Brain Health and Cognition in Older Adults: Roadmap and Milestones towards the Implementation of Preventive Strategies
Abstract
:1. Introduction
2. Brain Health as a New Target
3. Preventive Strategies: Who and How
3.1. Who: Subjective Cognitive Decline
3.2. How: Multidomain Personalized Preventive Strategies
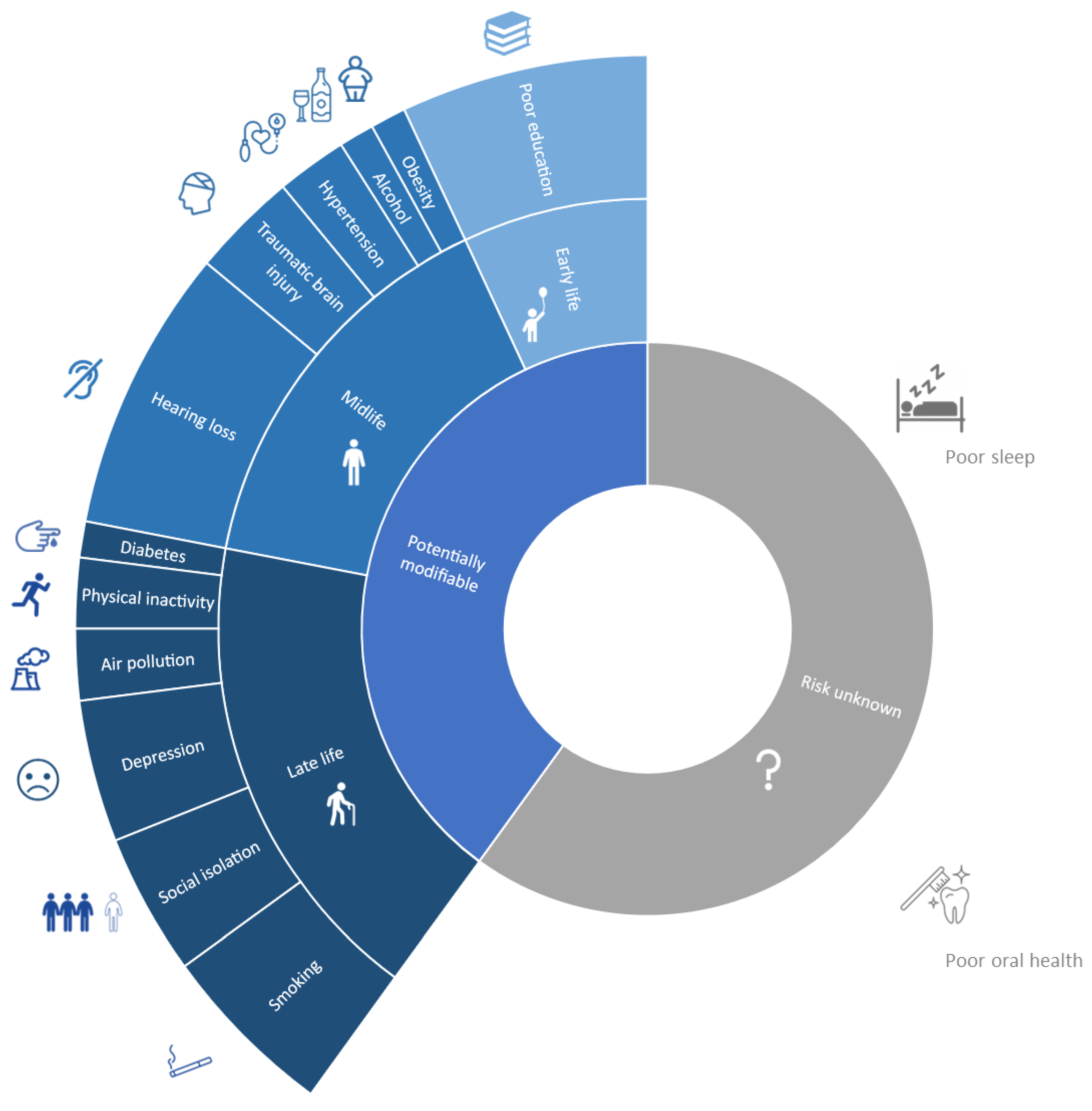
3.3. Beyond High-Risk: Universal Preventive Approach in Mid- and Latelife Cognitively Unimpaired
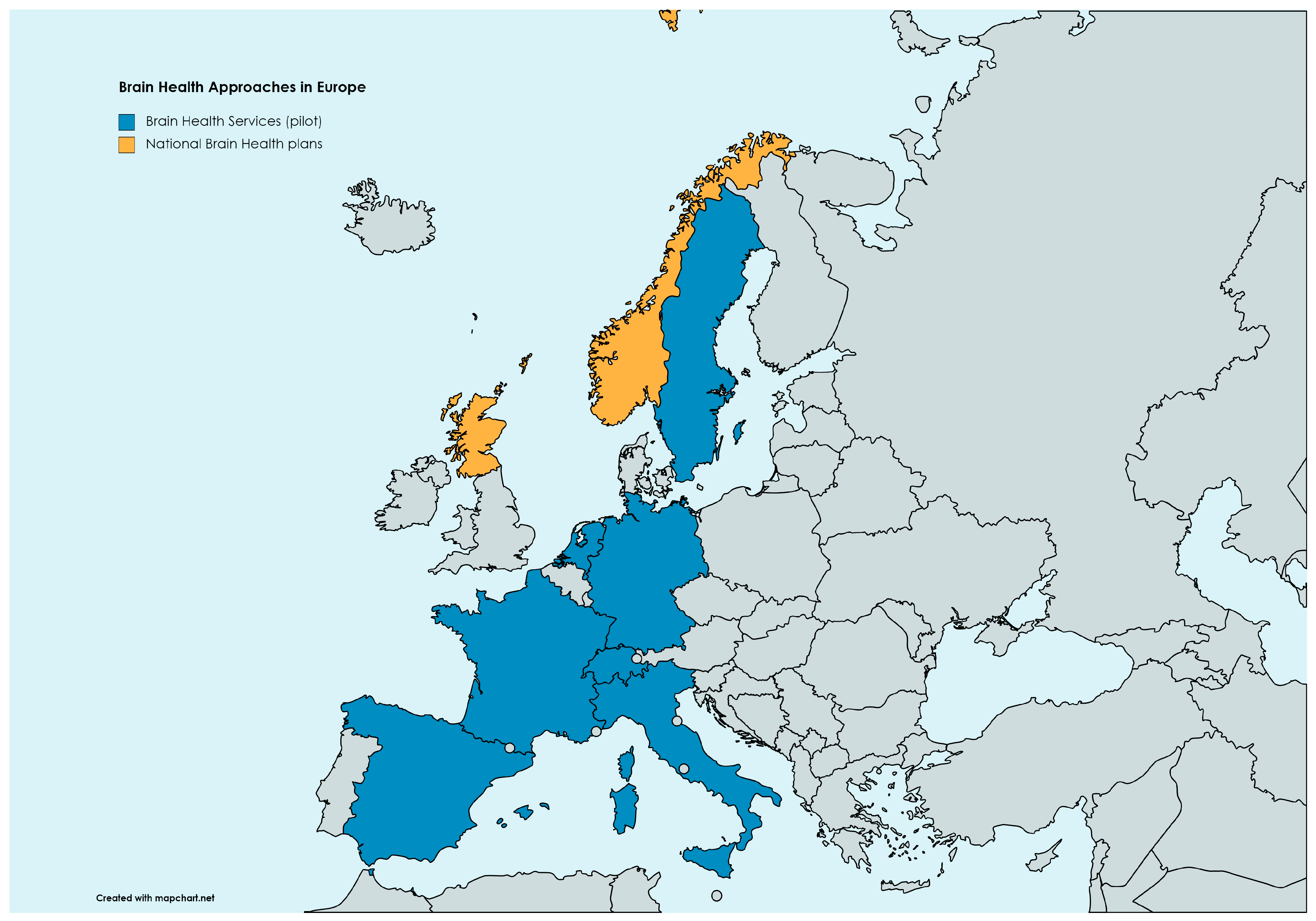
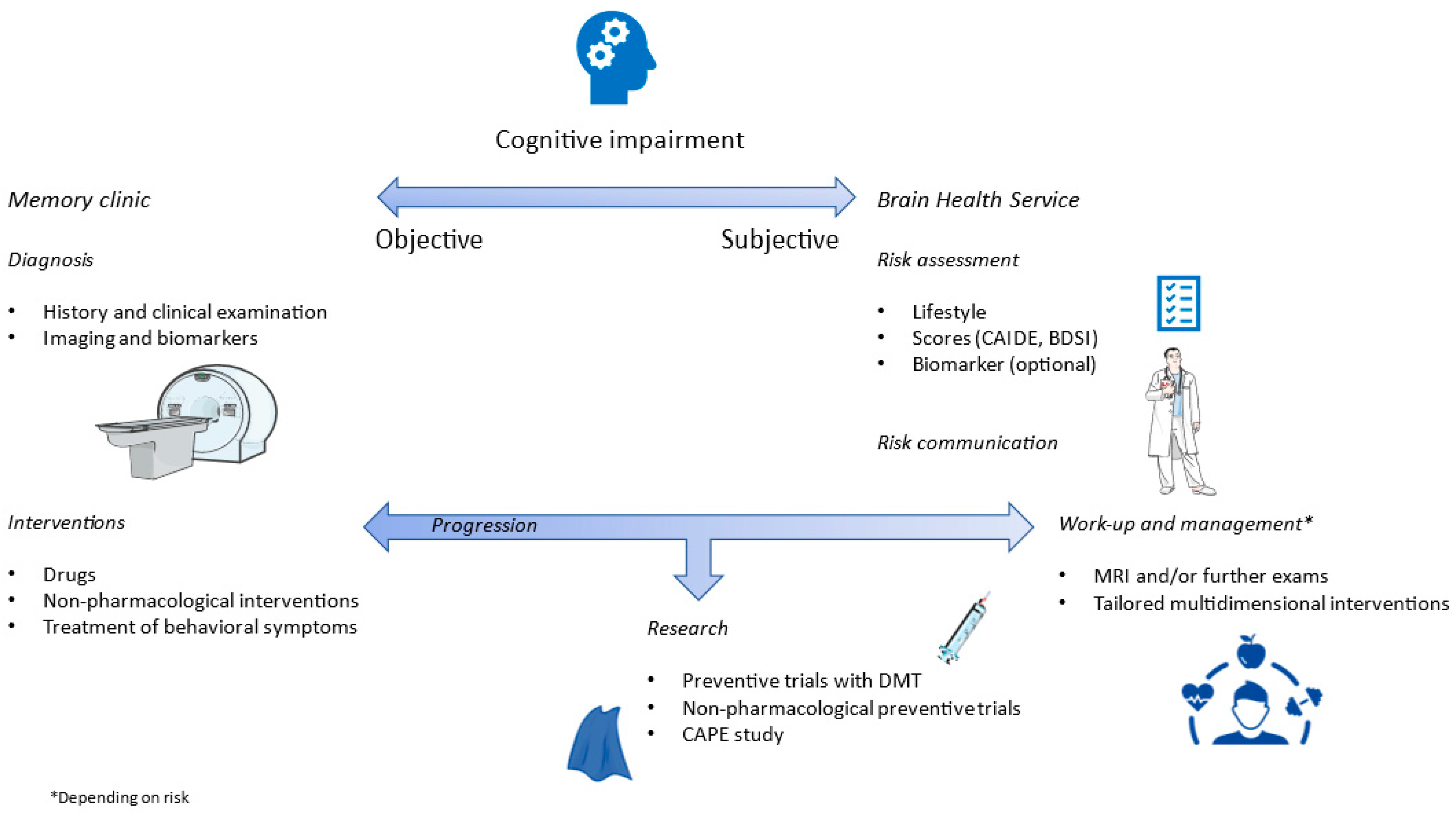
4. Brain Health Services and the Monza Experience
4.1. The Monza BHS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Optimizing Brain Health across the Life Course; World Health Organization: Geneva, Switzerland, 2022; ISBN 9789240054561.
- Chen, Y.; Demnitz, N.; Yamamoto, S.; Yaffe, K.; Lawlor, B.; Leroi, I. Defining brain health: A concept analysis. Int. J. Geriatr. Psychiatry 2022, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Simons, R.L.; Ong, M.L.; Beach, S.R.H.; Lei, M.K.; Philibert, R.; Mielke, M.M. Direct and indirect effects of socioeconomic status and discrimination on subjective cognitive decline: A longitudinal study of African American women. J. Gerontol. B. Psychol. Sci. Soc. Sci. 2023, 78, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Borelli, W.V.; Zimmer, E.R.; Bieger, A.; Coelho, B.; Pascoal, T.A.; Chaves, M.L.F.; Amariglio, R.; Castilhos, R.M. Subjective cognitive decline in Brazil: Prevalence and association with dementia modifiable risk factors in a population-based study. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2022, 14, e12368. [Google Scholar] [CrossRef] [PubMed]
- Sabayan, B.; Doyle, S.; Rost, N.S.; Sorond, F.A.; Lakshminarayan, K.; Launer, L.J. The role of population-level preventive care for brain health in ageing. Lancet Healthy Longev. 2023, 4, e274–e283. [Google Scholar] [CrossRef]
- Owolabi, M.O.; Leonardi, M.; Bassetti, C.; Jaarsma, J.; Hawrot, T.; Makanjuola, A.I.; Dhamija, R.K.; Feng, W.; Straub, V.; Camaradou, J.; et al. Global synergistic actions to improve brain health for human development. Nat. Rev. Neurol. 2023, 19, 371–383. [Google Scholar] [CrossRef]
- Hachinski, V.; Avan, A.; Gillilands, J.; Oveisgharan, S. A new definition of brain health. Lancet Neurol. 2021, 20, 335–336. [Google Scholar] [CrossRef]
- Lazar, R.M.; Howard, V.J.; Kernan, W.N.; Aparicio, H.J.; Levine, D.A.; Viera, A.J.; Jordan, L.C.; Nyenhuis, D.L.; Possin, K.L.; Sorond, F.A.; et al. A primary care agenda for brain health: A scientific statement from the American Heart Association. Stroke 2021, 52, E295–E308. [Google Scholar] [CrossRef]
- Bassetti, C.L.A.; Endres, M.; Sander, A.; Crean, M.; Subramaniam, S.; Carvalho, V.; Di Liberto, G.; Franco, O.H.; Pijnenburg, Y.; Leonardi, M.; et al. The European Academy of Neurology Brain Health Strategy: One brain, one life, one approach. Eur. J. Neurol. 2022, 29, 2559–2566. [Google Scholar] [CrossRef]
- Global prevention of stroke: Focus on the individual? Lancet Neurol. 2019, 18, 903. [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- “GBD 2019 Mental Disorders Collaborators” Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [CrossRef] [PubMed]
- The Lancet Neurology Sustainable development demands brain health. Lancet Neurol. 2023, 22, 871. [CrossRef] [PubMed]
- Jessen, F.; Amariglio, R.E.; Van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; Van Der Flier, W.M.; et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Pike, K.E.; Cavuoto, M.G.; Li, L.; Wright, B.J.; Kinsella, G.J. Subjective cognitive decline: Level of risk for future dementia and mild cognitive impairment, a meta-analysis of longitudinal studies. Neuropsychol. Rev. 2022, 32, 703–735. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.J.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Aaronson, A.; Ashford, M.T.; Jin, C.; Bride, J.; Decker, J.; DeNicola, A.; Turner, R.W.; Conti, C.; Tank, R.; Truran, D.; et al. Brain Health Registry Study Partner Portal: Novel infrastructure for digital, dyadic data collection. Alzheimer’s Dement. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Slot, R.E.R.; Verfaillie, S.C.J.; Overbeek, J.M.; Timmers, T.; Wesselman, L.M.P.; Teunissen, C.E.; Dols, A.; Bouwman, F.H.; Prins, N.D.; Barkhof, F.; et al. Subjective Cognitive Impairment Cohort (SCIENCe): Study design and first results. Alzheimer’s Res. Ther. 2018, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Spottke, A.; Boecker, H.; Brosseron, F.; Buerger, K.; Catak, C.; Fliessbach, K.; Franke, C.; Fuentes, M.; Heneka, M.T.; et al. Design and first baseline data of the DZNE multicenter observational study on predementia Alzheimer’s disease (DELCODE). Alzheimer’s Res. Ther. 2018, 10, 15. [Google Scholar] [CrossRef]
- Ebenau, J.L.; Timmers, T.; Wesselman, L.M.P.; Verberk, I.M.W.; Verfaillie, S.C.J.; Slot, R.E.R.; Van Harten, A.C.; Teunissen, C.E.; Barkhof, F.; Van Den Bosch, K.A.; et al. ATN classification and clinical progression in subjective cognitive decline: The SCIENCe project. Neurology 2020, 95, 46–58. [Google Scholar] [CrossRef]
- Pascual-Lucas, M.; Allué, J.A.; Sarasa, L.; Fandos, N.; Castillo, S.; Terencio, J.; Sarasa, M.; Tartari, J.P.; Sanabria, Á.; Tárraga, L.; et al. Clinical performance of an antibody-free assay for plasma Aβ42/Aβ40 to detect early alterations of Alzheimer’s disease in individuals with subjective cognitive decline. Alzheimer’s Res. Ther. 2023, 15, 2. [Google Scholar] [CrossRef]
- Hong, Y.J.; Ho, S.H.; Jeong, J.H.; Park, K.H.; Kim, S.Y.; Wang, M.J.; Choi, S.H.; Yang, D.W. Impacts of baseline biomarkers on cognitive trajectories in subjective cognitive decline: The CoSCo prospective cohort study. Alzheimer’s Res. Ther. 2023, 15, 132. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, S.; Lassi, M.; Padiglioni, S.; Vergani, A.A.; Moschini, V.; Scarpino, M.; Giacomucci, G.; Burali, R.; Morinelli, C.; Fabbiani, C.; et al. PRedicting the EVolution of SubjectIvE Cognitive Decline to Alzheimer’s Disease With machine learning: The PREVIEW study protocol. BMC Neurol. 2023, 23, 300. [Google Scholar] [CrossRef] [PubMed]
- De Simone, M.S.; Rodini, M.; De Tollis, M.; Fadda, L.; Caltagirone, C.; Carlesimo, G.A. The diagnostic usefulness of experimental memory tasks for detecting subjective cognitive decline: Preliminary results in an Italian sample. Neuropsychology 2022, 37, 636–649. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.; Wolfsgruber, S.; Kleineidam, L.; Frommann, I.; Altenstein, S.; Bartels, C.; Brosseron, F.; Buerger, K.; Burow, L.; Butryn, M.; et al. Relevance of minor neuropsychological deficits in patients with subjective cognitive decline. Neurology 2023, 101, e2185–e2196. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, F.; Tandetnik, C.; Gagliardi, G.; Bertin, H.; Habert, M.O.; Hampel, H.; Boukadida, L.; Révillon, M.; Epelbaum, S.; Dubois, B. Low cognitive awareness, but not complaint, is a good marker of preclinical Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 59, 753–762. [Google Scholar] [CrossRef]
- Gottesman, R.F.; Seshadri, S. Risk factors, lifestyle behaviors, and vascular brain health. Stroke 2022, 53, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Asher, S.; Stephen, R.; Mäntylä, P.; Suominen, A.L.; Solomon, A. Periodontal health, cognitive decline, and dementia: A systematic review and meta-analysis of longitudinal studies. J. Am. Geriatr. Soc. 2022, 70, 2695–2709. [Google Scholar] [CrossRef]
- Reddy, O.C.; van der Werf, Y.D. The sleeping brain: Harnessing the power of the glymphatic system through lifestyle choices. Brain Sci. 2020, 10, 868. [Google Scholar] [CrossRef]
- Mukadam, N.; Sommerlad, A.; Huntley, J.; Livingston, G. Population attributable fractions for risk factors for dementia in low-income and middle-income countries: An analysis using cross-sectional survey data. Lancet Glob. Health 2019, 7, e596–e603. [Google Scholar] [CrossRef]
- Besser, L.M.; Brenowitz, W.D.; Meyer, O.L.; Hoermann, S.; Renne, J. Methods to address self-selection and reverse causation in studies of neighborhood environments and brain health. Int. J. Environ. Res. Public Health 2021, 18, 6484. [Google Scholar] [CrossRef]
- Maasakkers, C.M.; Weijs, R.W.J.; Dekkers, C.; Gardiner, P.A.; Ottens, R.; Olde Rikkert, M.G.M.; Melis, R.J.F.; Thijssen, D.H.J.; Claassen, J.A.H.R. Sedentary behaviour and brain health in middle-aged and older adults: A systematic review. Neurosci. Biobehav. Rev. 2022, 140, 104802. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Geurts, L.; Hoyles, L.; Iozzo, P.; Kraneveld, A.D.; La Fata, G.; Miani, M.; Patterson, E.; Pot, B.; Shortt, C.; et al. The microbiota–gut–brain axis: Pathways to better brain health. Perspectives on what we know, what we need to investigate and how to put knowledge into practice. Cell. Mol. Life Sci. 2022, 79, 80. [Google Scholar] [CrossRef] [PubMed]
- Testai, F.D.; Gorelick, P.B.; Aparicio, H.J.; Filbey, F.M.; Gonzalez, R.; Gottesman, R.F.; Melis, M.; Piano, M.R.; Rubino, T.; Song, S.Y. Use of marijuana: Effect on brain health: A scientific statement from the American Heart Association. Stroke 2022, 53, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s disease drug development pipeline: 2023. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2023, 9, e12385. [Google Scholar] [CrossRef] [PubMed]
- Smart, C.M.; Karr, J.E.; Areshenkoff, C.N.; Rabin, L.A.; Hudon, C.; Gates, N.; Ali, J.I.; Arenaza-Urquijo, E.M.; Buckley, R.F.; Chetelat, G.; et al. Non-pharmacologic interventions for older adults with subjective cognitive decline: Systematic review, meta-analysis, and preliminary recommendations. Neuropsychol. Rev. 2017, 27, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Yang, K.; Wang, X.; Li, H.; Li, T.; Lin, L.; Liu, Y.; Yang, Q.; Wang, X.; Wang, X.; et al. Advances in non-pharmacological interventions for subjective cognitive decline: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2020, 77, 903–920. [Google Scholar] [CrossRef] [PubMed]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Wimo, A.; Handels, R.; Antikainen, R.; Eriksdotter, M.; Jönsson, L.; Knapp, M.; Kulmala, J.; Laatikainen, T.; Lehtisalo, J.; Peltonen, M.; et al. Dementia prevention: The potential long-term cost-effectiveness of the FINGER prevention program. Alzheimer’s Dement. 2022, 1–10. [Google Scholar] [CrossRef]
- Andrieu, S.; Guyonnet, S.; Coley, N.; Cantet, C.; Bonnefoy, M.; Bordes, S.; Bories, L.; Cufi, M.-N.M.-N.; Dantoine, T.; Dartigues, J.-F.J.-F.; et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol. 2017, 16, 377–389. [Google Scholar] [CrossRef]
- van Charante, E.P.M.; Richard, E.; Eurelings, L.S.; van Dalen, J.W.; Ligthart, S.A.; van Bussel, E.F.; Hoevenaar-Blom, M.P.; Vermeulen, M.; van Gool, W.A. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): A cluster-randomised controlled trial. Lancet 2016, 388, 797–805. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Molinuevo, J.L.; Altomare, D.; Carrera, E.; Barkhof, F.; Berkhof, J.; Delrieu, J.; Dubois, B.; Kivipelto, M.; Nordberg, A.; et al. Precision prevention of Alzheimer’s and other dementias: Anticipating future needs in the control of risk factors and implementation of disease-modifying therapies. Alzheimer’s Dement. 2020, 16, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Turunen, H.; Ngandu, T.; Peltonen, M.; Levälahti, E.; Helisalmi, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; et al. Effect of the apolipoprotein e genotype on cognitive change during a multidomain lifestyle intervention a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018, 75, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Smith, E.E.; Anrather, J.; Gu, C.; Mishra, A.; Misra, S.; Perez-Pinzon, M.A.; Shih, A.Y.; Sorond, F.A.; Van Veluw, S.J.; et al. The neurovasculome: Key roles in brain health and cognitive impairment: A scientific statement From the American Heart Association/American Stroke Association. Stroke 2023, 54, E251–E271. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012, 11, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Mungas, D.; Early, D.R.; Glymour, M.M.; Zeki Al Hazzouri, A.; Haan, M.N. Education, bilingualism, and cognitive trajectories: Sacramento Area Latino Aging Study (SALSA). Neuropsychology 2018, 32, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Lipton, R.B.; Sliwinski, M.; Katz, M.J.; Derby, C.A.; Verghese, J. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology 2009, 73, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.T.C.; Richards, M.; Chan, W.C.; Chiu, H.F.K.; Lee, R.S.Y.; Lam, L.C.W. Association of daily intellectual activities with lower risk of incident dementia among older Chinese adults. JAMA Psychiatry 2018, 75, 697–703. [Google Scholar] [CrossRef]
- Hotz, I.; Deschwanden, P.F.; Mérillat, S.; Jäncke, L. Entorhinal cortex thinning is related to white matter hyperintensity growth, memory decline, and leisure activity in cognitively healthy older adults. Neuroimage 2023, 284, 120461. [Google Scholar] [CrossRef]
- Valsdóttir, V.; Magnúsdóttir, B.B.; Chang, M.; Sigurdsson, S.; Gudnason, V.; Launer, L.J.; Jónsdóttir, M.K. Cognition and brain health among older adults in Iceland: The AGES-Reykjavik study. GeroScience 2022, 44, 2785–2800. [Google Scholar] [CrossRef]
- Berkes, M.; Calvo, N.; Anderson, J.A.E.; Bialystok, E. Poorer clinical outcomes for older adult monolinguals when matched to bilinguals on brain health. Brain Struct. Funct. 2021, 226, 415–424. [Google Scholar] [CrossRef]
- Berkes, M.; Bialystok, E. Bilingualism as a contributor to cognitive reserve: What it can do and what it cannot do. Am. J. Alzheimer’s Dis. Other Demen. 2022, 37, 15333175221091416. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Park, A.; Hong, R.K.; Ko, M.; Heo, M.; Kim, H.; Chung, J.Y. Efficacy of a mobile-based multidomain intervention to improve cognitive function and health-related outcomes among older Korean adults with subjective cognitive decline. J. Alzheimer’s Dis. 2023, 93, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Ternes, K.; Iyengar, V.; Lavretsky, H.; Dawson, W.D.; Booi, L.; Ibanez, A.; Vahia, I.; Reynolds, C.; DeKosky, S.; Cummings, J.; et al. Brain health INnovation Diplomacy: A model binding diverse disciplines to manage the promise and perils of technological innovation. Int. Psychogeriatr. 2020, 32, 955–979. [Google Scholar] [CrossRef] [PubMed]
- Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines; World Health Organization: Geneva, Switzerland, 2019; ISBN 9789241550543.
- Kivipelto, M.; Mangialasche, F.; Ngandu, T.; Eg Nuñez Martín, E.J.J.; Kivipelto, M.; Ngandu, T.; Soininen, H.; Tuomilehto, J.; Lindström, J.; Solomon, A.; et al. World Wide Fingers will advance dementia prevention. Lancet Neurol. 2018, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Mangialasche, F.; Snyder, H.M.; Allegri, R.; Andrieu, S.; Arai, H.; Baker, L.; Belleville, S.; Brodaty, H.; Brucki, S.M.; et al. World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimer’s Dement. 2020, 16, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Cody, K.A.; Koscik, R.L.; Erickson, C.M.; Berman, S.E.; Jonaitis, E.M.; Williams, V.J.; Mueller, K.D.; Christian, B.T.; Chin, N.A.; Clark, L.R.; et al. Associations of the Lifestyle for Brain Health index with longitudinal cognition and brain amyloid beta in clinically unimpaired older adults: Findings from the Wisconsin Registry for Alzheimer’s Prevention. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2022, 14, e12351. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-K.; Erickson, K.I.; Aghjayan, S.L.; Chen, F.-T.; Li, R.-H.; Shih, J.-R.; Chang, S.-H.; Huang, C.-M.; Chu, C.-H. The multi-domain exercise intervention for memory and brain function in late middle-aged and older adults at risk for Alzheimer’s disease: A protocol for Western–Eastern Brain Fitness Integration Training trial. Front. Aging Neurosci. 2022, 14, 929789. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, G.; Bartrés-Faz, D.; Morris, T.P.; Sánchez, J.S.; Macià, D.; Tarrero, C.; Tormos, J.M.; Pascual-Leone, A. The Barcelona brain health initiative: A cohort study to define and promote determinants of brain health. Front. Aging Neurosci. 2018, 10, 321. [Google Scholar] [CrossRef]
- España-Irla, G.; Gomes-Osman, J.; Cattaneo, G.; Albu, S.; Cabello-Toscano, M.; Solana-Sanchéz, J.; Redondo-Camós, M.; Delgado-Gallén, S.; Alviarez-Schulze, V.; Pachón-García, C.; et al. Associations between cardiorespiratory fitness, cardiovascular risk, and cognition are mediated by structural brain health in midlife. J. Am. Heart Assoc. 2021, 10, e020688. [Google Scholar] [CrossRef]
- Hussenoeder, F.S.; Riedel-Heller, S.G. Primary prevention of dementia: From modifiable risk factors to a public brain health agenda? Soc. Psychiatry Psychiatr. Epidemiol. 2018, 53, 1289–1301. [Google Scholar] [CrossRef]
- Namsrai, T.; Ambikairajah, A.; Cherbuin, N. Poorer sleep impairs brain health at midlife. Sci. Rep. 2023, 13, 1874. [Google Scholar] [CrossRef] [PubMed]
- Kolappa, K.; Seeher, K.; Dua, T. Brain health as a global priority. J. Neurol. Sci. 2022, 439, 120326. [Google Scholar] [CrossRef] [PubMed]
- Röhr, S.; Pabst, A.; Baber, R.; Engel, C.; Glaesmer, H.; Hinz, A.; Schroeter, M.L.; Witte, A.V.; Zeynalova, S.; Villringer, A.; et al. Social determinants and lifestyle factors for brain health: Implications for risk reduction of cognitive decline and dementia. Sci. Rep. 2022, 12, 12965. [Google Scholar] [CrossRef] [PubMed]
- Röhr, S.; Rodriguez, F.S.; Siemensmeyer, R.; Müller, F.; Romero-Ortuno, R.; Riedel-Heller, S.G. How can urban environments support dementia risk reduction? A qualitative study. Int. J. Geriatr. Psychiatry 2022, 37. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Maguire, B.; Brodaty, H.; O’Leary, F. Dietary patterns and cognitive health in older adults: A systematic review. J. Alzheimer’s Dis. 2019, 67, 583–619. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.J.; Blumenthal, J.A. Dietary factors and cognitive decline. J. Prev. Alzheimer’s Dis. 2015, 3, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Jaqua, E.; Biddy, E.; Moore, C.; Browne, G. The impact of the six pillars of lifestyle medicine on brain health. Cureus 2023, 15, e34605. [Google Scholar] [CrossRef]
- Halloway, S.; Wilbur, J.E.; Schoeny, M.E.; Arfanakis, K. Effects of endurance-focused physical activity interventions on brain health: A systematic review. Biol. Res. Nurs. 2017, 19, 53–64. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, C.; Chen, A. A systematic review and meta—Analysis of the effects of physical exercise on white matter integrity and cognitive function in older adults. GeroScience 2023. online ahead of print. [Google Scholar] [CrossRef]
- Venkataraman, A.; Kalk, N.; Sewell, G.; Ritchie, C.W.; Lingford-Hughes, A. Alcohol and Alzheimer’s disease-does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s disease? Alcohol Alcohol. 2017, 52, 151–158. [Google Scholar] [CrossRef]
- Zhong, G.; Wang, Y.; Zhang, Y.; Guo, J.J.; Zhao, Y. Smoking is associated with an increased risk of dementia: A meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS ONE 2015, 10, e0118333. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lin, L.; Xiong, M.; Sun, S.; Wu, S. cai Moderating effects of cognitive reserve on the relationship between brain structure and cognitive abilities in middle-aged and older adults. Neurobiol. Aging 2023, 128, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Elder, A.; Auten, R.L.; Bilbo, S.D.; Chen, H.; Chen, J.C.; Cory-Slechta, D.A.; Costa, D.; Diaz-Sanchez, D.; Dorman, D.C.; et al. The outdoor air pollution and brain health workshop. Neurotoxicology 2012, 33, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Avan, A.; Aamodt, A.H.; Selbæk, G.; Bovim, G.; Bassetti, C.L.A.; Boon, P.; Grisold, W.; Hachinski, V. Decreasing incidence of stroke, ischaemic heart disease and dementia in Norway, 1990–2019, a Global Burden of Disease study: An opportunity. Eur. J. Neurol. 2023, 30, 2267–2277. [Google Scholar] [CrossRef]
- Owolabi, M.O. Improving brain and vascular health at the population level: Lessons from the Norwegian success story. Eur. J. Neurol. 2023, 30, 2141–2143. [Google Scholar] [CrossRef]
- Helse-og Omsorgsdepartementet. National Brain Health Strategy (2018–2024). 2017. Available online: https://www.braincouncil.eu/wp-content/uploads/2018/04/Annex-Full-Norwegian-Brain-Health-Strategy.pdf (accessed on 28 November 2023).
- Kjelvik, G.; Rokstad, A.M.M.; Stuebs, J.; Thingstad, P.; Deckers, K.; Köhler, S.; Selbæk, G. Public knowledge about dementia risk reduction in Norway. BMC Public Health 2022, 22, 2046. [Google Scholar] [CrossRef] [PubMed]
- Zülke, A.E.; Luppa, M.; Köhler, S.; Riedel-Heller, S.G. Knowledge of risk and protective factors for dementia in older German adults A population-based survey on risk and protective factors for dementia and internet-based brain health interventions. PLoS ONE 2022, 17, e0277037. [Google Scholar] [CrossRef]
- Castellani, B.; Bartington, S.; Wistow, J.; Heckels, N.; Ellison, A.; Van Tongeren, M.; Arnold, S.R.; Barbrook-Johnson, P.; Bicket, M.; Pope, F.D.; et al. Mitigating the impact of air pollution on dementia and brain health: Setting the policy agenda. Environ. Res. 2022, 215, 114362. [Google Scholar] [CrossRef]
- Ibáñez, A.; Pina-Escudero, S.D.; Possin, K.L.; Quiroz, Y.T.; Peres, F.A.; Slachevsky, A.; Sosa, A.L.; Brucki, S.M.D.; Miller, B.L. Dementia caregiving across Latin America and the Caribbean and brain health diplomacy. Lancet Healthy Longev. 2021, 2, e222–e231. [Google Scholar] [CrossRef]
- Altomare, D.; Molinuevo, J.L.; Ritchie, C.; Ribaldi, F.; Carrera, E.; Dubois, B.; Jessen, F.; McWhirter, L.; Scheltens, P.; van der Flier, W.M.; et al. Brain Health Services: Organization, structure, and challenges for implementation. A user manual for Brain Health Services—Part 1 of 6. Alzheimer’s Res. Ther. 2021, 13, 168. [Google Scholar] [CrossRef]
- Ranson, J.M.; Rittman, T.; Hayat, S.; Brayne, C.; Jessen, F.; Blennow, K.; van Duijn, C.; Barkhof, F.; Tang, E.; Mummery, C.J.; et al. Modifiable risk factors for dementia and dementia risk profiling. A user manual for Brain Health Services—Part 2 of 6. Alzheimer’s Res. Ther. 2021, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Visser, L.N.C.; Minguillon, C.; Sánchez-Benavides, G.; Abramowicz, M.; Altomare, D.; Fauria, K.; Frisoni, G.B.; Georges, J.; Ribaldi, F.; Scheltens, P.; et al. Dementia risk communication. A user manual for Brain Health Services—Part 3 of 6. Alzheimer’s Res. Ther. 2021, 13, 170. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Stephen, R.; Altomare, D.; Carrera, E.; Frisoni, G.B.; Kulmala, J.; Molinuevo, J.L.; Nilsson, P.; Ngandu, T.; Ribaldi, F.; et al. Multidomain interventions: State-of-the-art and future directions for protocols to implement precision dementia risk reduction. A user manual for Brain Health Services—Part 4 of 6. Alzheimer’s Res. Ther. 2021, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Brioschi Guevara, A.; Bieler, M.; Altomare, D.; Berthier, M.; Csajka, C.; Dautricourt, S.; Démonet, J.F.; Dodich, A.; Frisoni, G.B.; Miniussi, C.; et al. Protocols for cognitive enhancement. A user manual for Brain Health Services—Part 5 of 6. Alzheimer’s Res. Ther. 2021, 13, 172. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.; Altomare, D.; Ribaldi, F.; Molinuevo, J.L.; Frisoni, G.B.; Brayne, C. Societal and equity challenges for Brain Health Services. A user manual for Brain Health Services—Part 6 of 6. Alzheimer’s Res. Ther. 2021, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, G.B.; Altomare, D.; Ribaldi, F.; Villain, N.; Brayne, C.; Mukadam, N.; Abramowicz, M.; Barkhof, F.; Berthier, M.; Bieler-Aeschlimann, M.; et al. Dementia prevention in memory clinics: Recommendations from the European task force for brain health services. Lancet Reg. Health Eur. 2023, 26, 100576. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2022, 388, 9–21. [Google Scholar] [CrossRef]
- McWhirter, L.; Ritchie, C.; Stone, J.; Carson, A. Functional cognitive disorders: A systematic review. Lancet Psychiatry 2020, 7, 191–207. [Google Scholar] [CrossRef]
- Crawford, C.; Boyd, C.; Avula, B.; Wang, Y.H.; Khan, I.A.; Deuster, P.A. A public health issue: Dietary supplements promoted for brain health and cognitive performance. J. Altern. Complement. Med. 2020, 26, 265–272. [Google Scholar] [CrossRef]
- Hellmuth, J.; Rabinovici, G.D.; Miller, B.L. The rise of pseudomedicine for dementia and brain health. JAMA 2019, 321, 543. [Google Scholar] [CrossRef]
- Kivipelto, M.; Ngandu, T.; Laatikainen, T.; Winblad, B.; Soininen, H.; Tuomilehto, J. Risk score for the prediction of dementia risk in 20 years among middle aged people: A longitudinal, population-based study. Lancet Neurol. 2006, 5, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Beiser, A.S.; Lee, A.; Langa, K.M.; Koyama, A.; Preis, S.R.; Neuhaus, J.; McCammon, R.J.; Yaffe, K.; Seshadri, S.; et al. Development and validation of a brief dementia screening indicator for primary care. Alzheimer’s Dement. 2014, 10, 656–665.e1. [Google Scholar] [CrossRef] [PubMed]
- Anstey, K.J.; Cherbuin, N.; Herath, P.M. Development of a new method for assessing global risk of Alzheimer’s disease for use in population health approaches to prevention. Prev. Sci. 2013, 14, 411–421. [Google Scholar] [CrossRef] [PubMed]
- van Maurik, I.S.; Visser, L.N.C.; Pel-Littel, R.E.; van Buchem, M.M.; Zwan, M.D.; Kunneman, M.; Pelkmans, W.; Bouwman, F.H.; Minkman, M.; Schoonenboom, N.; et al. Development and usability of ADappt: Web-based tool to support clinicians, patients, and caregivers in the diagnosis of mild cognitive impairment and alzheimer disease. JMIR Form. Res. 2019, 3, e13417. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Dubarbie, F.; Guerra-Ruiz, A.; López-García, S.; Lage, C.; Fernández-Matarrubia, M.; Infante, J.; Pozueta-Cantudo, A.; García-Martínez, M.; Corrales-Pardo, A.; Bravo, M.; et al. Accuracy of plasma Aβ40, Aβ42, and p-tau181 to detect CSF Alzheimer’s pathological changes in cognitively unimpaired subjects using the Lumipulse automated platform. Alzheimer’s Res. Ther. 2023, 15, 163. [Google Scholar] [CrossRef] [PubMed]
- Bemelmans, S.A.S.A.; Tromp, K.; Bunnik, E.M.; Milne, R.J.; Badger, S.; Brayne, C.; Schermer, M.H.; Richard, E. Psychological, behavioral and social effects of disclosing Alzheimer’s disease biomarkers to research participants: A systematic review. Alzheimer’s Res. Ther. 2016, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Largent, E.A.; Harkins, K.; Van Dyck, C.H.; Hachey, S.; Sankar, P.; Karlawish, J. Cognitively unimpaired adults’ reactions to disclosure of amyloid PET scan results. PLoS ONE 2020, 15, e0229137. [Google Scholar] [CrossRef]
- Ritchie, C.W.; Waymont, J.M.J.; Pennington, C.; Draper, K.; Borthwick, A.; Fullerton, N.; Chantler, M.; Porteous, M.E.; Danso, S.O.; Green, A.; et al. The Scottish Brain Health Service model: Rationale and scientific basis for a national care pathway of Brain Health Services in Scotland. J. Prev. Alzheimer’s Dis. 2022, 9, 348–358. [Google Scholar] [CrossRef]
- Fosnacht, A.M.; Patel, S.; Yucus, C.; Pham, A.; Rasmussen, E.; Frigerio, R.; Walters, S.; Maraganore, D. From brain disease to brain health: Primary prevention of Alzheimer’S disease and related disorders in a health system using an electronic medical record-based approach. J. Prev. Alzheimer’s Dis. 2017, 4, 157–164. [Google Scholar] [CrossRef]
- Morley, J.E.; Morris, J.C.; Berg-Weger, M.; Borson, S.; Carpenter, B.D.; del Campo, N.; Dubois, B.; Fargo, K.; Fitten, L.J.; Flaherty, J.H.; et al. Brain health: The importance of recognizing cognitive impairment: An IAGG Consensus Conference. J. Am. Med. Dir. Assoc. 2015, 16, 731–739. [Google Scholar] [CrossRef]
- Alchalabi, T.; Prather, C. Brain Health. Clin. Geriatr. Med. 2021, 37, 593–604. [Google Scholar] [CrossRef] [PubMed]
- WHO Guidelines on Physical Activity and Sedentary Behaviour: At a Glance; World Health Organization: Geneva, Switzerland, 2020; p. 535.
- Barha, C.K.; Galea, L.A.; Nagamatsu, L.S.; Erickson, K.I.; Liu-Ambrose, T. Personalising exercise recommendations for brain health: Considerations and future directions. Br. J. Sports Med. 2017, 51, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozzi, F.E.; Remoli, G.; Tremolizzo, L.; Appollonio, I.; Ferrarese, C.; Cuffaro, L. Brain Health and Cognition in Older Adults: Roadmap and Milestones towards the Implementation of Preventive Strategies. Brain Sci. 2024, 14, 55. https://doi.org/10.3390/brainsci14010055
Pozzi FE, Remoli G, Tremolizzo L, Appollonio I, Ferrarese C, Cuffaro L. Brain Health and Cognition in Older Adults: Roadmap and Milestones towards the Implementation of Preventive Strategies. Brain Sciences. 2024; 14(1):55. https://doi.org/10.3390/brainsci14010055
Chicago/Turabian StylePozzi, Federico Emanuele, Giulia Remoli, Lucio Tremolizzo, Ildebrando Appollonio, Carlo Ferrarese, and Luca Cuffaro. 2024. "Brain Health and Cognition in Older Adults: Roadmap and Milestones towards the Implementation of Preventive Strategies" Brain Sciences 14, no. 1: 55. https://doi.org/10.3390/brainsci14010055




