Johansen, P.B. Doxorubicin pharmacokinetics after intravenous and intraperitoneal administration in the nude mouse. Cancer Chemother. Pharmacol. 1981, 5, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Pisters, P.W.; Patel, S.R.; Prieto, V.G.; Thall, P.F.; Lewis, V.O.; Feig, B.W.; Hunt, K.K.; Yasko, A.W.; Lin, P.P.; Jacobson, M.G.; et al. Phase I trial of preoperative doxorubicin-based concurrent chemoradiation and surgical resection for localized extremity and body wall soft tissue sarcomas. J. Clin. Oncol. 2004, 22, 3375–3380. [Google Scholar] [CrossRef] [PubMed]
- Romesser, P.B.; Sherman, E.J.; Whiting, K.; Ho, M.L.; Shaha, A.R.; Sabra, M.M.; Riaz, N.; Waldenberg, T.E.; Sabol, C.R.; Ganly, I.; et al. Intensity-modulated radiation therapy and doxorubicin in thyroid cancer: A prospective phase 2 trial. Cancer 2021, 127, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Baghani, H.R.; Aghamiri, S.M.R.; Mahdavi, S.R.; Robatjazi, M.; Zadeh, A.R.; Akbari, M.E.; Mirzaei, H.R.; Nafissi, N.; Samsami, M. Dosimetric evaluation of Gafchromic EBT2 film for breast intraoperative electron radiotherapy verification. Phys. Med. 2015, 31, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Aglan, O. StatPearls. Radiation Therapy for Early-Stage Breast Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Dong, H.-W. Allen Reference Atlas: A Digital Color Brain Atlas of the C57Black/6J Male Mouse; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Vider, J.; Croaker, A.; Cox, A.J.; Raymond, E.; Rogers, R.; Adamson, S.; Doyle, M.; O’brien, B.; Cripps, A.W.; West, N.P. Comparison of skin biopsy sample processing and storage methods on high dimensional immune gene expression using the Nanostring nCounter system. Diagn. Pathol. 2020, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Nelson, M.; Basu, M.; Srinivasan, P.; Lazarski, C.; Zhang, P.; Zheng, P.; Sandler, A.D. MYC oncogene is associated with suppression of tumor immunity and targeting Myc induces tumor cell immunogenicity for therapeutic whole cell vaccination. J. Immunother. Cancer 2021, 9, e001388. [Google Scholar] [CrossRef]
- Ma, C.; Hunt, J.B.; Kovalenko, A.; Liang, H.; Selenica, M.-L.B.; Orr, M.B.; Zhang, B.; Gensel, J.C.; Feola, D.J.; Gordon, M.N.; et al. Myeloid Arginase 1 Insufficiency Exacerbates Amyloid-β Associated Neurodegenerative Pathways and Glial Signatures in a Mouse Model of Alzheimer’s Disease: A Targeted Transcriptome Analysis. Front. Immunol. 2021, 12, 628156. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’Ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin-An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jenrow, K.A.; Brown, S.L. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat. Oncol. J. 2014, 32, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; An, D.; Xu, H.; Cheng, X.; Wang, S.; Yu, W.; Yu, D.; Zhao, D.; Sun, Y.; Deng, W.; et al. Effects of social isolation and re-socialization on cognition and ADAR1 (p110) expression in mice. PeerJ 2016, 4, e2306. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yang, J.; Yu, W. Promoter Hypomethylation of TGFBR3 as a Risk Factor of Alzheimer’s Disease: An Integrated Epigenomic-Transcriptomic Analysis. Front. Cell Dev. Biol. 2021, 9, 825729. [Google Scholar] [CrossRef]
- Benton, C.S.; Miller, B.H.; Skwerer, S.; Suzuki, O.; Schultz, L.E.; Cameron, M.D.; Marron, J.S.; Pletcher, M.T.; Wiltshire, T. Evaluating genetic markers and neurobiochemical analytes for fluoxetine response using a panel of mouse inbred strains. Psychopharmacology 2012, 221, 297–315. [Google Scholar] [CrossRef]
- Anwar, M.J.; Pillai, K.K.; Khanam, R.; Akhtar, M.; Vohora, D. Effect of alprazolam on anxiety and cardiomyopathy induced by doxorubicin in mice. Fundam. Clin. Pharmacol. 2012, 26, 356–362. [Google Scholar] [CrossRef]
- Aziriova, S.; Bednarova, K.R.; Krajcirovicova, K.; Hrenak, J.; Rajkovicova, R.; Arendasova, K.; Kamodyova, N.; Celec, P.; Zorad, S.; Adamcova, M.; et al. Doxorubicin-induced behavioral disturbances in rats: Protective effect of melatonin and captopril. Pharmacol. Biochem. Behav. 2014, 124, 284–289. [Google Scholar] [CrossRef]
- Cavalier, A.N.; Clayton, Z.S.; Hutton, D.A.; Wahl, D.; Lark, D.S.; Reisz, J.A.; Melov, S.; Campisi, J.; Seals, D.R.; LaRocca, T.J. Accelerated aging of the brain transcriptome by the common chemotherapeutic doxorubicin. Exp. Gerontol. 2021, 152, 111451. [Google Scholar] [CrossRef]
- Liao, D.; Xiang, D.; Dang, R.; Xu, P.; Wang, J.; Han, W.; Fu, Y.; Yao, D.; Cao, L.; Jiang, P. Neuroprotective Effects of dl-3-n-Butylphthalide against Doxorubicin-Induced Neuroinflammation, Oxidative Stress, Endoplasmic Reticulum Stress, and Behavioral Changes. Oxid. Med. Cell Longev. 2018, 2018, 9125601. [Google Scholar] [CrossRef] [PubMed]
- Hol, E.M.; Pekny, M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr. Opin. Cell Biol. 2015, 32, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Castro, C.; Noori, A.; Magdamo, C.G.; Li, Z.; Marks, J.D.; Frosch, M.P.; Das, S.; Hyman, B.T.; Serrano-Pozo, A. Cyclic multiplex fluorescent immunohistochemistry and machine learning reveal distinct states of astrocytes and microglia in normal aging and Alzheimer’s disease. J. Neuroinflamm. 2022, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.A.; Goluszko, E.; Chen, H.-Z.; Leone, G.; Post, S.; Lozano, G.; Chen, Z.; Chauchereau, A. E2F3 is a mediator of DNA damage-induced apoptosis. Mol. Cell Biol. 2010, 30, 524–536. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McClellan, K.A.; Ruzhynsky, V.A.; Douda, D.N.; Vanderluit, J.L.; Ferguson, K.L.; Chen, D.; Bremner, R.; Park, D.S.; Leone, G.; Slack, R.S. Unique requirement for Rb/E2F3 in neuronal migration: Evidence for cell cycle-independent functions. Mol. Cell Biol. 2007, 27, 4825–4843. [Google Scholar] [CrossRef] [PubMed]
- Jamialahmadi, K.; Zahedipour, F.; Karimi, G. The role of microRNAs on doxorubicin drug resistance in breast cancer. J. Pharm. Pharmacol. 2021, 73, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Ji, H.; Lu, M.; Li, Z.; Qiao, X.; Sun, B.; Zhang, W.; Xue, D. Proteomic analysis of apoptotic and oncotic pancreatic acinar AR42J cells treated with caerulein. Mol. Cell Biochem. 2013, 382, 1–17. [Google Scholar] [CrossRef]
- Stankiewicz, E.; Mao, X.; Mangham, D.C.; Xu, L.; Yeste-Velasco, M.; Fisher, G.; North, B.; Chaplin, T.; Young, B.; Wang, Y.; et al. Identification of FBXL4 as a Metastasis Associated Gene in Prostate Cancer. Sci. Rep. 2017, 7, 5124. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Konishi, H.; Arima, C.; Tomida, S.; Takeuchi, T.; Shimada, Y.; Yatabe, Y.; Mitsudomi, T.; Osada, H.; Takahashi, T. Novel metastasis-related gene CIM functions in the regulation of multiple cellular stress-response pathways. Cancer Res. 2010, 70, 9949–9958. [Google Scholar] [CrossRef]
- Ryu, E.J.; Angelastro, J.M.; Greene, L.A. Analysis of gene expression changes in a cellular model of Parkinson disease. Neurobiol. Dis. 2005, 18, 54–74. [Google Scholar] [CrossRef]
- Sil, S.; Periyasamy, P.; Thangaraj, A.; Chivero, E.T.; Buch, S. PDGF/PDGFR axis in the neural systems. Mol. Aspects Med. 2018, 62, 63–74. [Google Scholar] [CrossRef]
- Järvelä, I. Genomics studies on musical aptitude, music perception, and practice. Ann. N. Y. Acad. Sci. 2018, 1423, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Tabaczar, S.; Czepas, J.; Koceva-Chyla, A.; Kilanczyk, E.; Piasecka-Zelga, J.; Gwozdzinski, K. The effect of the nitroxide pirolin on oxidative stress induced by doxorubicin and taxanes in the rat brain. J. Physiol. Pharmacol. 2017, 68, 295–308. [Google Scholar] [PubMed]
- Yuan, L.; Liu, C.; Wan, Y.; Yan, H.; Li, T. Effect of HDAC2/Inpp5f on neuropathic pain and cognitive function through regulating PI3K/Akt/GSK-3β signal pathway in rats with neuropathic pain. Exp. Ther. Med. 2019, 18, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.J.; Herskowitz, J.H. Perspectives on ROCK2 as a Therapeutic Target for Alzheimer’s Disease. Front. Cell Neurosci. 2021, 15, 636017. [Google Scholar] [CrossRef] [PubMed]
- Pranatharthi, A.; Thomas, P.; Udayashankar, A.H.; Bhavani, C.; Suresh, S.B.; Krishna, S.; Thatte, J.; Srikantia, N.; Ross, C.R.; Srivastava, S. RhoC regulates radioresistance via crosstalk of ROCK2 with the DNA repair machinery in cervical cancer. J. Exp. Clin. Cancer Res. 2019, 38, 392. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Lobachevsky, P.N.; MacManus, M.P.; Kron, T.; Möller, A.; Lobb, R.J.; Ventura, J.; Best, N.; Smith, J.; Ball, D.; et al. Radiotherapy for Non-Small Cell Lung Cancer Induces DNA Damage Response in Both Irradiated and Out-of-field Normal Tissues. Clin. Cancer Res. 2016, 22, 4817–4826. [Google Scholar] [CrossRef]
- Markarian, M.; Krattli, R.P.; Baddour, J.D.; Alikhani, L.; Giedzinski, E.; Usmani, M.T.; Agrawal, A.; Baulch, J.E.; Tenner, A.J.; Acharya, M.M. Glia-Selective Deletion of Complement. Cancer Res. 2021, 81, 1732–1744. [Google Scholar] [CrossRef]
- Son, M. Understanding the contextual functions of C1q and LAIR-1 and their applications. Exp. Mol. Med. 2022, 54, 567–572. [Google Scholar] [CrossRef]
- Katzeff, J.S.; Kim, W.S. ATP-binding cassette transporters and neurodegenerative diseases. Essays Biochem. 2021, 65, 1013–1024. [Google Scholar]
- Zeng, Y.; Callaghan, D.; Xiong, H.; Yang, Z.; Huang, P.; Zhang, W. Abcg2 deficiency augments oxidative stress and cognitive deficits in Tg-SwDI transgenic mice. J. Neurochem. 2012, 122, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Callaghan, D.; Jones, A.; Bai, J.; Rasquinha, I.; Smith, C.; Pei, K.; Walker, D.; Lue, L.F.; Stanimirovic, D.; et al. ABCG2 is upregulated in Alzheimer’s brain with cerebral amyloid angiopathy and may act as a gatekeeper at the blood-brain barrier for Abeta(1-40) peptides. J. Neurosci. 2009, 29, 5463–5475. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chung, Y.G.; Kim, C.Y.; Kim, H.K.; Lee, H.K. Upregulation of VEGF and FGF2 in normal rat brain after experimental intraoperative radiation therapy. J. Korean Med. Sci. 2004, 19, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Houchen, C.W.; George, R.J.; Sturmoski, M.A.; Cohn, S.M. FGF-2 enhances intestinal stem cell survival and its expression is induced after radiation injury. Am. J. Physiol. 1999, 276, G249–G258. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.; Ronai, Z.A. ATF2—At the crossroad of nuclear and cytosolic functions. J. Cell Sci. 2012, 125 Pt 12, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Miriyala, S.; Miao, L.; Mitov, M.; Schnell, D.; Dhar, S.; Cai, J.; Klein, J.; Sultana, R.; Butterfield, D.; et al. Redox proteomic identification of HNE-bound mitochondrial proteins in cardiac tissues reveals a systemic effect on energy metabolism after doxorubicin treatment. Free Radic. Biol. Med. 2014, 72, 55–65. [Google Scholar] [CrossRef]
- Vijay, V.; Moland, C.L.; Han, T.; Fuscoe, J.C.; Lee, T.; Herman, E.H.; Jenkins, G.R.; Lewis, S.M.; Cummings, C.A.; Gao, Y.; et al. Early transcriptional changes in cardiac mitochondria during chronic doxorubicin exposure and mitigation by dexrazoxane in mice. Toxicol. Appl. Pharmacol. 2016, 295, 68–84. [Google Scholar] [CrossRef]
- You, X.; Guo, W.; Wang, L.; Hou, Y.; Zhang, H.; Pan, Y.; Han, R.; Huang, M.; Liao, L.; Chen, Y. Subcellular distribution of RAD23B controls XPC degradation and DNA damage repair in response to chemotherapy drugs. Cell Signal 2017, 36, 108–116. [Google Scholar] [CrossRef]
- Ma, L.Y.; Liu, J.M.; Du, G.L.; Dang, X.B. Irisin attenuates lipopolysaccharide-induced acute lung injury by downregulating inflammatory cytokine expression through miR-199a-mediated Rad23b overexpression. Exp. Cell Res. 2021, 404, 112593. [Google Scholar] [CrossRef]
- Du, J.; Zhang, A.; Li, J.; Liu, X.; Wu, S.; Wang, B.; Wang, Y.; Jia, H. Doxorubicin-Induced Cognitive Impairment: The Mechanistic Insights. Front. Oncol. 2021, 11, 673340. [Google Scholar] [CrossRef]
- He, L.; Liu, F.; Li, J. Mitochondrial Sirtuins and Doxorubicin-induced Cardiotoxicity. Cardiovasc. Toxicol. 2021, 21, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Ma, L.; Li, Y.; Yang, J.; Yang, Q.; Yao, W.; Li, S. CoQ10 Improves Myocardial Damage in Doxorubicin-Induced Heart Failure in C57BL/6 Mice. Front. Biosci. (Landmark Ed.) 2022, 27, 244. [Google Scholar] [CrossRef]
- Eide, S.; Feng, Z.P. Doxorubicin chemotherapy-induced “chemo-brain”: Meta-analysis. Eur. J. Pharmacol. 2020, 881, 173078. [Google Scholar] [CrossRef] [PubMed]
- Bergbower, E.A.S.; Pierson, R.N.; Azimzadeh, A.M. Multi-gene technical assessment of qPCR and NanoString n-Counter analysis platforms in cynomolgus monkey cardiac allograft recipients. Cell Immunol. 2020, 347, 104019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Good, D.J. Comparison of hypothalamic mRNA levels in mice euthanized by CO₂ inhalation and focused-beam microwave irradiation. Lab Anim. 2011, 40, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Staib-Lasarzik, I.; Kriege, O.; Timaru-Kast, R.; Pieter, D.; Werner, C.; Engelhard, K.; Thal, S.C. Anesthesia for euthanasia influences mRNA expression in healthy mice and after traumatic brain injury. J. Neurotrauma 2014, 31, 1664–1671. [Google Scholar] [CrossRef]
- Scott, H.; Rogers, M.F.; Scott, H.L.; Campbell, C.; Warburton, E.C.; Uney, J.B. Recognition memory-induced gene expression in the perirhinal cortex: A transcriptomic analysis. Behav. Brain Res. 2017, 328, 1–12. [Google Scholar] [CrossRef]
- Mendez, M.; Arias, N.; Uceda, S.; Arias, J.L. c-Fos expression correlates with performance on novel object and novel place recognition tests. Brain Res. Bull. 2015, 117, 16–23. [Google Scholar] [CrossRef]
- Tangpong, J.; Cole, M.P.; Sultana, R.; Estus, S.; Vore, M.; Clair, W.S.; Ratanachaiyavong, S.; Clair, D.K.S.; Butterfield, D.A. Adriamycin-mediated nitration of manganese superoxide dismutase in the central nervous system: Insight into the mechanism of chemobrain. J. Neurochem. 2007, 100, 191–201. [Google Scholar] [CrossRef]


| Genes | Groups | Control | DOX | RT | DOX-RT |
|---|---|---|---|---|---|
| Atp2b2 | Mean | 8910 | 8749 | 9198 | 8298 |
| SEM | 149.0 | 233.8 | 64.26 | 167.6 | |
| DOX vs. RT | RT vs. DOX-RT | DOX-RT vs. DOX | |||
| Tukey p-value | 0.2512 | 0.0050 | 0.2462 | ||
| F-values | 5.190 | ||||
| p-value | 0.0082 | ||||
| DF | 23 | ||||
| Number of samples | 6 | 6 | 6 | 6 | |
| Cd74 | Mean | 123.3 | 126.8 | 104.1 | 166.5 |
| SEM | 18.75 | 11.14 | 10.00 | 11.01 | |
| DOX vs. RT | RT vs. DOX-RT | DOX-RT vs. DOX | |||
| Tukey p-value | 0.6240 | 0.0157 | 0.1783 | ||
| F-values | 3.943 | ||||
| p-value | 0.0232 | ||||
| DF | 23 | ||||
| Number of samples | 6 | 6 | 6 | 6 | |
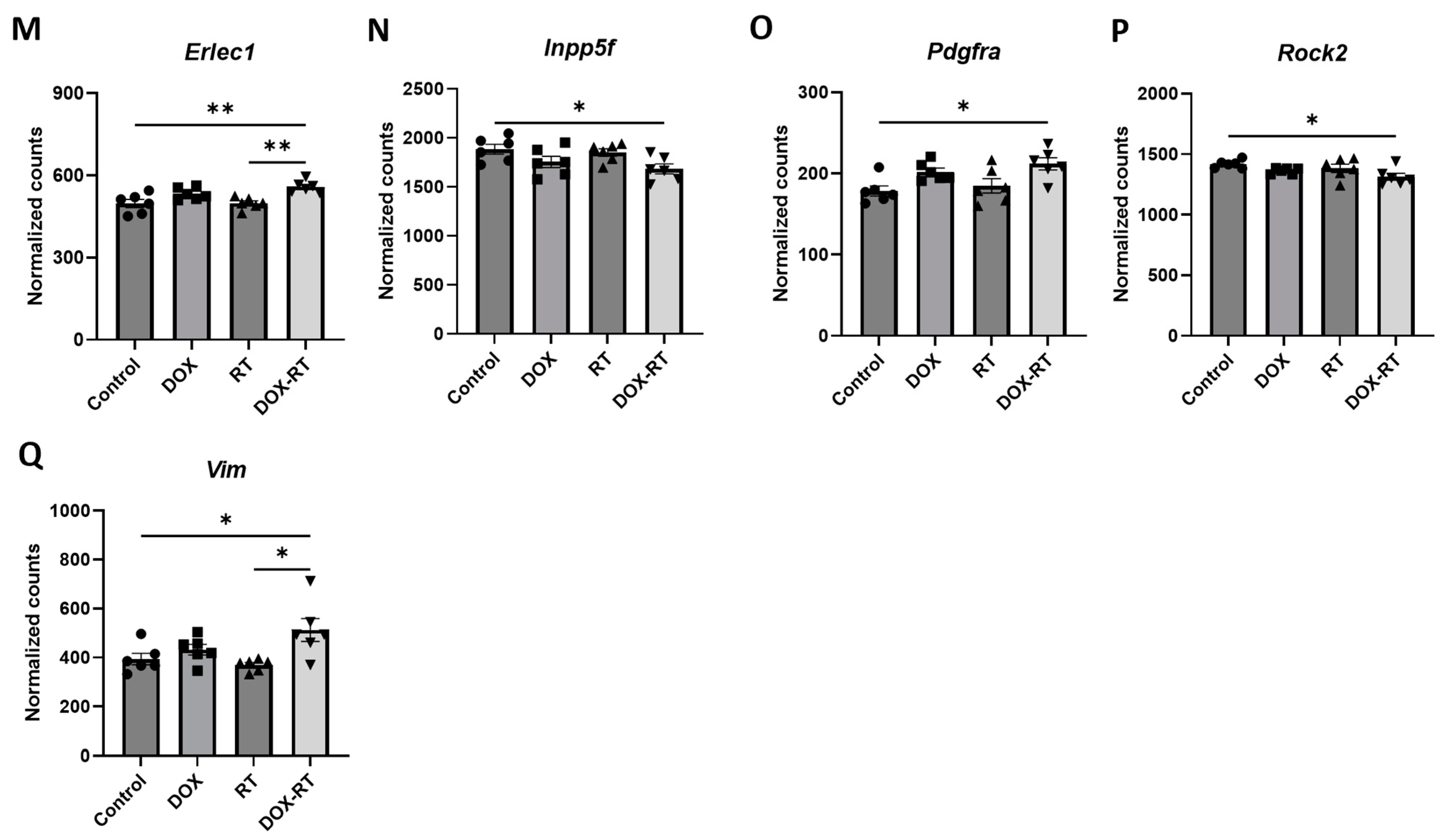
| Erlec1 | Mean | 497.7 | 533.0 | 498.3 | 558.3 |
| SEM | 14.78 | 8.970 | 8.815 | 8.698 | |
| DOX vs. RT | RT vs. DOX-RT | DOX-RT vs. DOX | |||
| Tukey p-value | 0.1298 | 0.0037 | 0.3597 | ||
| F-values | 7.634 | ||||
| p-value | 0.0014 | ||||
| DF | 23 | ||||
| Number of samples | 6 | 6 | 6 | 6 | |
| Gnb1 | Mean | 3234 | 3735 | 3529 | 3986 |
| SEM | 100.8 | 78.81 | 116.6 | 101.0 | |
| DOX vs. RT | RT vs. DOX-RT | DOX-RT vs. DOX | |||
| Tukey p-value | 0.4826 | 0.0202 | 0.3137 | ||
| F-values | 10.10 | ||||
| p-value | 0.0003 | ||||
| DF | 23 | ||||
| Number of samples | 6 | 6 | 6 | 6 | |
| Lyz2 | Mean | 71.09 | 64.61 | 58.89 | 87.30 |
| SEM | 3.388 | 4.631 | 5.420 | 10.26 | |
| DOX vs. RT | RT vs. DOX-RT | DOX-RT vs. DOX | |||
| Tukey p-value | 0.9228 | 0.0264 | 0.0942 | ||
| F-values | 3.597 | ||||
| p-value | 0.0316 | ||||
| DF | 23 | ||||
| Number of samples | 6 | 6 | 6 | 6 | |
| Olfml3 | Mean | 81.91 | 77.89 | 65.44 | 84.94 |
| SEM | 3.477 | 5.209 | 3.828 | 5.855 | |
| DOX vs. RT | RT vs. DOX-RT | DOX-RT vs. DOX | |||
| Tukey p-value | 0.2700 | 0.0375 | 0.7152 | ||
| F-values | 3.334 | ||||
| p-value | 0.0401 | ||||
| DF | 23 | ||||
| Number of samples | 6 | 6 | 6 | 6 | |
| Srpr | Mean | 568.2 | 624.2 | 598.0 | 644.8 |
| SEM | 13.81 | 8.784 | 11.27 | 12.06 | |
| DOX vs. RT | RT vs. DOX-RT | DOX-RT vs. DOX | |||
| Tukey p-value | 0.4047 | 0.0450 | 0.5991 | ||
| F-values | 8.153 | ||||
| p-value | 0.0010 | ||||
| DF | 23 | ||||
| Number of samples | 6 | 6 | 6 | 6 | |
| Vim | Mean | 393.7 | 432.1 | 369.7 | 512.2 |
| SEM | 23.30 | 21.63 | 9.866 | 46.34 | |
| DOX vs. RT | RT vs. DOX-RT | DOX-RT vs. DOX | |||
| Tukey p-value | 0.4290 | 0.0104 | 0.2272 | ||
| F-values | 4.781 | ||||
| p-value | 0.0114 | ||||
| DF | 23 | ||||
| Number of samples | 6 | 6 | 6 | 6 |
| Pathway Source | Pathway Name | p-Value | Adjusted p-Value | Odds Ratio | Combined Score | Genes Included in Pathway |
|---|---|---|---|---|---|---|
| WikiPathway 2023 Human [31,32,33] | Allograft rejection WP2328 | 8.724 × 10−7 | 0.0001003 | 71.19 | 993.21 | C1QB, C1QA, PDGFRA, VIM |
| KEGG 2021 Human [31,32,33] | Human cytomegalovirus infection | 9.552 × 10−7 | 0.00009552 | 37.43 | 518.83 | ATF2, PDGFRA, ROCK2, GNB1, E2F3 |
| BioPlanet 2019 [31,32,33] | Melanoma | 0.00002813 | 0.006385 | 62.76 | 657.62 | PDGFRA, E2F3, FGF2 |
| KEGG 2021 Human [31,32,33] | Melanoma | 0.00002934 | 0.001467 | 61.84 | 645.45 | PDGFRA, E2F3, FGF2 |
| Elsevier Pathway Collection [31,32,33] | Epithelial to mesenchymal transition in cancer: overview | 0.00005724 | 0.006158 | 49.00 | 478.69 | PDGFRA, VIM, FGF2 |
| KEGG 2021 Human [31,32,33] | Pathways in cancer | 0.00006148 | 0.002049 | 15.41 | 149.45 | PDGFRA, ROCK2, GNB1, E2F3, FGF2 |
| BioPlanet 2019 [31,32,33] | Complement activation, classical pathway | 0.00009179 | 0.01042 | 177.49 | 1649.98 | C1QB, C1QA |
| Elsevier Pathway Collection [31,32,33] | Proteins with altered expression in cancer metastases | 0.00009322 | 0.006158 | 41.36 | 383.84 | PDGFRA, VIM, FGF2 |
| Elsevier Pathway Collection [31,32,33] | CR3-mediated phagocytosis in neutrophils and macrophages | 0.0001032 | 0.006158 | 166.39 | 1527.26 | ROCK2, VIM |
| WikiPathway 2023 Human [31,32,33] | Focal adhesion-PI3K-Akt-mTOR-signaling pathway WP3932 | 0.0001039 | 0.003050 | 20.33 | 186.43 | ATF2, PDGFRA, GNB1, FGF2 |
| WikiPathway 2023 Human [31,32,33] | Imatinib and chronic myeloid leukemia WP3640 | 0.0001280 | 0.003050 | 147.89 | 1325.55 | PDGFRA, ABCG2 |
| WikiPathway 2023 Human [31,32,33] | Spinal cord injury WP2431 | 0.0001314 | 0.003050 | 36.70 | 328.00 | C1QB, ROCK2, VIM |
| WikiPathway 2023 Human [31,32,33] | PI3K-Akt signaling pathway WP4172 | 0.0001602 | 0.003050 | 18.10 | 158.18 | ATF2, PDGFRA, GNB1, FGF2 |
| BioPlanet 2019 [31,32,33] | Angiogenesis | 0.0001702 | 0.01288 | 126.74 | 1099.91 | PDGFRA, FGF2 |
| WikiPathway 2023 Human [31,32,33] | Complement activation WP545 | 0.0001702 | 0.003050 | 126.74 | 1099.91 | C1QB, C1QA |
| WikiPathway 2023 Human [31,32,33] | Angiogenesis WP1539 | 0.0001856 | 0.003050 | 120.98 | 1039.39 | PDGFRA, FGF2 |
| KEGG 2021 Human [31,32,33] | PI3K-Akt signaling pathway | 0.0001914 | 0.004784 | 17.26 | 147.77 | ATF2, PDGFRA, GNB1, FGF2 |
| Elsevier Pathway Collection [31,32,33] | Ca2+ toxicity in lens cells | 0.0002357 | 0.01055 | 106.44 | 889.10 | PDGFRA, VIM |
| WikiPathway 2023 Human [31,32,33] | Regulation of actin cytoskeleton WP51 | 0.0002603 | 0.003742 | 28.92 | 238.66 | PDGFRA, ROCK2, FGF2 |
| BioPlanet 2019 [31,32,33] | Prion diseases | 0.0003980 | 0.01912 | 80.61 | 631.07 | C1QB, C1QA |
| Elsevier Pathway Collection [31,32,33] | Proteins with altered expression in cancer-associated sustaining of proliferative signaling | 0.0004091 | 0.01454 | 24.68 | 192.55 | PDGFRA, E2F3, FGF2 |
| BioPlanet 2019 [31,32,33] | Phospholipids as signaling intermediaries | 0.0004212 | 0.01912 | 78.23 | 608.05 | PDGFRA, GNB1 |
| WikiPathway 2023 Human [31,32,33] | Microglia pathogen phagocytosis pathway WP3937 | 0.0005204 | 0.005985 | 69.98 | 529.13 | C1QB, C1QA |
| WikiPathway 2023 Human [31,32,33] | Oxidative damage WP3941 | 0.0005204 | 0.005985 | 69.98 | 529.13 | C1QB, C1QA |
| KEGG 2021 Human [31,32,33] | Kaposi sarcoma-associated herpesvirus infection | 0.0005445 | 0.01089 | 22.32 | 167.77 | GNB1, E2F3, FGF2 |
| BioPlanet 2019 [31,32,33] | Plasma membrane estrogen receptor signaling | 0.0005468 | 0.02069 | 68.18 | 512.16 | ROCK2, GNB1 |
| Elsevier Pathway Collection [31,32,33] | Proteins involved in erectile dysfunction | 0.0006299 | 0.01454 | 63.30 | 466.55 | ROCK2, FGF2 |
| BioPlanet 2019 [31,32,33] | RhoA signaling pathway | 0.0006589 | 0.02137 | 61.83 | 452.90 | ATF2, ROCK2 |
| Elsevier Pathway Collection [31,32,33] | Glioblastoma, primary | 0.0007496 | 0.01454 | 57.79 | 415.84 | PDGFRA, FGF2 |
| KEGG 2021 Human [31,32,33] | Regulation of actin cytoskeleton | 0.0007759 | 0.01161 | 19.70 | 141.10 | PDGFRA, ROCK2, FGF2 |
| Elsevier Pathway Collection [31,32,33] | CDH2 activation promotes cancer cell migration and survival | 0.0007811 | 0.01454 | 56.56 | 404.65 | PDGFRA, FGF2 |
| Elsevier Pathway Collection [31,32,33] | Glioblastoma, secondary | 0.0008133 | 0.01454 | 55.38 | 393.96 | PDGFRA, FGF2 |
| BioPlanet 2019 [31,32,33] | Actin cytoskeleton regulation | 0.0008612 | 0.02444 | 18.99 | 134.00 | PDGFRA, ROCK2, FGF2 |
| KEGG 2021 Human [31,32,33] | Coronavirus disease | 0.0009291 | 0.01161 | 18.48 | 129.05 | C1QB, C1QA, ADAR |
| KEGG 2021 Human [31,32,33] | Ras signaling pathway | 0.0009291 | 0.01161 | 18.48 | 129.05 | PDGFRA, GNB1, FGF2 |
| Elsevier Pathway Collection [31,32,33] | G0/G1 cell cycle phase transition activation in cancer | 0.0009481 | 0.01454 | 51.11 | 355.74 | PDGFRA, FGF2 |
| BioPlanet 2019 [31,32,33] | ATF2 transcription factor network | 0.001131 | 0.02851 | 46.61 | 316.24 | ATF2, PDGFRA |
| BioPlanet 2019 [31,32,33] | Glioma | 0.001371 | 0.02851 | 42.16 | 277.93 | PDGFRA, E2F3 |
| KEGG 2021 Human [31,32,33] | Prion disease | 0.001484 | 0.01556 | 15.65 | 101.89 | C1QB, ATF2, C1QA |
| KEGG 2021 Human [31,32,33] | Glioma | 0.001819 | 0.01556 | 36.37 | 229.44 | PDGFRA, E2F3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demos-Davies, K.; Lawrence, J.; Ferreira, C.; Seelig, D. The Distant Molecular Effects on the Brain by Cancer Treatment. Brain Sci. 2024, 14, 22. https://doi.org/10.3390/brainsci14010022
Demos-Davies K, Lawrence J, Ferreira C, Seelig D. The Distant Molecular Effects on the Brain by Cancer Treatment. Brain Sciences. 2024; 14(1):22. https://doi.org/10.3390/brainsci14010022
Chicago/Turabian StyleDemos-Davies, Kimberly, Jessica Lawrence, Clara Ferreira, and Davis Seelig. 2024. "The Distant Molecular Effects on the Brain by Cancer Treatment" Brain Sciences 14, no. 1: 22. https://doi.org/10.3390/brainsci14010022
APA StyleDemos-Davies, K., Lawrence, J., Ferreira, C., & Seelig, D. (2024). The Distant Molecular Effects on the Brain by Cancer Treatment. Brain Sciences, 14(1), 22. https://doi.org/10.3390/brainsci14010022






