Associations of Lipoprotein(a) Level with Cerebral Small Vessel Disease in Patients with Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Measurement
2.3. MRI Acquisition and Assessment
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Association of Lp(a) Levels with the Presence and Burden of CSVD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hiraga, T.; Shimada, M.; Okubo, M.; Nakanishi, K.; Kobayashi, T.; Murase, T. Lipoprotein(a) is an independent risk factor for multiple cerebral infarctions. Atherosclerosis 1996, 122, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Marcovina, S.M.; Koschinsky, M.L. Koschinsky, Lipoprotein(a) as a risk factor for coronary artery disease. Am. J. Cardiol. 1998, 82, 57U–66U, discussion 86U. [Google Scholar] [CrossRef] [PubMed]
- Sutton-Tyrrell, K.; Evans, R.W.; Meilahn, E.; Alcorn, H.G. Lipoprotein(a) and peripheral atherosclerosis in older adults. Atherosclerosis 1996, 122, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zenker, G.; Költringer, P.; Boné, G.; Niederkorn, K.; Pfeiffer, K.; Jürgens, G. Lipoprotein(a) as a strong indicator for cerebrovascular disease. Stroke 1986, 17, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Cai, X.; Jing, J.; Wang, S.; Meng, X.; Mei, L.; Yang, Y.; Jin, A.; DongXiao, Y.; Li, S.; et al. Differential associations of lipoprotein(a) level with cerebral large artery and small vessel diseases. Stroke Vasc. Neurol. 2022, 7, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, H.; Wang, Y.; Meng, X.; Wang, Y. Causal Effect of Lp(a) [Lipoprotein(a)] Level on Ischemic Stroke and Alzheimer Disease: A Mendelian Randomization Study. Stroke 2019, 50, 3532–3539. [Google Scholar] [CrossRef] [PubMed]
- Barage, S.H.; Sonawane, K.D. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides 2015, 52, 1–18. [Google Scholar] [CrossRef]
- Toledo, J.B.; Arnold, S.E.; Raible, K.; Brettschneider, J.; Xie, S.X.; Grossman, M.; Monsell, S.E.; Kukull, W.A.; Trojanowski, J.Q. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 2013, 136 Pt 9, 2697–2706. [Google Scholar] [CrossRef]
- Carare, R.O.; Hawkes, C.A.; Jeffrey, M.; Kalaria, R.N.; Weller, R.O. Review: Cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy. Neuropathol. Appl. Neurobiol. 2013, 39, 593–611. [Google Scholar] [CrossRef]
- Thomason, L.A.; Stefanovic, B.; McLaurin, J. Cerebrovascular contributions to Alzheimer’s disease pathophysiology and potential therapeutic interventions in mouse models. Eur. J. Neurosci. 2013, 37, 1994–2004. [Google Scholar] [CrossRef]
- Iwamoto, T.; Watanabe, D.; Umahara, T.; Sakurai, H.; Hanyu, H.; Kanaya, K. Dual inverse effects of lipoprotein(a) on the dementia process in Japanese late-onset Alzheimer’s disease. Psychogeriatrics 2004, 4, 64–71. [Google Scholar] [CrossRef]
- Urakami, K.; Wada-Isoe, K.; Wakutani, Y.; Ikeda, K.; Ji, Y.; Yamagata, K.; Kowa, H.; Okada, A.; Adachi, Y.; Nakashima, K. Lipoprotein(a) phenotypes in patients with vascular dementia. Dement. Geriatr. Cogn. Disord. 2000, 11, 135–138. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef] [PubMed]
- Maclullich, A.M.; Wardlaw, J.M.; Ferguson, K.J.; Starr, J.M.; Seckl, J.R.; Deary, I.J. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Vernooij, M.W.; Cordonnier, C.; Viswanathan, A.; Al-Shahi Salman, R.; Warach, S.; Launer, L.J.; Van Buchem, M.A.; Breteler, M.M. Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 2009, 8, 165–174. [Google Scholar] [CrossRef]
- Staals, J.; Makin, S.D.; Doubal, F.N.; Dennis, M.S.; Wardlaw, J.M. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014, 83, 1228–1234. [Google Scholar] [CrossRef]
- Steffen, B.T.; Thanassoulis, G.; Duprez, D.; Stein, J.H.; Karger, A.B.; Tattersall, M.C.; Kaufman, J.D.; Guan, W.; Tsai, M.Y. Race-Based Differences in Lipoprotein(a)-Associated Risk of Carotid Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 523–529. [Google Scholar] [CrossRef]
- van Dam-Nolen, D.H.K.; van Dijk, A.C.; Crombag, G.; Lucci, C.; Kooi, M.E.; Hendrikse, J.; Nederkoorn, P.J.; Daemen, M.; van der Steen, A.F.W.; Koudstaal, P.J.; et al. Lipoprotein(a) levels and atherosclerotic plaque characteristics in the carotid artery: The Plaque at RISK (PARISK) study. Atherosclerosis 2021, 329, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, P.; Imperiale, D.; Fornengo, P.; Bruno, G.; Cassader, M.; Maffeis, P.; Cavallo Perin, P.; Pagano, G.; Bergamasco, B. Higher lipoprotein (a) levels in atherothrombotic than lacunar ischemic cerebrovascular disease. Neurology 2002, 58, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Iskra, T.; Turaj, W.; Słowik, A.; Szczudlik, A.; Dembińska-Kieć, A. Lipoprotein (a) in stroke patients with large and small vessel disease. Przegl Lek. 2002, 59, 877–880. [Google Scholar] [PubMed]
- Yuan, B.B.; Luo, G.G.; Gao, J.X.; Qiao, J.; Yang, J.B.; Huo, K.; Li, Y.B.; Liu, Y. Variance of Serum Lipid Levels in Stroke Subtypes. Clin. Lab. 2015, 61, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Enkhmaa, B.; Anuurad, E.; Berglund, L. Lipoprotein (a): Impact by ethnicity and environmental and medical conditions. J. Lipid Res. 2016, 57, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Brenowitz, W.D.; Nelson, P.T.; Besser, L.M.; Heller, K.B.; Kukull, W.A. Cerebral amyloid angiopathy and its co-occurrence with Alzheimer’s disease and other cerebrovascular neuropathologic changes. Neurobiol. Aging 2015, 36, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Charidimou, A.; Meegahage, R.; Fox, Z.; Peeters, A.; Vandermeeren, Y.; Laloux, P.; Baron, J.C.; Jäger, H.R.; Werring, D.J. Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: A multicentre MRI cohort study. J. Neurol. Neurosurg. Psychiatry 2013, 84, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Riba-Llena, I.; Jiménez-Balado, J.; Castañé, X.; Girona, A.; López-Rueda, A.; Mundet, X.; Jarca, C.I.; Álvarez-Sabin, J.; Montaner, J.; Delgado, P. Arterial Stiffness Is Associated with Basal Ganglia Enlarged Perivascular Spaces and Cerebral Small Vessel Disease Load. Stroke 2018, 49, 1279–1281. [Google Scholar] [CrossRef]
- Gregoire, S.M.; Chaudhary, U.J.; Brown, M.M.; Yousry, T.A.; Kallis, C.; Jäger, H.R.; Werring, D.J. The Microbleed Anatomical Rating Scale (MARS): Reliability of a tool to map brain microbleeds. Neurology 2009, 73, 1759–1766. [Google Scholar] [CrossRef]
- Rabelo, A.G.; Teixeira, C.V.; Magalhães, T.N.; Carletti-Cassani, A.F.M.; Amato Filho, A.C.; Joaquim, H.P.; Talib, L.L.; Forlenza, O.; Ribeiro, P.A.; Secolin, R.; et al. Is cerebral microbleed prevalence relevant as a biomarker in amnestic mild cognitive impairment and mild Alzheimer’s disease? Neuroradiol. J. 2017, 30, 477–485. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Kantarci, K.; Weigand, S.D.; Lundt, E.S.; Gunter, J.L.; Duffy, J.R.; Strand, E.A.; Machulda, M.M.; Spychalla, A.J.; Drubach, D.A.; et al. Microbleeds in atypical presentations of Alzheimer’s disease: A comparison to dementia of the Alzheimer’s type. J. Alzheimers Dis. 2015, 45, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Panza, F.; D’Introno, A.; Colacicco, A.M.; Capurso, C.; Basile, A.M.; Capurso, A. Lipoprotein(a), apolipoprotein E genotype, and risk of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2002, 72, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Chimienti, G.; Liuzzi, G.M.; Lamanuzzi, B.L.; Nardulli, M.; Lolli, F.; Anglés-Cano, E.; Matà, S. Lipoprotein(a) in the cerebrospinal fluid of neurological patients with blood-cerebrospinal fluid barrier dysfunction. Clin. Chem. 2006, 52, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Bergmark, C.; Dewan, A.; Orsoni, A.; Merki, E.; Miller, E.R.; Shin, M.J.; Binder, C.J.; Hörkkö, S.; Krauss, R.M.; Chapman, M.J.; et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J. Lipid Res. 2008, 49, 2230–2239. [Google Scholar] [CrossRef] [PubMed]
- Saczynski, J.S.; White, L.; Peila, R.L.; Rodriguez, B.L.; Launer, L.J. The relation between apolipoprotein A-I and dementia: The Honolulu-Asia aging study. Am. J. Epidemiol. 2007, 165, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Khan, H.; Nyyssönen, K.; Laukkanen, J.A. Is lipoprotein (a) protective of dementia? Eur. J. Epidemiol. 2016, 31, 1149–1152. [Google Scholar] [CrossRef] [PubMed]
- Mooser, V.; Helbecque, N.; Miklossy, J.; Marcovina, S.M.; Nicod, P.; Amouyel, P. Interactions between apolipoprotein E and apolipoprotein(a) in patients with late-onset Alzheimer disease. Ann. Intern. Med. 2000, 132, 533–537. [Google Scholar] [CrossRef]
- Bard, J.M.; Delattre-Lestavel, S.; Clavey, V.; Pont, P.; Derudas, B.; Parra, H.J.; Fruchart, J.C. Isolation and characterization of two sub-species of Lp(a), one containing apo E and one free of apo E. Biochim. Biophys. Acta 1992, 1127, 124–130. [Google Scholar] [CrossRef]
- van Barlingen, H.H.; Kleinveld, H.A.; Erkelens, D.W.; de Bruin, T.W. Lipoprotein lipase-enhanced binding of lipoprotein(a) [Lp(a)] to heparan sulfate is improved by apolipoprotein E (apoE) saturation: Secretion-capture process of apoE is a possible route for the catabolism of Lp(a). Metabolism 1997, 46, 650–655. [Google Scholar] [CrossRef]
- Moriarty, P.M.; Varvel, S.A.; Gordts, P.L.; McConnell, J.P.; Tsimikas, S. Lipoprotein(a) Mass Levels Increase Significantly According to APOE Genotype: An Analysis of 431239 Patients. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 580–588. [Google Scholar] [CrossRef]
- Schilling, S.; DeStefano, A.L.; Sachdev, P.S.; Choi, S.H.; Mather, K.A.; DeCarli, C.D.; Wen, W.; Høgh, P.; Raz, N.; Au, R.; et al. APOE genotype and MRI markers of cerebrovascular disease: Systematic review and meta-analysis. Neurology 2013, 81, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Schilling, S.; Tzourio, C.; Dufouil, C.; Zhu, Y.; Berr, C.; Alpérovitch, A.; Crivello, F.; Mazoyer, B.; Debette, S. Plasma lipids and cerebral small vessel disease. Neurology 2014, 83, 1844–1852. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 111) | Tertiles of Lp(a) Level | p Value | ||
|---|---|---|---|---|---|
| T1 (n = 37) | T2 (n = 37) | T3 (n = 37) | |||
| Demographic data | |||||
| Age, means ± SD | 75.27 ± 9.70 | 75.46 ± 8.12 | 76.57 ± 9.83 | 73.78 ± 10.86 | 0.467 |
| Male, n (%) | 44 (39.6) | 16 (43.2) | 13 (35.1) | 15 (40.5) | 0.768 |
| Current smoking, n (%) | 49 (44.1) | 16 (43.2) | 17 (45.9) | 16 (43.2) | 0.964 |
| Alcohol drinkers, n (%) | 22 (19.8) | 9 (24.3) | 8 (21.6) | 5(13.5) | 0.479 |
| BMI, kg/m2, means ± SD | 23.5 ± 3.2 | 23.0 ± 2.9 | 23.9 ± 3.0 | 23.6 ± 3.4 | 0.175 |
| FPG, mmol/L, means ± SD | 5.55 ± 1.30 | 5.55 ± 1.17 | 5.71 ± 1/37 | 5.40 ± 1.35 | 0.606 |
| TC, mmol/L, means ± SD | 4.63 ± 3.95 | 4.11 ± 1.31 | 5.28 ± 6.58 | 4.50 ± 1.24 | 0.437 |
| TG, mmol/L, means ± SD | 1.41 ± 1.14 | 1.66 ± 1.72 | 1.30 ± 0.72 | 1.30 ± 0.60 | 0.289 |
| LDL-C, mmol/L, means ± SD | 2.48 ± 0.96 | 2.27 ± 0.96 | 2.41 ± 0.76 | 2.76 ± 1.08 | 0.076 |
| HDL-C, mmol/L, means ± SD | 1.16 ± 0.36 | 1.09 ± 0.32 | 1.23 ± 0.44 | 1.16 ± 0.32 | 0.282 |
| BUN, mmol/L, means ± SD | 6.46 ± 3.73 | 7.08 ± 4.75 | 5.87 ± 3.56 | 6.41 ± 2.46 | 0.141 |
| Scr, umol/L, means ± SD | 78.60 ± 44.42 | 81.65 ± 65.61 | 78.61 ± 28.51 | 75.53 ± 28.95 | 0.706 |
| HbA1c (%), means ± SD | 6.23 ± 1.06 | 6.28 ± 0.89 | 6.08 ± 1.18 | 5.95 ± 0.77 | 0.375 |
| Medical history, n (%) | |||||
| Coronary heart disease | 55 (49.5) | 19 (51.3) | 15 (40.5) | 21 (56.8) | 0.510 |
| Atrial fibrillation | 10 (9.0) | 3 (8.1) | 5 (13.5) | 2 (5.4) | 0.463 |
| Hypertension | 54 (48.6) | 17 (45.9) | 21 (56.8) | 16 (43.2) | 0.469 |
| Diabetes | 40 (36.0) | 12 (32.4) | 15 (40.5) | 13 (35.1) | 0.761 |
| Dyslipidemia | 22 (19.8) | 7 (18.9) | 7 (18.9) | 8 (21.6) | 0.945 |
| Imaging markers, n (%) | |||||
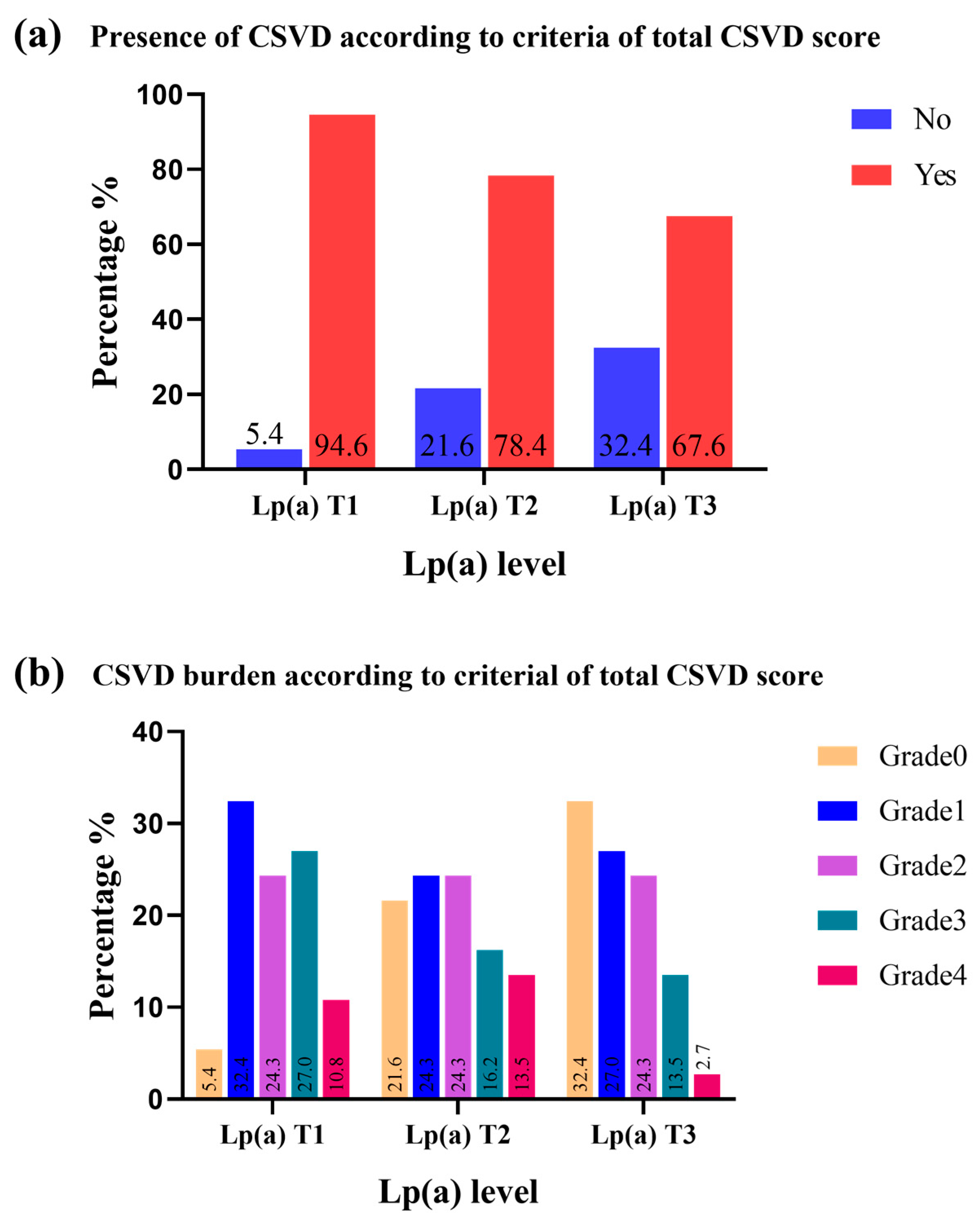
| Cerebral small vessel diseases | 89 (80.2) | 35 (94.6) | 29 (78.4) | 25 (67.6) | 0.013 |
| BG-EPVS > 10 | 77 (69.4) | 30 (81.1) | 25 (67.6) | 22 (59.5) | 0.125 |
| CSO-EPVS > 10 | 85 (76.6) | 36 (97.3) | 27 (73.0) | 22 (59.5) | 0.001 |
| Lacunes | 47 (42.3) | 20 (54.1) | 15 (40.5) | 12 (32.4) | 0.164 |
| Confluent WMH | 41 (36.9) | 15 (40.5) | 16 (43.2) | 10 (27.0) | 0.302 |
| CMBs | 21 (18.9) | 10 (27.0) | 8 (21.6) | 3 (8.1) | 0.111 |
| Outcome | Lp(a) Categories | CSVD (n%) | Unadjusted cOR (95% CI) | p Value | Model 1 * | Model 2 † | ||
|---|---|---|---|---|---|---|---|---|
| Adjusted cOR (95% CI) | p Value | Adjusted cOR (95% CI) | p Value | |||||
| Total CSVD Score ‡ | T1 | 35 (94.6) | Ref | Ref | Ref | |||
| T2 | 29 (78.4) | 0.207 (0.041–1.053) | 0.058 | 0.141 (0.023–0.841) | 0.032 | 0.132 (0.018–0.946) | 0.044 | |
| T3 | 25 (66.2) | 0.119 (0.024–0.579) | 0.008 | 0.116 (0.021–0.655) | 0.015 | 0.109 (0.016–0.737) | 0.023 | |
| Outcome | Lp(a) Category | Unadjusted cOR (95% CI) | p Value | Model 1 * | Model 2 † | ||
|---|---|---|---|---|---|---|---|
| Adjusted cOR (95% CI) | p Value | Adjusted cOR (95% CI) | p Value | ||||
| Total CSVD score ‡ | T1 | Ref | Ref | Ref | |||
| T2 | 0.743 (0.431–1.279) | 0.284 | 0.696 (0.427–1.134) | 0.145 | 0.722 (0.452–1.154) | 0.174 | |
| T3 | 0.457 (0.265–0.787) | 0.005 | 0.501 (0.308–0.815) | 0.005 | 0.576 (0.362–0. 915) | 0.019 |
| Outcome | Lp(a) Category | Unadjusted cOR (95%CI) | p Value | Model 1 * | Model 2 † | ||
|---|---|---|---|---|---|---|---|
| Adjusted cOR (95% CI) | p Value | Adjusted cOR (95% CI) | p Value | ||||
| BG-EPVS > 10 (moderate-to-severe) and | T1 | ref | ref | ref | |||
| T2 | 0.486 (0.166–1.421) | 0.188 | 0.377 (0.780–1.231) | 0.115 | 0.582 (0.154–2.200) | 0.425 | |
| T3 | 0.342 (0.119–0.980) | 0.046 | 0.366 (0.113–1.091) | 0.095 | 0.530 (0.143–1.964) | 0.342 | |
| CSO-EPVS >10 (moderate-to-severe) and | T1 | ref | ref | ref | |||
| T2 | 0.075 (0.009–0.622) | 0.016 | 0.070 (0.008–0.583) | 0.014 | 0.059 (0.006–0.542) | 0.012 | |
| T3 | 0.041 (0.005–0.330) | 0.003 | 0.041 (0.005–0.583) | 0.003 | 0.029 (0.003–0.273) | 0.002 | |
| Presence of Lacunes | T1 | ref | ref | ref | |||
| T2 | 0.580 (0.231–1.456) | 0.246 | 0.439 (0.159–1.217) | 0.114 | 0.636 (0.168–2.407) | 0.505 | |
| T3 | 0.408 (0.159–1.049) | 0.063 | 0.383 (0.135–1.090) | 0.072 | 0.536 (0.149–1.928) | 0.340 | |
| Periventricular WMH | T1 | ref | ref | ref | |||
| T2 | 1.135 (0.424–3.039) | 0.802 | 0.976 (0.344–2.767) | 0.964 | 0.869 (0.270–2.799) | 0.814 | |
| T3 | 0.457 (0.149–1.406) | 0.172 | 0.437 (0.133–1.431) | 0.171 | 0.496 (0.140–1.760) | 0.278 | |
| Deep WMH | T1 | ref | ref | ref | |||
| T2 | 0.704 (0.272–1.822) | 0.469 | 0.635 (0.240–1.684) | 0.362 | 0.594 (0.206–1.711) | 0.334 | |
| T3 | 0.471 (0.174–1.278) | 0.139 | 0.473 (0.171–1.312) | 0.150 | 0.505 (0.173–1.472) | 0.211 | |
| Confluent WMH # | T1 | ref | ref | ref | |||
| T2 | 1.117 (0.444–2.815) | 0.814 | 0.903 (0.386–2.608) | 0.995 | 0.940 (0.384–3.116) | 0.867 | |
| T3 | 0.543 (0.204–1.445) | 0.221 | 0.542 (0.197–1.493) | 0.236 | 0.673 (0.230–1.971) | 0.470 | |
| Presence of CMBs ‡ | T1 | ref | ref | ref | |||
| T2 | 0.745 (0.350–1.584) | 0.588 | 0.802 (0.268–2.395) | 0.692 | 0.652 (0.193–2.205) | 0.491 | |
| T3 | 0.238 (0.089–0.635) | 0.042 | 0.233 (0.057–0.935) | 0.043 | 0.144 (0.029–0.716) | 0.018 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, N.; Jiang, F.; Chen, X.; Zhu, L.; Qiao, N.; Zhou, J.; Zhang, Y. Associations of Lipoprotein(a) Level with Cerebral Small Vessel Disease in Patients with Alzheimer’s Disease. Brain Sci. 2024, 14, 34. https://doi.org/10.3390/brainsci14010034
Chen N, Jiang F, Chen X, Zhu L, Qiao N, Zhou J, Zhang Y. Associations of Lipoprotein(a) Level with Cerebral Small Vessel Disease in Patients with Alzheimer’s Disease. Brain Sciences. 2024; 14(1):34. https://doi.org/10.3390/brainsci14010034
Chicago/Turabian StyleChen, Nihong, Fuping Jiang, Xiangliang Chen, Lin Zhu, Na Qiao, Junshan Zhou, and Yingdong Zhang. 2024. "Associations of Lipoprotein(a) Level with Cerebral Small Vessel Disease in Patients with Alzheimer’s Disease" Brain Sciences 14, no. 1: 34. https://doi.org/10.3390/brainsci14010034
APA StyleChen, N., Jiang, F., Chen, X., Zhu, L., Qiao, N., Zhou, J., & Zhang, Y. (2024). Associations of Lipoprotein(a) Level with Cerebral Small Vessel Disease in Patients with Alzheimer’s Disease. Brain Sciences, 14(1), 34. https://doi.org/10.3390/brainsci14010034







