Chronic Nicotine Consumption and Withdrawal Regulate Melanocortin Receptor, CRF, and CRF Receptor mRNA Levels in the Rat Brain
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Forced Oral Nicotine Treatment
2.4. Tissue Dissection
2.5. RNA Extraction and cDNA Synthesis
2.6. Quantitative Real-Time PCR (qPCR)
2.7. Statistical Analysis
3. Results
3.1. Effect of Chronic Nicotine Exposure and Withdrawal on Body Weight
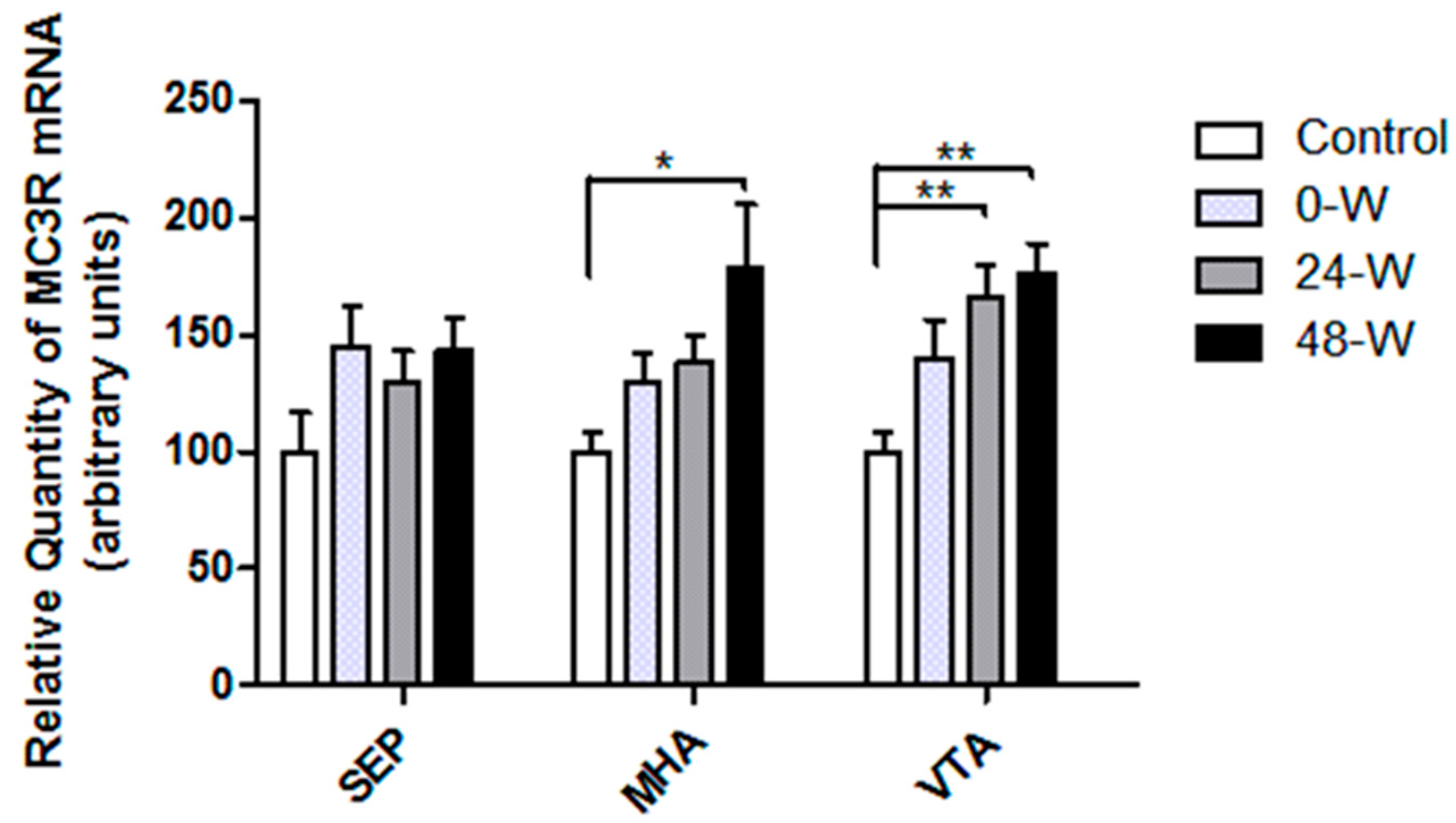
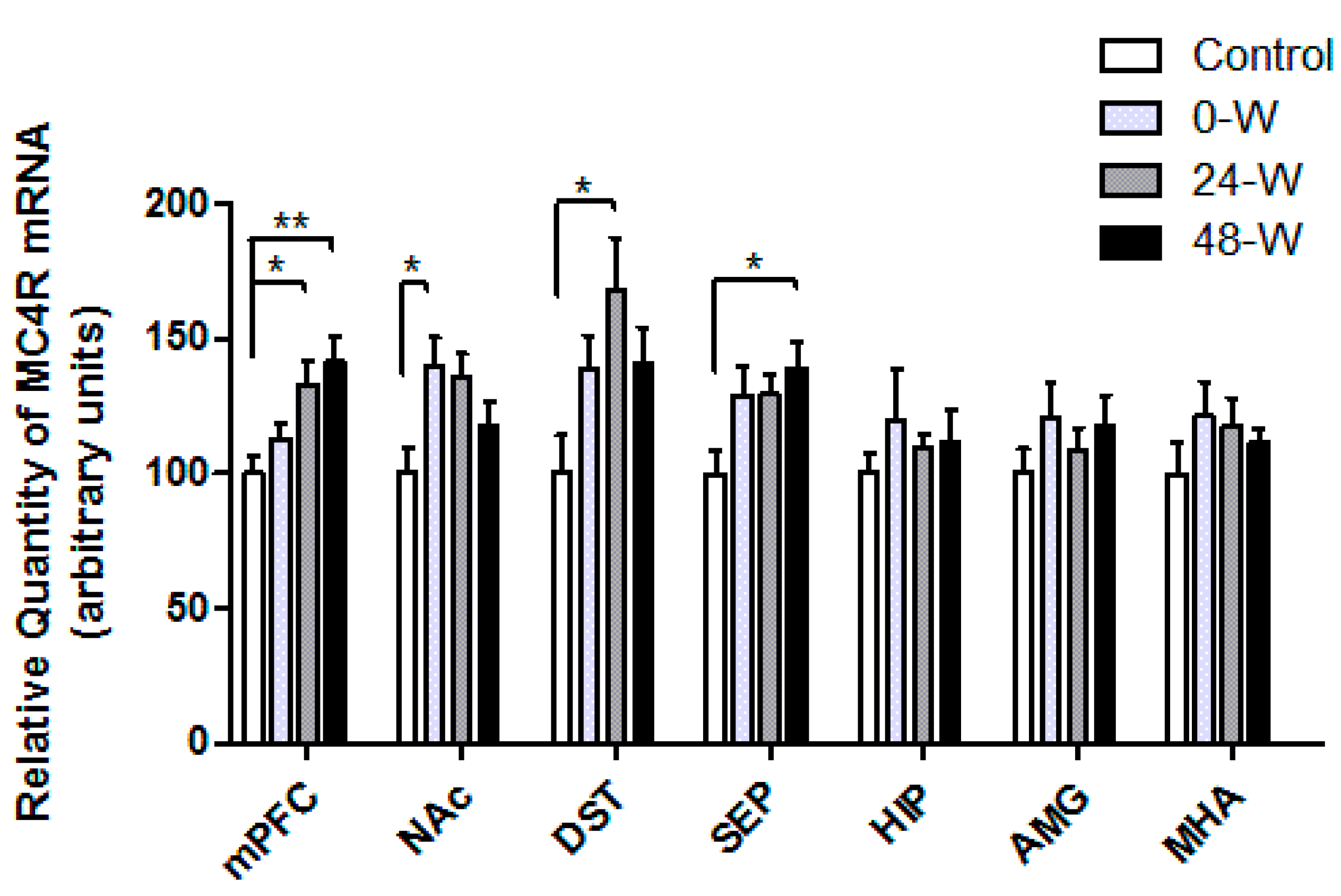
3.2. MC3R and MC4R Transcript Levels following Chronic Nicotine Administration and Withdrawal
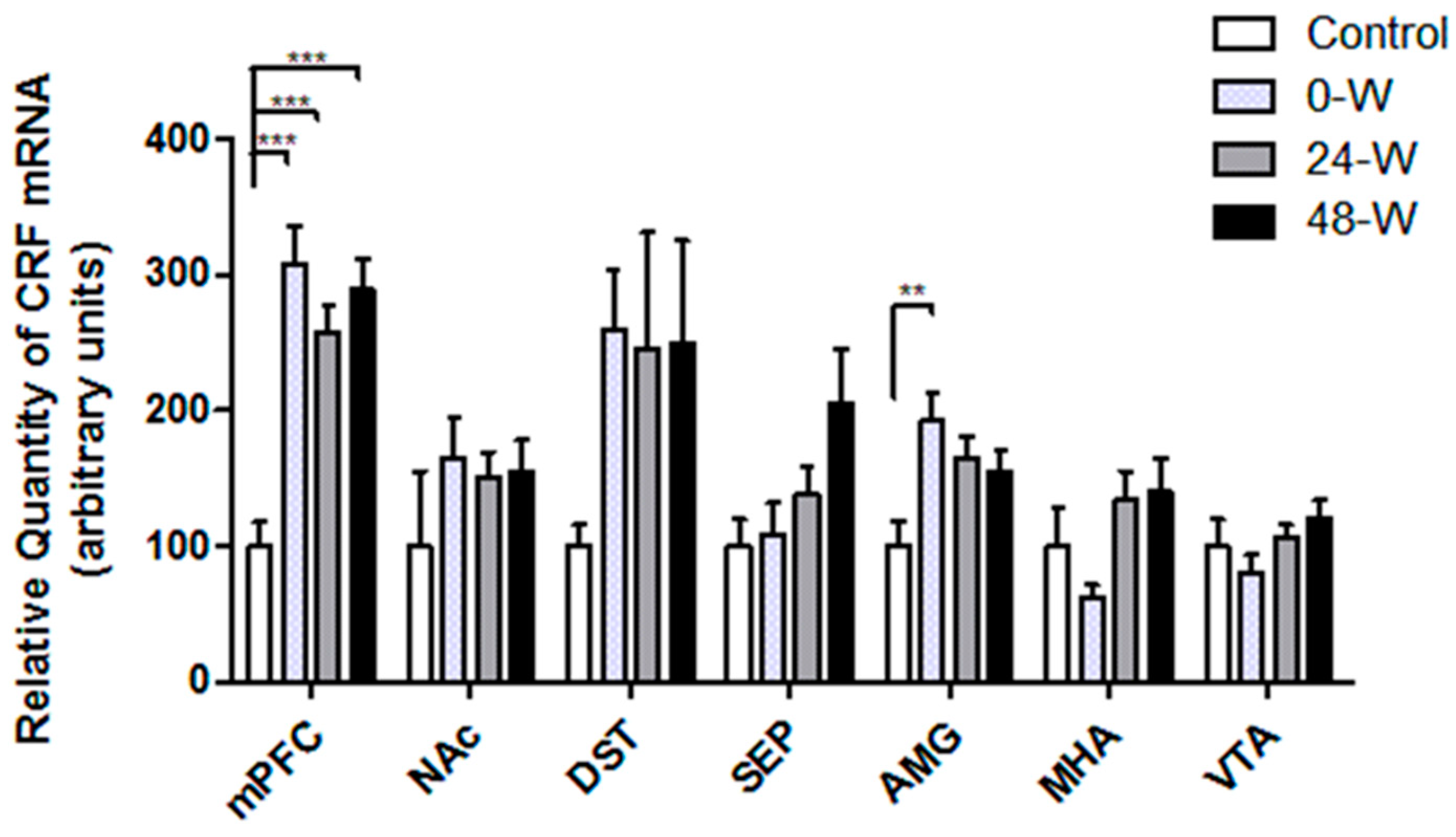
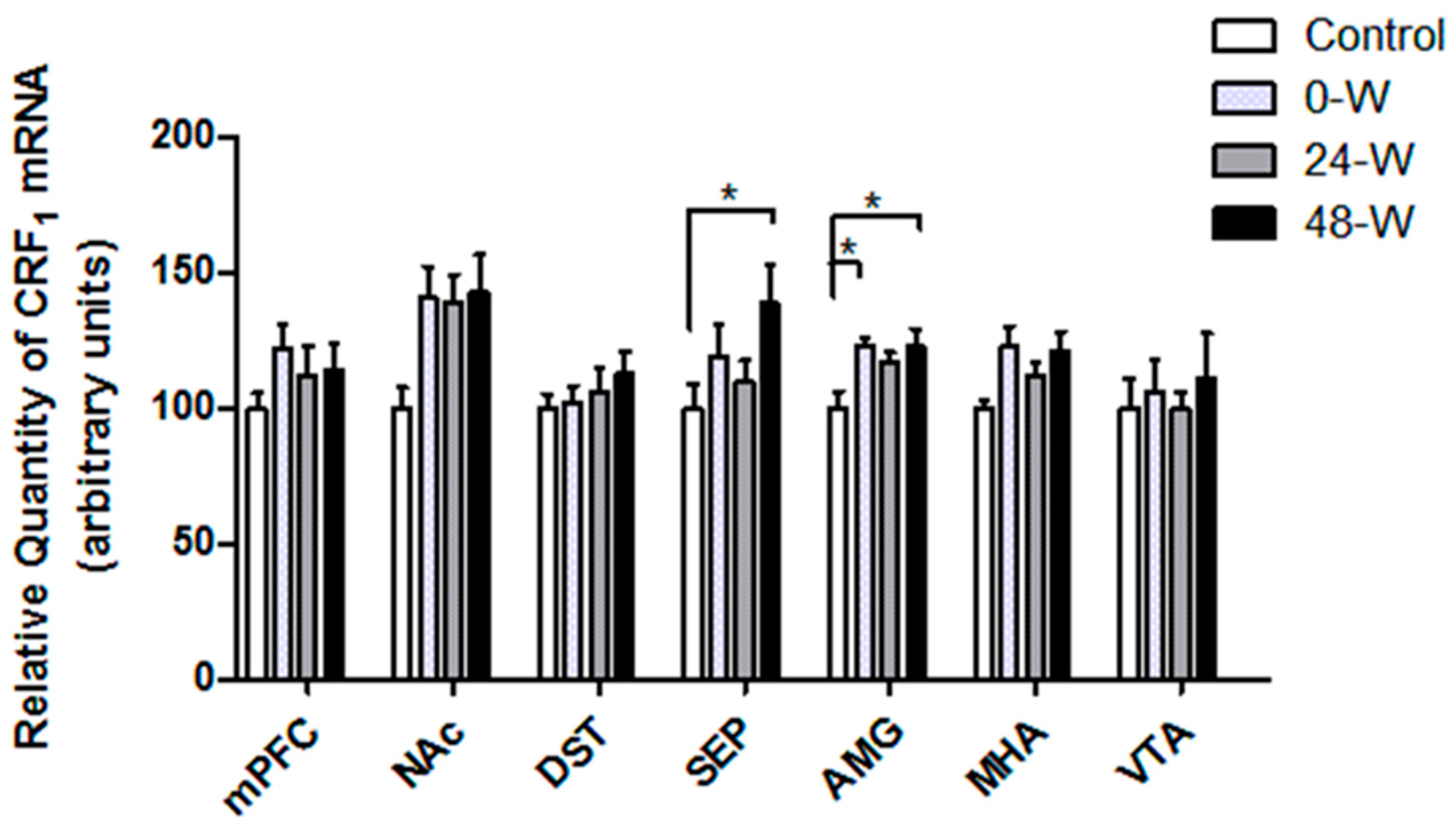
3.3. CRF and CRFR Transcript Levels following Chronic Nicotine Administration and Withdrawal
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pistillo, F.; Clementi, F.; Zoli, M.; Gotti, C. Nicotinic, glutamatergic and dopaminergic synaptic transmission and plasticity in the mesocorticolimbic system: Focus on nicotine effects. Prog. Neurobiol. 2015, 124, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Malin, D.H.; Goyarzu, P. Rodent models of nicotine withdrawal syndrome. In Nicotine Psychopharmacology; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 401–434. [Google Scholar] [CrossRef]
- Wise, R.A.; Koob, G.F. The development and maintenance of drug addiction. Neuropsychopharmacology 2014, 39, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Malin, D.H.; Lake, J.R.; Newlin-Maultsby, P.; Roberts, L.K.; Lanier, J.G.; Carter, V.A.; Cunningham, J.S.; Wilson, O.B. Rodent model of nicotine abstinence syndrome. Pharmacol. Biochem. Behav. 1992, 43, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, C.; Tedesco, M.; Bellomo, M.; Caputi, A.P.; Calapai, G. Long-term effects of nicotine on the forced swimming test in mice: An experimental model for the study of depression caused by smoke. Neurochem. Int. 2006, 49, 481–486. [Google Scholar] [CrossRef]
- Epping-Jordan, M.P.; Watkins, S.S.; Koob, G.F.; Markou, A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature 1998, 393, 76–79. [Google Scholar] [CrossRef]
- Grieder, T.E.; George, O.; Tan, H.; George, S.R.; Le Foll, B.; Laviolette, S.R.; van der Kooy, D. Phasic D1 and tonic D2 dopamine receptor signaling double dissociate the motivational effects of acute nicotine and chronic nicotine withdrawal. Proc. Natl. Acad. Sci. USA 2012, 109, 3101–3106. [Google Scholar] [CrossRef]
- Natividad, L.A.; Tejeda, H.A.; Torres, O.V.; O’Dell, L.E. Nicotine withdrawal produces a decrease in extracellular levels of dopamine in the nucleus accumbens that is lower in adolescent versus adult male rats. Synapse 2010, 64, 136–145. [Google Scholar] [CrossRef]
- Rada, P.; Jensen, K.; Hoebel, B.G. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology 2001, 157, 105–110. [Google Scholar] [CrossRef]
- Hadjiconstantinou, M.; Duchemin, A.M.; Zhang, H.; Neff, N.H. Enhanced dopamine transporter function in striatum during nicotine withdrawal. Synapse 2011, 65, 91–98. [Google Scholar] [CrossRef]
- Roselli-Rehfuss, L.; Mountjoy, K.G.; Robbins, L.S.; Mortrud, M.T.; Low, M.J.; Tatro, J.B.; Entwistle, M.L.; Simerly, R.B.; Cone, R.D. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc. Natl. Acad. Sci. USA 1993, 90, 8856–8860. [Google Scholar] [CrossRef]
- Kishi, T.; Aschkenasi, C.J.; Lee, C.E.; Mountjoy, K.G.; Saper, C.B.; Elmquist, J.K. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J. Comp. Neurol. 2003, 457, 213–235. [Google Scholar] [CrossRef]
- Goyal, S.N.; Kokare, D.M.; Chopde, C.T.; Subhedar, N.K. Alpha-melanocyte stimulating hormone antagonizes antidepressant-like effect of neuropeptide Y in Porsolt’s test in rats. Pharmacol. Biochem. Behav. 2006, 85, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Kokare, D.M.; Chopde, C.T.; Subhedar, N.K. Participation of alpha-melanocyte stimulating hormone in ethanol-induced anxiolysis and withdrawal anxiety in rats. Neuropharmacology 2006, 51, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.K.; Huang, K.W.; Grueter, B.A.; Rothwell, P.E.; Malenka, R.C. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 2012, 487, 183–189. [Google Scholar] [CrossRef]
- Qi, X.; Yamada, H.; Corrie, L.W.; Ji, Y.; Bauzo, R.M.; Alexander, J.C.; Bruijnzeel, A.W. A critical role for the melanocortin 4 receptor in stress-induced relapse to nicotine seeking in rats. Addict. Biol. 2015, 20, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Lindblom, J.; Opmane, B.; Mutulis, F.; Mutule, I.; Petrovska, R.; Klusa, V.; Bergstrom, L.; Wikberg, J.E. The MC4 receptor mediates alpha-MSH induced release of nucleus accumbens dopamine. Neuroreport 2001, 12, 2155–2158. [Google Scholar] [CrossRef]
- Sanchez, M.S.; Barontini, M.; Armando, I.; Celis, M.E. Correlation of increased grooming behavior and motor activity with alterations in nigrostriatal and mesolimbic catecholamines after alpha-melanotropin and neuropeptide glutamine-isoleucine injection in the rat ventral tegmental area. Cell. Mol. Neurobiol. 2001, 21, 523–533. [Google Scholar] [CrossRef]
- Pandit, R.; Omrani, A.; Luijendijk, M.C.; de Vrind, V.A.; Van Rozen, A.J.; Ophuis, R.J.; Garner, K.; Kallo, I.; Ghanem, A.; Liposits, Z.; et al. Melanocortin 3 Receptor Signaling in Midbrain Dopamine Neurons Increases the Motivation for Food Reward. Neuropsychopharmacology 2016, 41, 2241–2251. [Google Scholar] [CrossRef]
- Lindblom, J.; Wikberg, J.E.; Bergstrom, L. Alcohol-preferring AA rats show a derangement in their central melanocortin signalling system. Pharmacol. Biochem. Behav. 2002, 72, 491–496. [Google Scholar] [CrossRef]
- Dedic, N.; Chen, A.; Deussing, J.M. The CRF Family of Neuropeptides and their Receptors—Mediators of the Central Stress Response. Curr. Mol. Pharmacol. 2018, 11, 4–31. [Google Scholar] [CrossRef]
- Van Pett, K.; Viau, V.; Bittencourt, J.C.; Chan, R.K.; Li, H.Y.; Arias, C.; Prins, G.S.; Perrin, M.; Vale, W.; Sawchenko, P.E. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000, 428, 191–212. [Google Scholar] [CrossRef]
- Cador, M.; Ahmed, S.H.; Koob, G.F.; Le Moal, M.; Stinus, L. Corticotropin-releasing factor induces a place aversion independent of its neuroendocrine role. Brain Res. 1992, 597, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Britton, K.T.; Akwa, Y.; Spina, M.G.; Koob, G.F. Neuropeptide Y blocks anxiogenic-like behavioral action of corticotropin-releasing factor in an operant conflict test and elevated plus maze. Peptides 2000, 21, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, V.P.; Newman, S.M.; Smith-Roe, S.; Jochman, K.A.; Kalin, N.H. Stimulation of lateral septum CRF2 receptors promotes anorexia and stress-like behaviors: Functional homology to CRF1 receptors in basolateral amygdala. J. Neurosci. 2007, 27, 10568–10577. [Google Scholar] [CrossRef] [PubMed]
- George, O.; Ghozland, S.; Azar, M.R.; Cottone, P.; Zorrilla, E.P.; Parsons, L.H.; O’Dell, L.E.; Richardson, H.N.; Koob, G.F. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc. Natl. Acad. Sci. USA 2007, 104, 17198–17203. [Google Scholar] [CrossRef]
- Miguel, T.T.; Gomes, K.S.; Nunes-de-Souza, R.L. Tonic modulation of anxiety-like behavior by corticotropin-releasing factor (CRF) type 1 receptor (CRF1) within the medial prefrontal cortex (mPFC) in male mice: Role of protein kinase A (PKA). Horm. Behav. 2014, 66, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.L.; Lu, Y.L.; Baynes, B.B.; Richardson, H.N.; Gilpin, N.W. Corticotropin-releasing factor in ventromedial prefrontal cortex mediates avoidance of a traumatic stress-paired context. Neuropharmacology 2017, 113, 323–330. [Google Scholar] [CrossRef]
- Baiamonte, B.A.; Valenza, M.; Roltsch, E.A.; Whitaker, A.M.; Baynes, B.B.; Sabino, V.; Gilpin, N.W. Nicotine dependence produces hyperalgesia: Role of corticotropin-releasing factor-1 receptors (CRF1Rs) in the central amygdala (CeA). Neuropharmacology 2014, 77, 217–223. [Google Scholar] [CrossRef]
- Grieder, T.E.; Herman, M.A.; Contet, C.; Tan, L.A.; Vargas-Perez, H.; Cohen, A.; Chwalek, M.; Maal-Bared, G.; Freiling, J.; Schlosburg, J.E.; et al. VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nat. Neurosci. 2014, 17, 1751–1758. [Google Scholar] [CrossRef]
- Bruijnzeel, A.W.; Prado, M.; Isaac, S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol. Psychiatry 2009, 66, 110–117. [Google Scholar] [CrossRef]
- Cohen, A.; Treweek, J.; Edwards, S.; Leao, R.M.; Schulteis, G.; Koob, G.F.; George, O. Extended access to nicotine leads to a CRF1 receptor dependent increase in anxiety-like behavior and hyperalgesia in rats. Addict. Biol. 2015, 20, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Bagosi, Z.; Palotai, M.; Simon, B.; Bokor, P.; Buzas, A.; Balango, B.; Pinter, D.; Jaszberenyi, M.; Csabafi, K.; Szabo, G. Selective CRF2 receptor agonists ameliorate the anxiety- and depression-like state developed during chronic nicotine treatment and consequent acute withdrawal in mice. Brain Res. 2016, 1652, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Carboni, L.; Ponzoni, L.; Braida, D.; Sala, M.; Gotti, C.; Zoli, M. Altered mRNA Levels of Stress-Related Peptides in Mouse Hippocampus and Caudate-Putamen in Withdrawal after Long-Term Intermittent Exposure to Tobacco Smoke or Electronic Cigarette Vapour. Int. J. Mol. Sci. 2021, 22, 599. [Google Scholar] [CrossRef] [PubMed]
- Torres, O.V.; Gentil, L.G.; Natividad, L.A.; Carcoba, L.M.; O’Dell, L.E. Behavioral, Biochemical, and Molecular Indices of Stress are Enhanced in Female Versus Male Rats Experiencing Nicotine Withdrawal. Front. Psychiatry 2013, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Birdogan, A.; Salur, E.; Tuzcu, F.; Gokmen, R.C.; Ozturk Bintepe, M.; Aypar, B.; Keser, A.; Balkan, B.; Koylu, E.O.; Kanit, L.; et al. Chronic oral nicotine administration and withdrawal regulate the expression of neuropeptide Y and its receptors in the mesocorticolimbic system. Neuropeptides 2021, 90, 102184. [Google Scholar] [CrossRef] [PubMed]
- Cadoni, C.; Di Chiara, G. Differential changes in accumbens shell and core dopamine in behavioral sensitization to nicotine. Eur. J. Pharmacol. 2000, 387, R23–R25. [Google Scholar] [CrossRef] [PubMed]
- Pfarr, S.; Schaaf, L.; Reinert, J.K.; Paul, E.; Herrmannsdorfer, F.; Rossmanith, M.; Kuner, T.; Hansson, A.C.; Spanagel, R.; Korber, C.; et al. Choice for Drug or Natural Reward Engages Largely Overlapping Neuronal Ensembles in the Infralimbic Prefrontal Cortex. J. Neurosci. 2018, 38, 3507–3519. [Google Scholar] [CrossRef]
- Huang, L.Z.; Parameswaran, N.; Bordia, T.; Michael McIntosh, J.; Quik, M. Nicotine is neuroprotective when administered before but not after nigrostriatal damage in rats and monkeys. J. Neurochem. 2009, 109, 826–837. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Hall, S.M.; Herning, R.I.; Jacob, P., 3rd; Jones, R.T.; Osman, A.L. Smokers of low-yield cigarettes do not consume less nicotine. N. Engl. J. Med. 1983, 309, 139–142. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Sweeney, P.; Bedenbaugh, M.N.; Maldonado, J.; Pan, P.; Fowler, K.; Williams, S.Y.; Gimenez, L.E.; Ghamari-Langroudi, M.; Downing, G.; Gui, Y.; et al. The melanocortin-3 receptor is a pharmacological target for the regulation of anorexia. Sci. Transl. Med. 2021, 13, abd6434. [Google Scholar] [CrossRef]
- Lippert, R.N.; Ellacott, K.L.; Cone, R.D. Gender-specific roles for the melanocortin-3 receptor in the regulation of the mesolimbic dopamine system in mice. Endocrinology 2014, 155, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- West, K.S.; Lu, C.; Olson, D.P.; Roseberry, A.G. Alpha-melanocyte stimulating hormone increases the activity of melanocortin-3 receptor-expressing neurons in the ventral tegmental area. J. Physiol. 2019, 597, 3217–3232. [Google Scholar] [CrossRef]
- Tapinc, D.E.; Ilgin, R.; Kaya, E.; Gozen, O.; Ugur, M.; Koylu, E.O.; Kanit, L.; Keser, A.; Balkan, B. Gene expression of pro-opiomelanocortin and melanocortin receptors is regulated in the hypothalamus and mesocorticolimbic system following nicotine administration. Neurosci. Lett. 2017, 637, 75–79. [Google Scholar] [CrossRef]
- Dunigan, A.I.; Swanson, A.M.; Olson, D.P.; Roseberry, A.G. Whole-brain efferent and afferent connectivity of mouse ventral tegmental area melanocortin-3 receptor neurons. J. Comp. Neurol. 2021, 529, 1157–1183. [Google Scholar] [CrossRef]
- Kim, S.R.; Kim, S.Y. Functional Dissection of Glutamatergic and GABAergic Neurons in the Bed Nucleus of the Stria Terminalis. Mol. Cells 2021, 44, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Lammel, S.; Lim, B.K.; Ran, C.; Huang, K.W.; Betley, M.J.; Tye, K.M.; Deisseroth, K.; Malenka, R.C. Input-specific control of reward and aversion in the ventral tegmental area. Nature 2012, 491, 212–217. [Google Scholar] [CrossRef]
- Sharp, B.M. Basolateral amygdala, nicotinic cholinergic receptors, and nicotine: Pharmacological effects and addiction in animal models and humans. Eur. J. Neurosci. 2019, 50, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Wirtshafter, H.S.; Wilson, M.A. Lateral septum as a nexus for mood, motivation, and movement. Neurosci. Biobehav. Rev. 2021, 126, 544–559. [Google Scholar] [CrossRef]
- Morales, M.; Margolis, E.B. Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 2017, 18, 73–85. [Google Scholar] [CrossRef]
- Roseberry, A.G. Altered feeding and body weight following melanocortin administration to the ventral tegmental area in adult rats. Psychopharmacology 2013, 226, 25–34. [Google Scholar] [CrossRef]
- Shanmugarajah, L.; Dunigan, A.I.; Frantz, K.J.; Roseberry, A.G. Altered sucrose self-administration following injection of melanocortin receptor agonists and antagonists into the ventral tegmental area. Psychopharmacology 2017, 234, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.H.; Roseberry, A.G. Decreased consumption of rewarding sucrose solutions after injection of melanocortins into the ventral tegmental area of rats. Psychopharmacology 2015, 232, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Bagnol, D.; Lu, X.Y.; Kaelin, C.B.; Day, H.E.; Ollmann, M.; Gantz, I.; Akil, H.; Barsh, G.S.; Watson, S.J. Anatomy of an endogenous antagonist: Relationship between Agouti-related protein and proopiomelanocortin in brain. J. Neurosci. 1999, 19, RC26. [Google Scholar] [CrossRef]
- Mounien, L.; Bizet, P.; Boutelet, I.; Vaudry, H.; Jegou, S. Expression of melanocortin MC3 and MC4 receptor mRNAs by neuropeptide Y neurons in the rat arcuate nucleus. Neuroendocrinology 2005, 82, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Renquist, B.J.; Lippert, R.N.; Sebag, J.A.; Ellacott, K.L.; Cone, R.D. Physiological roles of the melanocortin MC(3) receptor. Eur. J. Pharmacol. 2011, 660, 13–20. [Google Scholar] [CrossRef][Green Version]
- Lee, M.; Kim, A.; Conwell, I.M.; Hruby, V.; Mayorov, A.; Cai, M.; Wardlaw, S.L. Effects of selective modulation of the central melanocortin-3-receptor on food intake and hypothalamic POMC expression. Peptides 2008, 29, 440–447. [Google Scholar] [CrossRef]
- Kamdi, S.P.; Nakhate, K.T.; Dandekar, M.P.; Kokare, D.M.; Subhedar, N.K. Participation of corticotropin-releasing factor type 2 receptors in the acute, chronic and withdrawal actions of nicotine associated with feeding behavior in rats. Appetite 2009, 53, 354–362. [Google Scholar] [CrossRef]
- Kramer, P.R.; Kramer, S.F.; Marr, K.; Guan, G.; Wellman, P.J.; Bellinger, L.L. Nicotine administration effects on feeding and cocaine-amphetamine-regulated transcript (CART) expression in the hypothalamus. Regul. Pept. 2007, 138, 66–73. [Google Scholar] [CrossRef]
- Hsu, R.; Taylor, J.R.; Newton, S.S.; Alvaro, J.D.; Haile, C.; Han, G.; Hruby, V.J.; Nestler, E.J.; Duman, R.S. Blockade of melanocortin transmission inhibits cocaine reward. Eur. J. Neurosci. 2005, 21, 2233–2242. [Google Scholar] [CrossRef]
- Klawonn, A.M.; Fritz, M.; Nilsson, A.; Bonaventura, J.; Shionoya, K.; Mirrasekhian, E.; Karlsson, U.; Jaarola, M.; Granseth, B.; Blomqvist, A.; et al. Motivational valence is determined by striatal melanocortin 4 receptors. J. Clin. Investig. 2018, 128, 3160–3170. [Google Scholar] [CrossRef]
- Cui, H.; Lutter, M. The expression of MC4Rs in D1R neurons regulates food intake and locomotor sensitization to cocaine. Genes Brain Behav. 2013, 12, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.; van der Zwaal, E.M.; Luijendijk, M.C.; Brans, M.A.; van Rozen, A.J.; Oude Ophuis, R.J.; Vanderschuren, L.J.; Adan, R.A.; la Fleur, S.E. Central melanocortins regulate the motivation for sucrose reward. PLoS ONE 2015, 10, e0121768. [Google Scholar] [CrossRef] [PubMed]
- Kokare, D.M.; Dandekar, M.P.; Singru, P.S.; Gupta, G.L.; Subhedar, N.K. Involvement of alpha-MSH in the social isolation induced anxiety- and depression-like behaviors in rat. Neuropharmacology 2010, 58, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Itoga, C.A.; Chen, Y.; Fateri, C.; Echeverry, P.A.; Lai, J.M.; Delgado, J.; Badhon, S.; Short, A.; Baram, T.Z.; Xu, X. New viral-genetic mapping uncovers an enrichment of corticotropin-releasing hormone-expressing neuronal inputs to the nucleus accumbens from stress-related brain regions. J. Comp. Neurol. 2019, 527, 2474–2487. [Google Scholar] [CrossRef] [PubMed]
- Rodaros, D.; Caruana, D.A.; Amir, S.; Stewart, J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience 2007, 150, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Refojo, D.; Schweizer, M.; Kuehne, C.; Ehrenberg, S.; Thoeringer, C.; Vogl, A.M.; Dedic, N.; Schumacher, M.; von Wolff, G.; Avrabos, C.; et al. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science 2011, 333, 1903–1907. [Google Scholar] [CrossRef]
- Lemos, J.C.; Wanat, M.J.; Smith, J.S.; Reyes, B.A.; Hollon, N.G.; Van Bockstaele, E.J.; Chavkin, C.; Phillips, P.E. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature 2012, 490, 402–406. [Google Scholar] [CrossRef]
- Yarur, H.E.; Zegers, J.; Vega-Quiroga, I.; Novoa, J.; Ciruela, F.; Andres, M.E.; Gysling, K. Functional Interplay of Type-2 Corticotrophin Releasing Factor and Dopamine Receptors in the Basolateral Amygdala-Medial Prefrontal Cortex Circuitry. Int. J. Neuropsychopharmacol. 2021, 24, 221–228. [Google Scholar] [CrossRef]
- Wanat, M.J.; Hopf, F.W.; Stuber, G.D.; Phillips, P.E.; Bonci, A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J. Physiol. 2008, 586, 2157–2170. [Google Scholar] [CrossRef]
- Verharen, J.P.H.; Zhu, Y.; Lammel, S. Aversion hot spots in the dopamine system. Curr. Opin. Neurobiol. 2020, 64, 46–52. [Google Scholar] [CrossRef]
- Cipriano, A.C.; Gomes, K.S.; Nunes-de-Souza, R.L. CRF receptor type 1 (but not type 2) located within the amygdala plays a role in the modulation of anxiety in mice exposed to the elevated plus maze. Horm. Behav. 2016, 81, 59–67. [Google Scholar] [CrossRef]
- Chen, Y.W.; Rada, P.V.; Butzler, B.P.; Leibowitz, S.F.; Hoebel, B.G. Corticotropin-releasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance. Neuroscience 2012, 206, 155–166. [Google Scholar] [CrossRef]
- Marcinkiewcz, C.A.; Prado, M.M.; Isaac, S.K.; Marshall, A.; Rylkova, D.; Bruijnzeel, A.W. Corticotropin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacology 2009, 34, 1743–1752. [Google Scholar] [CrossRef]
- Bruijnzeel, A.W.; Ford, J.; Rogers, J.A.; Scheick, S.; Ji, Y.; Bishnoi, M.; Alexander, J.C. Blockade of CRF1 receptors in the central nucleus of the amygdala attenuates the dysphoria associated with nicotine withdrawal in rats. Pharmacol. Biochem. Behav. 2012, 101, 62–68. [Google Scholar] [CrossRef]
- Qi, X.; Shan, Z.; Ji, Y.; Guerra, V.; Alexander, J.C.; Ormerod, B.K.; Bruijnzeel, A.W. Sustained AAV-mediated overexpression of CRF in the central amygdala diminishes the depressive-like state associated with nicotine withdrawal. Transl. Psychiatry 2014, 4, e385. [Google Scholar] [CrossRef]
- Chen, P.; Lou, S.; Huang, Z.H.; Wang, Z.; Shan, Q.H.; Wang, Y.; Yang, Y.; Li, X.; Gong, H.; Jin, Y.; et al. Prefrontal Cortex Corticotropin-Releasing Factor Neurons Control Behavioral Style Selection under Challenging Situations. Neuron 2020, 106, 301–315.e7. [Google Scholar] [CrossRef]
- Baumgartner, H.M.; Schulkin, J.; Berridge, K.C. Activating Corticotropin-Releasing Factor Systems in the Nucleus Accumbens, Amygdala, and Bed Nucleus of Stria Terminalis: Incentive Motivation or Aversive Motivation? Biol. Psychiatry 2021, 89, 1162–1175. [Google Scholar] [CrossRef]
- Semba, J.; Wakuta, M.; Maeda, J.; Suhara, T. Nicotine withdrawal induces subsensitivity of hypothalamic-pituitary-adrenal axis to stress in rats: Implications for precipitation of depression during smoking cessation. Psychoneuroendocrinology 2004, 29, 215–226. [Google Scholar] [CrossRef]


| Gene | Sense | Antisense | Probe |
|---|---|---|---|
| CRF (NM_031019) | cccaagtacgttgagaaa | ctctcttctcctcccttg | tgagcccgcactgttgttct |
| CRF1 (NM_001301812) | gtccagtcaggagataac | gcagaatagtaagagtctaatg | agcattatcagaccgcactcca |
| CRF2 (NM_022714) | gaaggtccctactcctac | atgccgttgaagtattcg | caacacgaccttggaccagat |
| MC3R (NM_001025270) | gccgataaccatgaactc | tctggcttgatgaaaacc | cctcttatccgacgctgcctaa |
| MC4R (NM_013099) | gagtgaatactacggctaa | tgctcatcatttctttagaag | ctctcggctgaccagtctgc |
| Actb (NM_031144) | cccgcgagtacaaccttct | cgtcatccatggcgaact | agctcctccgtcgccggtcca |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gozen, O.; Aypar, B.; Ozturk Bintepe, M.; Tuzcu, F.; Balkan, B.; Koylu, E.O.; Kanit, L.; Keser, A. Chronic Nicotine Consumption and Withdrawal Regulate Melanocortin Receptor, CRF, and CRF Receptor mRNA Levels in the Rat Brain. Brain Sci. 2024, 14, 63. https://doi.org/10.3390/brainsci14010063
Gozen O, Aypar B, Ozturk Bintepe M, Tuzcu F, Balkan B, Koylu EO, Kanit L, Keser A. Chronic Nicotine Consumption and Withdrawal Regulate Melanocortin Receptor, CRF, and CRF Receptor mRNA Levels in the Rat Brain. Brain Sciences. 2024; 14(1):63. https://doi.org/10.3390/brainsci14010063
Chicago/Turabian StyleGozen, Oguz, Buket Aypar, Meliha Ozturk Bintepe, Fulya Tuzcu, Burcu Balkan, Ersin O. Koylu, Lutfiye Kanit, and Aysegul Keser. 2024. "Chronic Nicotine Consumption and Withdrawal Regulate Melanocortin Receptor, CRF, and CRF Receptor mRNA Levels in the Rat Brain" Brain Sciences 14, no. 1: 63. https://doi.org/10.3390/brainsci14010063
APA StyleGozen, O., Aypar, B., Ozturk Bintepe, M., Tuzcu, F., Balkan, B., Koylu, E. O., Kanit, L., & Keser, A. (2024). Chronic Nicotine Consumption and Withdrawal Regulate Melanocortin Receptor, CRF, and CRF Receptor mRNA Levels in the Rat Brain. Brain Sciences, 14(1), 63. https://doi.org/10.3390/brainsci14010063






