Localization of Vestibular Cortex Using Electrical Cortical Stimulation: A Systematic Literature Review
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Data Extraction Evidence Synthesis
2.5. Quality Evaluation
3. Results
3.1. Literature Search
3.2. Quality of the Evidence
| Penfield (1957, Canada) [2] | |
| Methods | Retrospective case series |
| Participants | 108 patients undergoing exploratory craniotomy |
| Interventions | Gentle electrical stimulation of the cerebral cortex |
| Outcome | Vestibular sensation |
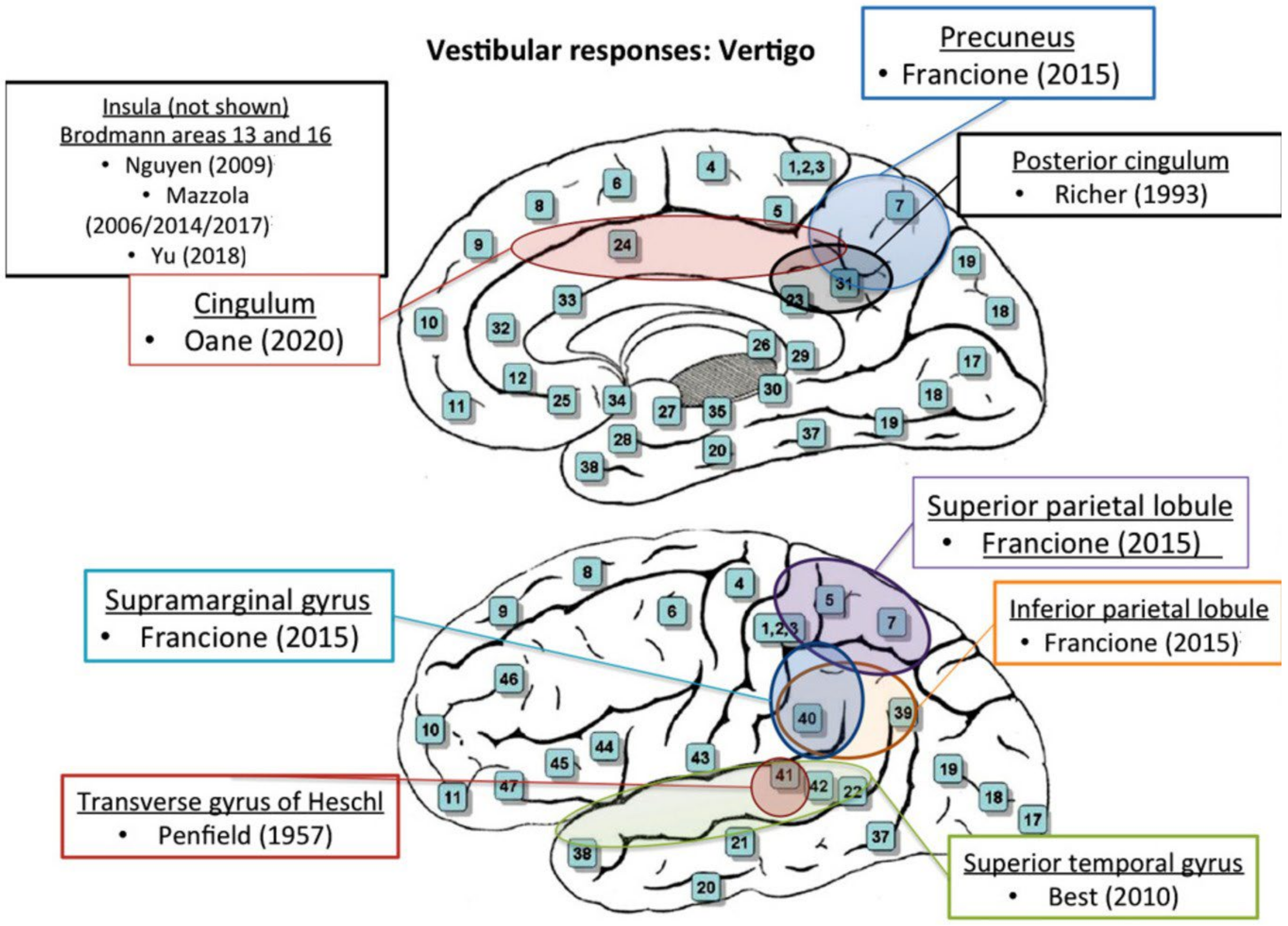
| Notes | Stimulation of the transverse gyrus, the parietal lobe, and occipital cortex elicited dizziness, sensation of rotation or body displacement, and conjugate deviation of the eyes with dizziness, respectively |
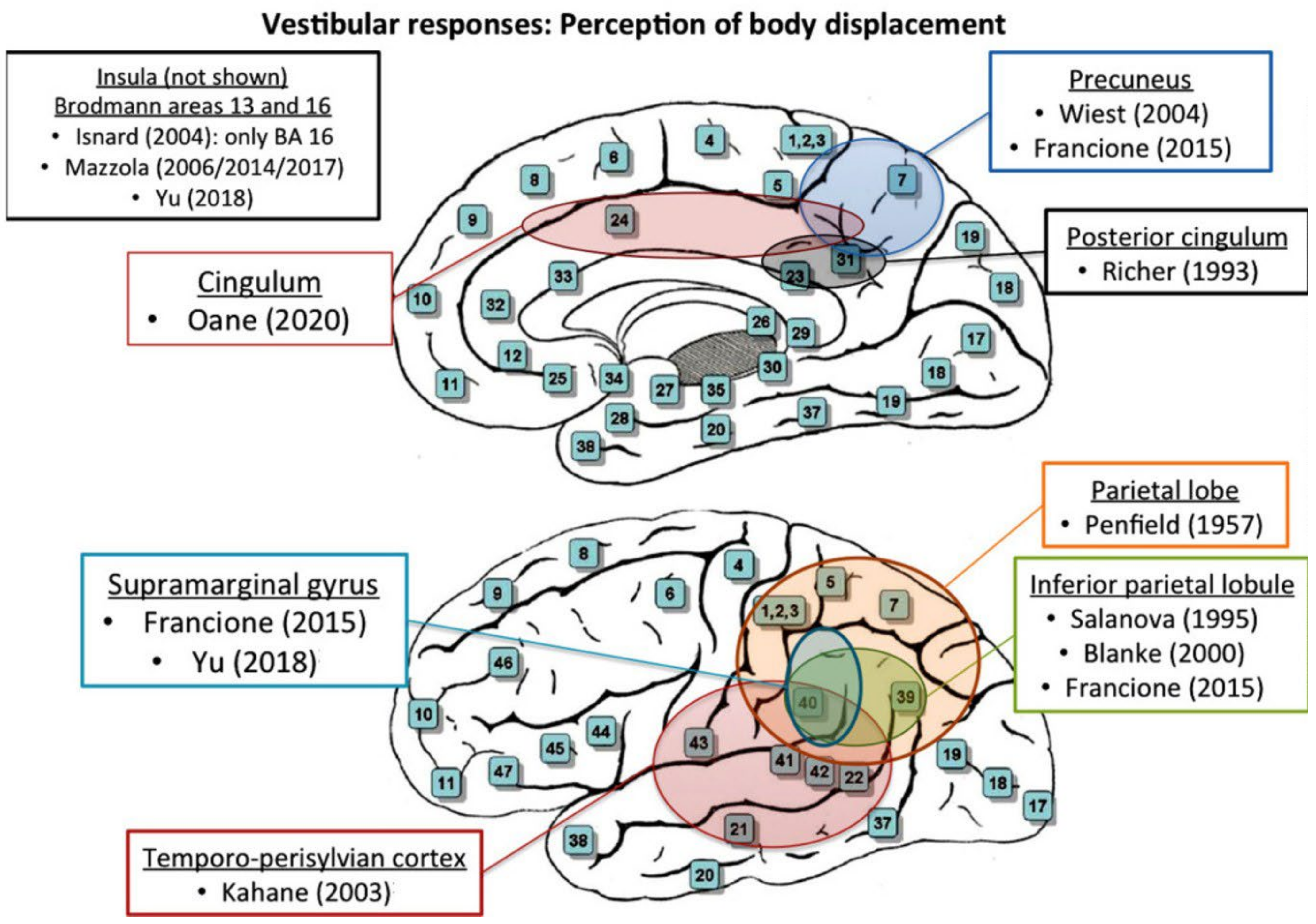
| Richer (1993, Canada) [18] | |
| Methods | Retrospective case series |
| Participants | 40 patients undergoing preoperative evaluation for DRE |
| Interventions | Bipolar stimulation of the somatosensory cortex (rolandic and parietal regions) |
| Outcome | Somatosensory responses |
| Notes | 3 out of 12 stimulation sites in medial parietal regions elicited perceptions of altered body position |
| Fish (1994, Canada) [19] | |
| Methods | Retrospective case series |
| Participants | 75 patients undergoing preoperative evaluation for DRE |
| Interventions | Bipolar stimulation of the temporal and frontal lobes |
| Outcome | Responses without after-discharge spreading beyond the site of stimulation |
| Notes | Vague cephalic sensations or dizziness occurred in seven patients, in one with hippocampal stimulation, and in six with stimulation of the amygdala. Five out of seven patients reported similar findings during spontaneous seizures |
| Salanova (1995, Canada) [20] | |
| Methods | Retrospective case series |
| Participants | 80 patients with parietal lobe epilepsy |
| Interventions | Parietal lobe resections |
| Outcome | Clinical manifestations and outcomes |
| Notes | During intra-operative monopolar cortical stimulation of the parietal lobe, one patient reported a sensation of ‘rolling’ off the table. Two patients developed a disturbance of body image; one felt a twisting sensation in the contralateral extremity, and another stated that ‘I just swayed’ following stimulation of the non-dominant inferior parietal lobule |
| Blanke (2000, Switzerland) [28] | |
| Methods | Retrospective case report |
| Participants | 1 patient undergoing preoperative evaluation for DRE |
| Interventions | Electrical cortical stimulation using subdural grid electrodes covering the covering parts of the left frontal, parietal, and temporal lobe |
| Outcome | Vestibular responses |
| Notes | Vestibular responses were evoked after stimulation of the inferior parietal lobule at the anterior part of the intraparietal sulcus |
| Kahane (2003, France) [12] | |
| Methods | Retrospective case series |
| Participants | 260 patients undergoing preoperative evaluation for DRE |
| Interventions | Electrical cortical stimulation using 5 to 16 semirigid electrodes implanted per patient, in various cortical areas depending on the suspected origin of seizures |
| Outcome | Vestibular responses |
| Notes | Vestibular symptoms were induced on 44 anatomical sites in 28 patients. The patients experienced illusions of rotation (n = 30), translations (n = 6), or indefinable feelings of body motion (n = 8). Almost all vestibular sites were located in the cortex (41/44): in the temporal (n = 19), parietal (n = 14), frontal (n = 5), occipital (n = 2), and insular (n = 1) lobes. Among these sites, the authors identified a lateral cortical temporo-parietal area we called the temporo-peri-Sylvian vestibular cortex, from which vestibular symptoms, and above all rotatory sensations, were particularly easily elicited (24/41 cortical sites, 58.5%) |
| Wiest (2004, Austria) [29] | |
| Methods | Retrospective case report |
| Participants | 1 patient undergoing preoperative evaluation for DRE due to an ependymoma |
| Interventions | Resective surgery |
| Outcome | Clinical manifestations, electrophysiologic findings, overall outcome |
| Notes | Electrical cortical stimulation of the precuneus could reproduce the vestibular sensations of linear self-motion and occasionally body tilts, which ceased after lesionectomy |
| Isnard (2004, France) [21] | |
| Methods | Retrospective case series |
| Participants | 50 patients undergoing preoperative evaluation for DRE |
| Interventions | Direct electric stimulation of the insular cortex using implanted transopercular electrodes |
| Outcome | Responses that were evoked in the absence of any after-discharge |
| Notes | From a total of 125 responses, whole-body sensations (n = 5; 4%) occurred in six patients, as a sudden sensation of displacement of their body in space |
| Mazzola (2006, France) [17] | |
| Methods | Retrospective cohort study |
| Participants | 14 patients undergoing preoperative evaluation for DRE |
| Interventions | Direct electrical cortical stimulation using transopercular electrodes exploring the following:
|
| Outcome | Somatosensory and pain responses |
| Notes | Multiple non-somatosensory responses were obtained after stimulation of the insula, including vestibular sensations such as vertigo or horizontal rotation of the body |
| Nguyen (2009, Canada) [22] | |
| Methods | Retrospective case series |
| Participants | 10 patients undergoing preoperative evaluation for DRE |
| Interventions | Direct current insular stimulation using depth electrodes, placed under direct vision after microsurgical opening of the Sylvian fissure |
| Outcome | Elicited responses |
| Notes | Electrical cortical stimulation performed in 9 of 10 patients with insular electrodes elicited, in decreasing order of frequency, somatosensory, viscero-sensory, motor, auditory, vestibular, and speech symptoms. Vertigo (3%) was rare |
| Best (2010, Germany) [30] | |
| Methods | Retrospective case report |
| Participants | 1 patient undergoing preoperative stimulation for DRE |
| Interventions | Electrical cortical stimulation using three subdural electrodes to the temporal cortex and temporo-occipital junction, and one depth electrode toward the posterior insular cortex |
| Outcome | Clinical manifestations after cortical stimulation |
| Notes | Stimulation over the medial part of the STG elicited intracranial numbness, ipsilateral headache, and a subjective unidirectional vertigo |
| Mazzola (2014, France) [23] | |
| Methods | Retrospective case series |
| Participants | 219 patients undergoing preoperative evaluation for DRE |
| Interventions | 642 electrical stimulations of the insula, using stereotactically implanted depth electrodes |
| Outcome | Insular mapping of vestibular sensations |
| Notes | Vestibular sensations occurred in 7.6% of the 541 evoked sensations after electrical stimulations of the insula. They were mostly obtained after stimulation of the posterior insula, that is, in the granular insular cortex and the postcentral insular gyrus. The authors suggested a spatial segregation of the responses in the insula, with the rotatory and translational vestibular sensations being evoked at more posterior stimulation sites than other less definable vestibular sensations. No left–right differences were observed. |
| Francione (2015, Italy) [25] | |
| Methods | Retrospective case series |
| Participants | 40 patients undergoing preoperative evaluation for DRE of surgical resections strictly confined to the parietal lobe |
| Interventions | Surgical resection of parietal lobe structures |
| Outcome | Anatomo-electro-clinical features and clinical outcome |
| Notes | The most frequent responses induced by electrical cortical stimulations in the parietal lobe structures, besides the simple somatosensory manifestations, consisted of vertiginous sensations and visual illusions. Vestibular sensations were clinically variable |
| Mazzola (2017, France) [24] | |
| Methods | Retrospective case series |
| Participants | 222 patients undergoing preoperative evaluation for DRE |
| Interventions | 669 electrical stimulations of the insula, using stereotactically implanted depth electrodes |
| Outcome | Clinical manifestations after insular stimulation |
| Notes | Somatosensory responses (61% of evoked sensations) including pain and visceral sensations (14.9%) were the most frequent, followed by auditory sensations (8%), vestibular illusions (7.5%), speech impairment (5%), gustatory, (2.7%), and olfactory (1%) sensations. The authors reported that although these responses showed some functional segregation (in particular, a privileged pain representation in the postero-superior quadrant), there was a clear spatial overlap between representations of the different modalities |
| Yu (2018, China) [26] | |
| Methods | Retrospective case series |
| Participants | 43 patients undergoing preoperative evaluation for DRE |
| Interventions | Bipolar electrical stimulation with at least 1 electrode inserted into the insula or opercula via an oblique approach |
| Outcome | Clinical manifestations after electrical stimulation |
| Notes | A total of 6 responses involving vestibular symptoms out of 142 were related to left insula. Stimulation of the opercula evoked 10 vestibular responses; 2 cases with vertigo at 1.0 mA; 3 cases were sensations of deficiency of the contralateral limb or trunk at 1.0–2.0 mA; and 5 changes in the perception of the body’s location, at 1.0–4.0 mA |
| Oane (2020, Romania) [27] | |
| Methods | Retrospective case series |
| Participants | 47 patients undergoing preoperative evaluation for DRE |
| Interventions | Depth electrodes in the cingulate cortex stereotactically placed using Leksel frame |
| Outcome | Cingulate cortex function and multi-modal connectivity mapping |
| Notes | Vestibular responses, defined as vertigo and dizziness, were elicited in 8 sites in the ACC, anterior MCC and PCC areas a24, p24, RSC, 31a, 23d. Connectivity analysis of these areas highlights important connections with lateral and mesial parietal regions, parietal operculum, and prefrontal cortex. Body perception responses, defined as altered perception related to location, gravity, or displacement of whole-body or body-parts, were elicited by stimulating 8 sites in MCC, PCC, and RSC. |
3.3. Elicited Vestibular Responses (Q1)
3.4. Anatomic Correlations (Q2)
3.5. Left vs. Right-Sided Stimulation
3.6. Intensity and Frequency of Stimulation (Q3)
3.7. Other Non-Vestibular Manifestations (Q4)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gizzi, M.; Diamond, S. Dizziness of Vestibular Problems Resembling Seizures. In Imitators of Epilepsy, 2nd ed.; Springer Publishing Company: New York, NY, USA, 2005; pp. 145–169. [Google Scholar]
- Penfield, W. Vestibular Sensation and the Cerebral Cortex. Ann. Otol. Rhinol. Laryngol. 1957, 66, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Zu Eulenburg, P.; Caspers, S.; Roski, C.; Eickhoff, S.B. Meta-Analytical Definition and Functional Connectivity of the Human Vestibular Cortex. Neuroimage 2012, 60, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, M.; Brandt, T. Functional Brain Imaging of Peripheral and Central Vestibular Disorders. Brain 2008, 131 Pt 10, 2538–2552. [Google Scholar] [CrossRef]
- Lopez, C.; Blanke, O. The Thalamocortical Vestibular System in Animals and Humans. Brain Res. Rev. 2011, 67, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Alpers, B.J. Vertiginous Epilepsy. Laryngoscope 1960, 70, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Barba, C.; Barbati, G.; Minotti, L.; Hoffmann, D.; Kahane, P. Ictal Clinical and Scalp-EEG Findings Differentiating Temporal Lobe Epilepsies from Temporal “plus” Epilepsies. Brain 2007, 130, 1957–1967. [Google Scholar] [CrossRef]
- Bartolomei, F.; Gavaret, M.; Hewett, R.; Valton, L.; Aubert, S.; Régis, J.; Wendling, F.; Chauvel, P. Neural Networks Underlying Parietal Lobe Seizures: A Quantified Study from Intracerebral Recordings. Epilepsy Res. 2011, 93, 164–176. [Google Scholar] [CrossRef]
- Berkovic, S.F.; Crompton, D.E. The Borderland of Epilepsy: A Clinical and Molecular View, 100 Years On. Epilepsia 2010, 51 (Suppl. S1), 3–4. [Google Scholar] [CrossRef]
- Bladin, P.F. History of “Epileptic Vertigo”: Its Medical, Social, and Forensic Problems. Epilepsia 1998, 39, 442–447. [Google Scholar] [CrossRef]
- Gordon, A.G. Link between Vertigo and Epilepsy. Epilepsia 1999, 40, 1168–1169. [Google Scholar] [CrossRef]
- Kahane, P.; Hoffmann, D.; Minotti, L.; Berthoz, A. Reappraisal of the Human Vestibular Cortex by Cortical Electrical Stimulation Study. Ann. Neurol. 2003, 54, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Hewett, R.; Bartolomei, F. Epilepsy and the Cortical Vestibular System: Tales of Dizziness and Recent Concepts. Front. Integr. Neurosci. 2013, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Duque-Parra, J.E. Perspective on the Vestibular Cortex throughout History. Anat. Rec. Part B New Anat. 2004, 280, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Wieling, W.; Thijs, R.D.; van Dijk, N.; Wilde, A.A.M.; Benditt, D.G.; van Dijk, J.G. Symptoms and Signs of Syncope: A Review of the Link between Physiology and Clinical Clues. Brain 2009, 132 Pt 10, 2630–2642. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, L.; Isnard, J.; Mauguière, F. Somatosensory and Pain Responses to Stimulation of the Second Somatosensory Area (SII) in Humans. A Comparison with SI and Insular Responses. Cereb. Cortex 2006, 16, 960–968. [Google Scholar] [CrossRef]
- Richer, F.; Martinez, M.; Robert, M.; Bouvier, G.; Saint-Hilaire, J.M. Stimulation of Human Somatosensory Cortex: Tactile and Body Displacement Perceptions in Medial Regions. Exp. Brain Res. 1993, 93, 173–176. [Google Scholar] [CrossRef]
- Fish, D.R.; Gloor, P.; Quesney, F.L.; Oliver, A. Clinical Responses to Electrical Brain Stimulation of the Temporal and Frontal Lobes in Patients with Epilepsy: Pathophysiological Implications. Brain 1993, 116, 397–414. [Google Scholar] [CrossRef]
- Salanova, V.; Andermann, F.; Rasmussen, T.; Olivier, A.; Quesney, L.F. Parietal Lobe Epilepsy Clinical Manifestations and Outcome in 82 Patients Treated Surgically between 1929 and 1988. Brain 1995, 118, 607–627. [Google Scholar] [CrossRef]
- Isnard, J.; Guénot, M.; Sindou, M.; Mauguière, F. Clinical Manifestations of Insular Lobe Seizures: A Stereo- Electroencephalographic Study. Epilepsia 2004, 45, 1079–1090. [Google Scholar] [CrossRef]
- Nguyen, D.K.; Nguyen, D.B.; Malak, R.; Leroux, J.M.; Carmant, L.; Saint-Hilaire, J.M.; Giard, N.; Cossette, P.; Bouthillier, A. Revisiting the Role of the Insula in Refractory Partial Epilepsy. Epilepsia 2009, 50, 510–520. [Google Scholar] [CrossRef]
- Mazzola, L.; Lopez, C.; Faillenot, I.; Chouchou, F.; Mauguière, F.; Isnard, J. Vestibular Responses to Direct Stimulation of the Human Insular Cortex. Ann. Neurol. 2014, 76, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, L.; Mauguière, F.; Isnard, J. Electrical Stimulations of the Human Insula: Their Contribution to the Ictal Semiology of Insular Seizures. J. Clin. Neurophysiol. 2017, 34, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Francione, S.; Liava, A.; Mai, R.; Nobili, L.; Sartori, I.; Tassi, L.; Scarpa, P.; Cardinale, F.; Castana, L.; Cossu, M.; et al. Drug-Resistant Parietal Epilepsy: Polymorphic Ictal Semiology Does Not Preclude Good Post-Surgical Outcome. Epileptic Disord. 2015, 17, 32–46. [Google Scholar] [CrossRef]
- Yu, K.; Yu, T.; Qiao, L.; Liu, C.; Wang, X.; Zhou, X.; Ni, D.; Zhang, G.; Li, Y. Electrical Stimulation of the Insulo-Opercular Region: Visual Phenomena and Altered Body-Ownership Symptoms. Epilepsy Res. 2018, 148, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Oane, I.; Barborica, A.; Chetan, F.; Donos, C.; Maliia, M.D.; Arbune, A.A.; Daneasa, A.; Pistol, C.; Nica, A.E.; Bajenaru, O.A.; et al. Cingulate Cortex Function and Multi-Modal Connectivity Mapped Using Intracranial Stimulation. Neuroimage 2020, 220, 117059. [Google Scholar] [CrossRef] [PubMed]
- Blanke, O.; Perrig, S.; Thut, G.; Landis, T.; Seeck, M. Simple and Complex Vestibular Responses Induced by Electrical Cortical Stimulation of the Parietal Cortex in Humans. J. Neurol. Neurosurg. Psychiatry 2000, 69, 553–556. [Google Scholar] [CrossRef]
- Wiest, G.; Zimprich, F.; Prayer, D.; Czech, T.; Serles, W.; Baumgartner, C. Vestibular Processing in Human Paramedian Precuneus as Shown by Electrical Cortical Stimulation. Neurology 2004, 62, 473–475. [Google Scholar] [CrossRef]
- Best, C.; Stefan, H.; Hopfengaertner, R.; Dieterich, M. Effects of Electrical Stimulation in Vestibular Cortex Areas in Humans. J. Neurol. Sci. 2010, 290, 157–162. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE Guidelines: 1. Introduction-GRADE Evidence Profiles and Summary of Findings Tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Blanke, O.; Ortigue, S.; Landis, T.; Seeck, M. Stimulating Illusory Own-Body Perceptions. Nature 2002, 419, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Guldin, W.O.; Grüsser, O.J. Is There a Vestibular Cortex? Trends Neurosci. 1998, 21, 254–259. [Google Scholar] [CrossRef]
- Lopez, C.; Blanke, O.; Mast, F.W. The Human Vestibular Cortex Revealed by Coordinate-Based Activation Likelihood Estimation Meta-Analysis. Neuroscience 2012, 212, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Baloh, R.W. Prosper Ménière and His Disease. Arch. Neurol. 2001, 58, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Hewett, R.; Guye, M.; Gavaret, M.; Bartolomei, F. Benign Temporo-Parieto-Occipital Junction Epilepsy with Vestibular Disturbance: An Underrecognized Form of Epilepsy? Epilepsy Behav. 2011, 21, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, S.L.; DellaBadia, J.; Erbayat-Altay, E.; Thio, L.L. Rotational Vestibular Epilepsy from the Temporo-Parieto-Occipital Junction. Neurology 2006, 67, 368. [Google Scholar] [CrossRef]
- Jepsen, O.; Pedersen, E. Epileptic Vertigo. Acta Psychiatr. Neurol. Scand. Suppl. 1956, 108, 301–310. [Google Scholar] [CrossRef]
- Karbowski, K. Epileptic Seizures Induced by Vestibular and Auditory Stimuly. In Medicine and Hygiene; Médecine & Hygiène: Geneve, Switzerland, 1989. [Google Scholar]
- Maillard, L.; Vignal, J.-P.; Gavaret, M.; Guye, M.; Biraben, A.; McGonigal, A.; Chauvel, P.; Bartolomei, F. Semiologic and Electrophysiologic Correlations in Temporal Lobe Seizure Subtypes. Epilepsia 2004, 45, 1590–1599. [Google Scholar] [CrossRef]
- Brandt, T. Phobic Postural Vertigo. Neurology 1996, 46, 1515–1519. [Google Scholar] [CrossRef]
- Hughlings Jackson, J. On the Scientific and Empirical Investigations of Epilepsies, London, UK, 1931.
- Foerster, O. Sensible Corticale Felder. Handb. Neurol. 1936, 6, 358–448. [Google Scholar]
- Brandt, T.; Dieterich, M. The Vestibular Cortex. Its Locations, Functions, and Disorders. Ann. N. Y. Acad. Sci. 1999, 871, 293–312. [Google Scholar] [CrossRef]
- Della-Justina, H.M.; Gamba, H.R.; Lukasova, K.; Nucci-da-Silva, M.P.; Winkler, A.M.; Amaro, E. Interaction of Brain Areas of Visual and Vestibular Simultaneous Activity with fMRI. Exp. Brain Res. 2015, 233, 237–252. [Google Scholar] [CrossRef]
- Brandt, T.; Glasauer, S.; Stephan, T.; Bense, S.; Yousry, T.A.; Deutschlander, A.; Dieterich, M. Visual-Vestibular and Visuovisual Cortical Interaction: New Insights from fMRI and Pet. Ann. N. Y. Acad. Sci. 2002, 956, 230–241. [Google Scholar] [CrossRef]
- Karim, H.T.; Fuhrman, S.I.; Furman, J.M.; Huppert, T.J. Neuroimaging to Detect Cortical Projection of Vestibular Response to Caloric Stimulation in Young and Older Adults Using Functional Near-Infrared Spectroscopy (fNIRS). Neuroimage 2013, 76, 1–10. [Google Scholar] [CrossRef]
- Aedo-Jury, F.; Cottereau, B.R.; Celebrini, S.; Séverac Cauquil, A. Antero-Posterior vs. Lateral Vestibular Input Processing in Human Visual Cortex. Front. Integr. Neurosci. 2020, 14, 43. [Google Scholar] [CrossRef]
- Devantier, L.; Hansen, A.K.; Mølby-Henriksen, J.; Christensen, C.B.; Pedersen, M.; Hansen, K.V.; Magnusson, M.; Ovesen, T.; Borghammer, P. Positron Emission Tomography Visualized Stimulation of the Vestibular Organ Is Localized in Heschl’s Gyrus. Hum. Brain Mapp. 2020, 41, 185–193. [Google Scholar] [CrossRef]
- Di Marco, S.; Sulpizio, V.; Bellagamba, M.; Fattori, P.; Galati, G.; Galletti, C.; Lappe, M.; Maltempo, T.; Pitzalis, S. Multisensory Integration in Cortical Regions Responding to Locomotion-related Visual and Somatomotor Signals. Neuroimage 2021, 244, 118581. [Google Scholar] [CrossRef]
- Ibitoye, R.T.; Mallas, E.-J.; Bourke, N.J.; Kaski, D.; Bronstein, A.M.; Sharp, D.J. The Human Vestibular Cortex: Functional Anatomy of OP2, Its Connectivity and the Effect of Vestibular Disease. Cereb. Cortex 2023, 33, 567–582. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Takakura, H.; Nishijo, H.; Ueda, N.; Ito, S.; Fujisaka, M.; Akaogi, K.; Shojaku, H. Cerebral Hemodynamic Responses to the Sensory Conflict Between Visual and Rotary Vestibular Stimuli: An Analysis With a Multichannel Near-Infrared Spectroscopy (NIRS) System. Front. Hum. Neurosci. 2020, 14, 125. [Google Scholar] [CrossRef]
- Ruehl, R.M.; Flanagin, V.L.; Ophey, L.; Raiser, T.M.; Seiderer, K.; Ertl, M.; Conrad, J.; Zu Eulenburg, P. The Human Egomotion Network. NeuroImage 2022, 264, 119715. [Google Scholar] [CrossRef] [PubMed]
- Beer, A.L.; Becker, M.; Frank, S.M.; Greenlee, M.W. Vestibular and Visual Brain Areas in the Medial Cortex of the Human Brain. J. Neurophysiol. 2023, 129, 948–962. [Google Scholar] [CrossRef]
- Dlugaiczyk, J.; Gensberger, K.D.; Straka, H. Galvanic Vestibular Stimulation: From Basic Concepts to Clinical Applications. J. Neurophysiol. 2019, 121, 2237–2255. [Google Scholar] [CrossRef]
- Hernández-Román, J.; Montero-Hernández, S.; Vega, R.; Orihuela-Espina, F.; Soto, E. Galvanic Vestibular Stimulation Activates the Parietal and Temporal Cortex in Humans: A Functional near-Infrared Spectroscopy (fNIRS) Study. Eur. J. Neurosci. 2023, 58, 2267–2277. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Taoka, M.; Obayashi, S.; Hara, Y.; Tanaka, M.; Iriki, A. Modulation of Cortical Vestibular Processing by Somatosensory Inputs in the Posterior Insula. Brain Inj. 2013, 27, 1685–1691. [Google Scholar] [CrossRef]
- Baier, B.; Zu Eulenburg, P.; Best, C.; Geber, C.; Müller-Forell, W.; Birklein, F.; Dieterich, M. Posterior Insular Cortex—A Site of Vestibular-Somatosensory Interaction? Brain Behav. 2013, 3, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Boiten, J.; Wilmink, J.; Kingma, H. Acute Rotatory Vertigo Caused by a Small Haemorrhage of the Vestibular Cortex. J. Neurol. Neurosurg. Psychiatry 2003, 74, 388. [Google Scholar] [CrossRef] [PubMed]
- Cullen, K.E. The Vestibular System: Multimodal Integration and Encoding of Self-Motion for Motor Control. Trends Neurosci. 2012, 35, 185–196. [Google Scholar] [CrossRef]
- Lopez, C.; Heydrich, L.; Seeck, M.; Blanke, O. Abnormal Self-Location and Vestibular Vertigo in a Patient with Right Frontal Lobe Epilepsy. Epilepsy Behav. 2010, 17, 289–292. [Google Scholar] [CrossRef]
- McCarthy, B.; Datta, S.; Sesa-Ashton, G.; Wong, R.; Henderson, L.A.; Dawood, T.; Macefield, V.G. Top-down Control of Vestibular Inputs by the Dorsolateral Prefrontal Cortex. Exp. Brain Res. 2023, 241, 2845–2853. [Google Scholar] [CrossRef]
- Indovina, I.; Bosco, G.; Riccelli, R.; Maffei, V.; Lacquaniti, F.; Passamonti, L.; Toschi, N. Structural Connectome and Connectivity Lateralization of the Multimodal Vestibular Cortical Network. Neuroimage 2020, 222, 117247. [Google Scholar] [CrossRef]
- Li, Y. A Vertigo Network Derived from Human Brain Lesions and Brain Stimulation. Brain Commun. 2023, 5, fcad071. [Google Scholar] [CrossRef]
- Bisdorff, A.R. Management of Vestibular Migraine. Ther. Adv. Neurol. Disord. 2011, 4, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.-H. Migraine-Associated Vertigo: Diagnosis and Treatment. Semin. Neurol. 2010, 30, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Zhe, X.; Gao, J.; Chen, L.; Zhang, D.; Tang, M.; Yan, X.; Bai, F.; Zhang, X.; Zou, Z.; Chen, W.; et al. Altered Structure of the Vestibular Cortex in Patients with Vestibular Migraine. Brain Behav. 2020, 10, e01572. [Google Scholar] [CrossRef]
- Zhe, X.; Zhang, X.; Chen, L.; Zhang, L.; Tang, M.; Zhang, D.; Li, L.; Lei, X.; Jin, C. Altered Gray Matter Volume and Functional Connectivity in Patients With Vestibular Migraine. Front. Neurosci. 2021, 15, 683802. [Google Scholar] [CrossRef] [PubMed]
- Raiser, T.M.; Flanagin, V.L.; Duering, M.; Van Ombergen, A.; Ruehl, R.M.; Zu Eulenburg, P. The Human Corticocortical Vestibular Network. Neuroimage 2020, 223, 117362. [Google Scholar] [CrossRef]
- Dieterich, M.; Bense, S.; Lutz, S.; Drzezga, A.; Stephan, T.; Bartenstein, P.; Brandt, T. Dominance for Vestibular Cortical Function in the Non-Dominant Hemisphere. Cereb. Cortex 2003, 13, 994–1007. [Google Scholar] [CrossRef]
- Dieterich, M.; Brandt, T. Global Orientation in Space and the Lateralization of Brain Functions. Curr. Opin. Neurol. 2018, 31, 96–104. [Google Scholar] [CrossRef]
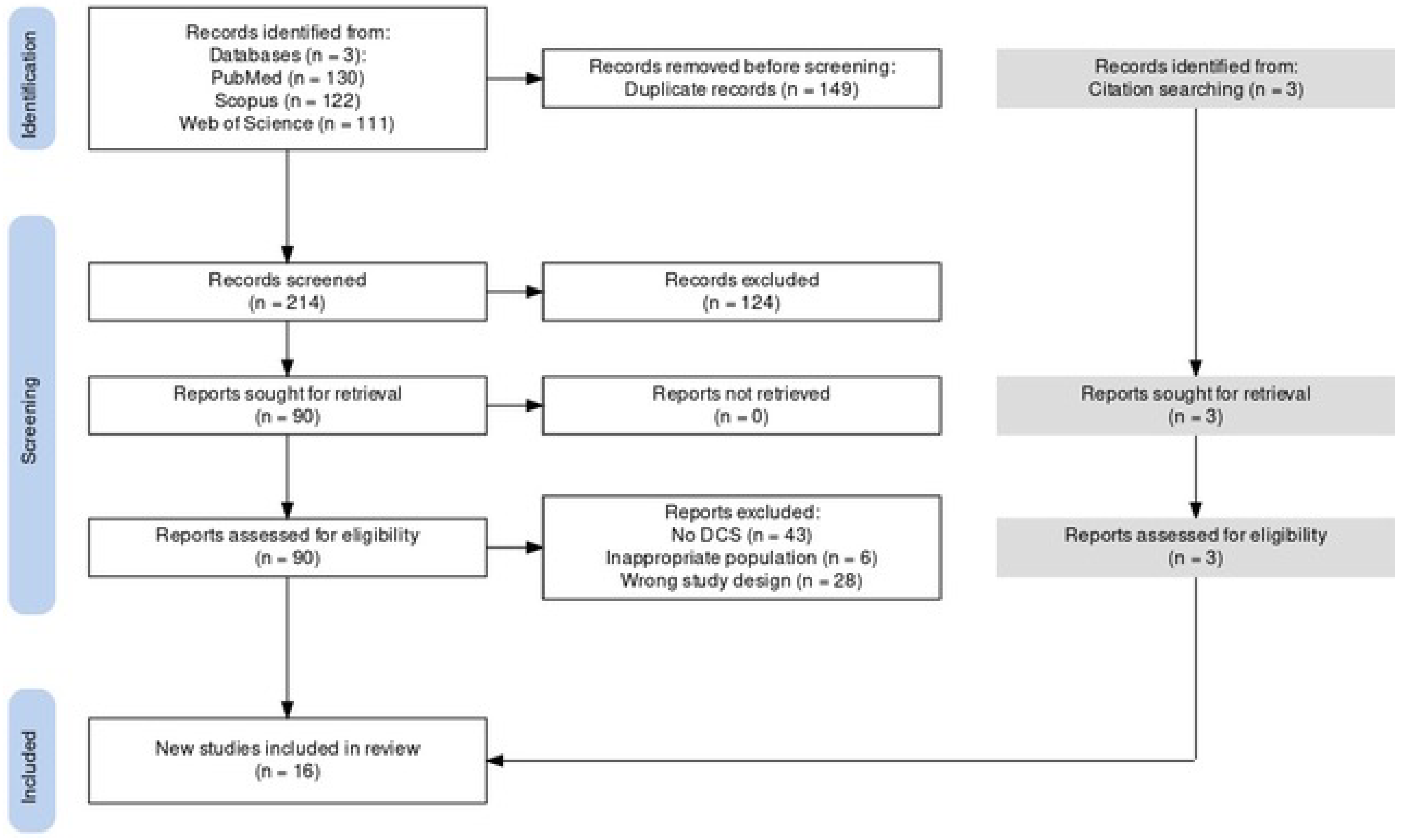
| Frame | P (Participants) | I (Intervention) | C (Comparator) | O (Outcome) | Time |
|---|---|---|---|---|---|
| Mesh terms | Any | Electrical stimulation of brain structures | None | Vestibular response | Intra-operatively or during the preoperative evaluation for drug resistance epilepsy (DRE) |
| Search | PubMed: (((direct current stimulation) OR (electrodes)) AND ((Vestibular) OR (vertiginous))) AND (cortex) Scopus: ((TITLE-ABS-KEY (vestibular) OR TITLE-ABS-KEY (vertiginous))) AND ((TITLE-ABS-KEY (direct AND current AND stimulation) OR TITLE-ABS-KEY (electrodes))) AND (TITLE-ABS-KEY (cortex)) Web of Science: https://www.webofscience.com/wos/woscc/summary/accf5915-f542-43db-ac83-d52ad90ff489-be1316c5/relevance/1 accessed on 30 November 2023 | ||||
| Exclusion Criteria | Irrelevant title or abstract, irrelevant full-text, editorial, reviews, meta-analysis, neonatal studies, experimental/non-human studies, non-English studies, stimulation other than electrical (caloric, galvanic), responses not including vertigo or perception of body movement | ||||
| Sources | Databases (PubMed, Scopus, Web of Science) Reference list | ||||
| Time limits | The search period: any until July 2023 | Last search: 30 November 2023 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arvaniti, C.K.; Brotis, A.G.; Paschalis, T.; Kapsalaki, E.Z.; Fountas, K.N. Localization of Vestibular Cortex Using Electrical Cortical Stimulation: A Systematic Literature Review. Brain Sci. 2024, 14, 75. https://doi.org/10.3390/brainsci14010075
Arvaniti CK, Brotis AG, Paschalis T, Kapsalaki EZ, Fountas KN. Localization of Vestibular Cortex Using Electrical Cortical Stimulation: A Systematic Literature Review. Brain Sciences. 2024; 14(1):75. https://doi.org/10.3390/brainsci14010075
Chicago/Turabian StyleArvaniti, Christina K., Alexandros G. Brotis, Thanasis Paschalis, Eftychia Z. Kapsalaki, and Kostas N. Fountas. 2024. "Localization of Vestibular Cortex Using Electrical Cortical Stimulation: A Systematic Literature Review" Brain Sciences 14, no. 1: 75. https://doi.org/10.3390/brainsci14010075
APA StyleArvaniti, C. K., Brotis, A. G., Paschalis, T., Kapsalaki, E. Z., & Fountas, K. N. (2024). Localization of Vestibular Cortex Using Electrical Cortical Stimulation: A Systematic Literature Review. Brain Sciences, 14(1), 75. https://doi.org/10.3390/brainsci14010075








