Brain Frontal-Lobe Misery Perfusion in COVID-19 ICU Survivors: An MRI Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI Experiments
2.3. aTRUPC Data Processing
2.3.1. Iterative Maxwell and Eddy-Current Correction
2.3.2. Semi-Automatic ROI Analysis
2.4. pCASL Data Processing
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Venkatesan, P. NICE guideline on long COVID. Lancet Respir. Med. 2021, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, F.; Piva, S.; Stampatori, C.; Righetti, F.; Mega, I.; Peli, E.; Sala, E.; Tomasi, C.; Indelicato, A.M.; Latronico, N.; et al. Neurologic and cognitive sequelae after SARS-CoV2 infection: Different impairment for ICU patients. J. Neurol. Sci. 2022, 432, 120061. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Perl, D.P.; Steiner, J.; Pasternack, N.; Li, W.; Maric, D.; Safavi, F.; Horkayne-Szakaly, I.; Jones, R.; Stram, M.N.; et al. Neurovascular injury with complement activation and inflammation in COVID-19. Brain 2022, 145, 2555–2568. [Google Scholar] [CrossRef] [PubMed]
- Leng, A.; Shah, M.; Ahmad, S.A.; Premraj, L.; Wildi, K.; Li Bassi, G.; Pardo, C.A.; Choi, A.; Cho, S.M. Pathogenesis Underlying Neurological Manifestations of Long COVID Syndrome and Potential Therapeutics. Cells 2023, 12, 816. [Google Scholar] [CrossRef]
- Grubb, R.L., Jr.; Derdeyn, C.P.; Fritsch, S.M.; Carpenter, D.A.; Yundt, K.D.; Videen, T.O.; Spitznagel, E.L.; Powers, W.J. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 1998, 280, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, H.; Higashi, T.; Kagawa, S.; Nishii, R.; Kudo, T.; Sugimoto, K.; Okazawa, H.; Fukuyama, H. Is misery perfusion still a predictor of stroke in symptomatic major cerebral artery disease? Brain 2012, 135, 2515–2526. [Google Scholar] [CrossRef]
- Stone, A.J.; Harston, G.W.J.; Carone, D.; Okell, T.W.; Kennedy, J.; Blockley, N.P. Prospects for investigating brain oxygenation in acute stroke: Experience with a non-contrast quantitative BOLD based approach. Hum. Brain Mapp. 2019, 40, 2853–2866. [Google Scholar] [CrossRef]
- Fan, A.P.; Khalil, A.A.; Fiebach, J.B.; Zaharchuk, G.; Villringer, A.; Villringer, K.; Gauthier, C.J. Elevated brain oxygen extraction fraction measured by MRI susceptibility relates to perfusion status in acute ischemic stroke. J. Cereb. Blood Flow Metab. 2020, 40, 539–551. [Google Scholar] [CrossRef]
- An, H.; Ford, A.L.; Chen, Y.; Zhu, H.; Ponisio, R.; Kumar, G.; Shanechi, A.M.; Khoury, N.; Vo, K.D.; Williams, J.; et al. Defining the ischemic penumbra using magnetic resonance oxygen metabolic index. Stroke 2015, 46, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Nezu, T.; Yokota, C.; Uehara, T.; Yamauchi, M.; Fukushima, K.; Toyoda, K.; Matsumoto, M.; Iide, H.; Minematsu, K. Preserved acetazolamide reactivity in lacunar patients with severe white-matter lesions: 15O-labeled gas and H2O positron emission tomography studies. J. Cereb. Blood Flow Met. 2012, 32, 844–850. [Google Scholar] [CrossRef]
- Jiang, D.; Lu, H.; Parkinson, C.; Su, P.; Wei, Z.; Pan, L.; Tekes, A.; Huisman, T.; Golden, W.C.; Liu, P. Vessel-specific quantification of neonatal cerebral venous oxygenation. Magn. Reson. Med. 2019, 82, 1129–1139. [Google Scholar] [CrossRef]
- Alsop, D.C.; Detre, J.A.; Golay, X.; Gunther, M.; Hendrikse, J.; Hernandez-Garcia, L.; Lu, H.; MacIntosh, B.J.; Parkes, L.M.; Smits, M.; et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 2015, 73, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Garcia, D.; de Bazelaire, C.; Alsop, D.C. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn. Reson. Med. 2008, 60, 1488–1497. [Google Scholar] [CrossRef]
- Zhao, L.; Vidorreta, M.; Soman, S.; Detre, J.A.; Alsop, D.C. Improving the robustness of pseudo-continuous arterial spin labeling to off-resonance and pulsatile flow velocity. Magn. Reson. Med. 2017, 78, 1342–1351. [Google Scholar] [CrossRef]
- Lu, H.Z.; Xu, F.; Grgac, K.; Liu, P.Y.; Qin, Q.; van Zijl, P. Calibration and validation of TRUST MRI for the estimation of cerebral blood oxygenation. Magn. Reson. Med. 2012, 67, 42–49. [Google Scholar] [CrossRef]
- Jiang, D.; Liu, P.; Lin, Z.; Hazel, K.; Pottanat, G.; Lucke, E.; Moghekar, A.; Pillai, J.J.; Lu, H. MRI assessment of cerebral oxygen extraction fraction in the medial temporal lobe. Neuroimage 2023, 266, 119829. [Google Scholar] [CrossRef]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man. Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Bernstein, M.A.; Zhou, X.H.J.; Polzin, J.A.; King, K.F.; Ganin, A.; Pelc, N.J.; Glover, G.H. Concomitant gradient terms in phase contrast MR: Analysis and correction. Magn. Reson. Med. 1998, 39, 300–308. [Google Scholar] [CrossRef]
- Jiang, D.R.; Deng, S.W.; Franklin, C.G.; O’Boyle, M.; Zhang, W.; Heyl, B.L.; Pan, L.; Jerabek, P.A.; Fox, P.T.; Lu, H.Z. Validation of T2-based oxygen extraction fraction measurement with 15O positron emission tomography. Magn. Reson. Med. 2021, 85, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.A.-O.; Liu, P.; Li, Y.; Fan, H.; Su, P.; Peng, S.A.-O.; Park, D.C.; Rodrigue, K.M.; Jiang, H.; Faria, A.V.; et al. ASL-MRICloud: An online tool for the processing of ASL MRI data. NMR Biomed. 2019, 32, e4051. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Wu, D.; Ceritoglu, C.; Li, Y.; Kolasny, A.; Vaillant, M.A.; Faria, A.V.; Oishi, K.; Miller, M.I. MRICloud: Delivering High-Throughput MRI Neuroinformatics as Cloud-Based Software as a Service. Comput. Sci. Eng. 2016, 18, 21–35. [Google Scholar] [CrossRef]
- Rhoton, A.L., Jr. The cerebral veins. Neurosurgery 2002, 51, S159–S205. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, X.; Yuan, C.; Zhao, X.; van Osch, M.J.P. Measuring the labeling efficiency of pseudocontinuous arterial spin labeling. Magn. Reason Med. 2017, 77, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, X.; Zhang, X.; Guo, R.; Teeuwisse, W.M.; Zhang, B.; Koken, P.; Smink, J.; Yuan, C.; van Osch, M.J.P. Simultaneous measurement of brain perfusion and labeling efficiency in a single pseudo-continuous arterial spin labeling scan. Magn. Reson. Med. 2018, 79, 1922–1930. [Google Scholar] [CrossRef]
- Friedman, N.P.; Robbins, T.W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef]
- Becker, J.H.; Lin, J.J.; Doernberg, M.; Stone, K.; Navis, A.; Festa, J.R.; Wisnivesky, J.P. Assessment of Cognitive Function in Patients After COVID-19 Infection. JAMA Netw. Open 2021, 4, e2130645. [Google Scholar] [CrossRef]
- Zhao, S.; Shibata, K.; Hellyer, P.J.; Trender, W.; Manohar, S.; Hampshire, A.; Husain, M. Rapid vigilance and episodic memory decrements in COVID-19 survivors. Brain Commun. 2022, 4, fcab295. [Google Scholar] [CrossRef]
- Hosp, J.A.; Dressing, A.; Blazhenets, G.; Bormann, T.; Rau, A.; Schwabenland, M.; Thurow, J.; Wagner, D.; Waller, C.; Niesen, W.D.; et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain 2021, 144, 1263–1276. [Google Scholar] [CrossRef]
- Paniz-Mondolfi, A.; Bryce, C.; Grimes, Z.; Gordon, R.E.; Reidy, J.; Lednicky, J.; Sordillo, E.M.; Fowkes, M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J. Med. Virol. 2020, 92, 699–702. [Google Scholar] [CrossRef]
- Franceschi, A.M.; Arora, R.; Wilson, R.; Giliberto, L.; Libman, R.B.; Castillo, M. Neurovascular Complications in COVID-19 Infection: Case Series. AJNR Am. J. Neuroradiol. 2020, 41, 1632–1640. [Google Scholar] [CrossRef]
- Agarwal, S.; Jain, R.; Dogra, S.; Krieger, P.; Lewis, A.; Nguyen, V.; Melmed, K.; Galetta, S. Cerebral Microbleeds and Leukoencephalopathy in Critically Ill Patients With COVID-19. Stroke 2020, 51, 2649–2655. [Google Scholar] [CrossRef]
- Shi, W.; Jiang, D.; Rando, H.; Khanduja, S.; Lin, Z.; Hazel, K.; Pottanat, G.; Jones, E.; Xu, C.; Lin, D.; et al. Blood-brain barrier breakdown in COVID-19 ICU survivors: An MRI pilot study. NeuroImmune Pharm. Ther. 2023, 2, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Matschke, J.; Lutgehetmann, M.; Hagel, C.; Sperhake, J.P.; Schroder, A.S.; Edler, C.; Mushumba, H.; Fitzek, A.; Allweiss, L.; Dandri, M.; et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020, 19, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.T.; Miller, E.H.; Glendinning, M.D.; Al-Dalahmah, O.; Banu, M.A.; Boehme, A.K.; Boubour, A.L.; Bruce, S.S.; Chong, A.M.; Claassen, J.; et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain 2021, 144, 2696–2708. [Google Scholar] [CrossRef]
- Meyer, P.T.; Hellwig, S.; Blazhenets, G.; Hosp, J.A. Molecular Imaging Findings on Acute and Long-Term Effects of COVID-19 on the Brain: A Systematic Review. J. Nucl. Med. 2022, 63, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Guedj, E.; Campion, J.Y.; Dudouet, P.; Kaphan, E.; Bregeon, F.; Tissot-Dupont, H.; Guis, S.; Barthelemy, F.; Habert, P.; Ceccaldi, M.; et al. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2823–2833. [Google Scholar] [CrossRef]
- Ajcevic, M.; Iscra, K.; Furlanis, G.; Michelutti, M.; Miladinovic, A.; Buoite Stella, A.; Ukmar, M.; Cova, M.A.; Accardo, A.; Manganotti, P. Cerebral hypoperfusion in post-COVID-19 cognitively impaired subjects revealed by arterial spin labeling MRI. Sci. Rep. 2023, 13, 5808. [Google Scholar] [CrossRef]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef]
- Qin, Y.; Wu, J.; Chen, T.; Li, J.; Zhang, G.; Wu, D.; Zhou, Y.; Zheng, N.; Cai, A.; Ning, Q.; et al. Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J. Clin. Investig. 2021, 131, e147329. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Emst, T.; Liang, H.; Jiang, D.; Cunningham, E.; Lin, Z.; Ryan, M.C.; Lu, H.; Wilson, E.; Levine, A.; et al. Lower Cerebral Venous Oxygenation in Post-acute Sequelae of COVID-19. In Proceedings of the 30th Annual Meeting of ISMRM, London, UK, 7–12 May 2022; p. 2207. [Google Scholar]
- Lin, Z.; Lim, C.; Jiang, D.; Soldan, A.; Pettigrew, C.; Oishi, K.; Zhu, Y.; Moghekar, A.; Liu, P.; Albert, M.; et al. Longitudinal changes in brain oxygen extraction fraction (OEF) in older adults: Relationship to markers of vascular and Alzheimer’s pathology. Alzheimer’s Dement. 2023, 19, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Catchlove, S.J.; Macpherson, H.; Hughes, M.E.; Chen, Y.; Parrish, T.B.; Pipingas, A. An investigation of cerebral oxygen utilization, blood flow and cognition in healthy aging. PLoS ONE 2018, 13, e0197055. [Google Scholar] [CrossRef]
- Aanerud, J.; Borghammer, P.; Chakravarty, M.M.; Vang, K.; Rodell, A.B.; Jonsdottir, K.Y.; Moller, A.; Ashkanian, M.; Vafaee, M.S.; Iversen, P.; et al. Brain energy metabolism and blood flow differences in healthy aging. J. Cereb. Blood Flow Metab. 2012, 32, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Staffaroni, A.M.; Cobigo, Y.; Elahi, F.M.; Casaletto, K.B.; Walters, S.M.; Wolf, A.; Lindbergh, C.A.; Rosen, H.J.; Kramer, J.H. A longitudinal characterization of perfusion in the aging brain and associations with cognition and neural structure. Hum. Brain Mapp. 2019, 40, 3522–3533. [Google Scholar] [CrossRef] [PubMed]
- Juttukonda, M.R.; Li, B.; Almaktoum, R.; Stephens, K.A.; Yochim, K.M.; Yacoub, E.; Buckner, R.L.; Salat, D.H. Characterizing cerebral hemodynamics across the adult lifespan with arterial spin labeling MRI data from the Human Connectome Project-Aging. Neuroimage 2021, 230, 117807. [Google Scholar] [CrossRef]
- Chen, J.J.; Rosas, H.D.; Salat, D.H. Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage 2011, 55, 468–478. [Google Scholar] [CrossRef]
- Pagani, M.; Salmaso, D.; Jonsson, C.; Hatherly, R.; Jacobsson, H.; Larsson, S.A.; Wägner, A. Regional cerebral blood flow as assessed by principal component analysis and 99mTc-HMPAO SPET in healthy subjects at rest: Normal distribution and effect of age and gender. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 67–75. [Google Scholar]
- Lee, C.; Lopez, O.L.; Becker, J.T.; Raji, C.; Dai, W.; Kuller, L.H.; Gach, H.M. Imaging cerebral blood flow in the cognitively normal aging brain with arterial spin labeling: Implications for imaging of neurodegenerative disease. J. Neuroimaging 2009, 19, 344–352. [Google Scholar] [CrossRef]
- Mintun, M.A.; Raichle, M.E.; Martin, W.R.; Herscovitch, P. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J. Nucl. Med. 1984, 25, 177–187. [Google Scholar]
- Fox, P.T.; Raichle, M.E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl. Acad. Sci. USA 1986, 83, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Frackowiak, R.S.; Pozzilli, C.; Legg, N.J.; Du Boulay, G.H.; Marshall, J.; Lenzi, G.L.; Jones, T. Regional cerebral oxygen supply and utilization in dementia. A clinical and physiological study with oxygen-15 and positron tomography. Brain 1981, 104, 753–778. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Koehler, R.C.; Liu, X.; Kulikowicz, E.; Lee, J.K.; Lu, H.; Liu, P. Quantitative validation of MRI mapping of cerebral venous oxygenation with direct blood sampling: A graded-O(2) study in piglets. Magn. Reason. Med 2021, 86, 1445–1453. [Google Scholar] [CrossRef]
- Puig, O.; Henriksen, O.M.; Vestergaard, M.B.; Hansen, A.E.; Andersen, F.L.; Ladefoged, C.N.; Rostrup, E.; Larsson, H.B.; Lindberg, U.; Law, I. Comparison of simultaneous arterial spin labeling MRI and 15O-H2O PET measurements of regional cerebral blood flow in rest and altered perfusion states. J. Cereb. Blood Flow Metab. 2020, 40, 1621–1633. [Google Scholar] [CrossRef]
- Fan, A.P.; Jahanian, H.; Holdsworth, S.J.; Zaharchuk, G. Comparison of cerebral blood flow measurement with [15O]-water positron emission tomography and arterial spin labeling magnetic resonance imaging: A systematic review. J. Cereb. Blood Flow Metab. 2016, 36, 842–861. [Google Scholar] [CrossRef]
- Blazhenets, G.; Schroeter, N.; Bormann, T.; Thurow, J.; Wagner, D.; Frings, L.; Weiller, C.; Meyer, P.T.; Dressing, A.; Hosp, J.A. Slow but Evident Recovery from Neocortical Dysfunction and Cognitive Impairment in a Series of Chronic COVID-19 Patients. J. Nucl. Med. 2021, 62, 910–915. [Google Scholar] [CrossRef]
- Hyder, F.; Herman, P.; Bailey, C.J.; Moller, A.; Globinsky, R.; Fulbright, R.K.; Rothman, D.L.; Gjedde, A. Uniform distributions of glucose oxidation and oxygen extraction in gray matter of normal human brain: No evidence of regional differences of aerobic glycolysis. J. Cereb. Blood Flow Metab. 2016, 36, 903–916. [Google Scholar] [CrossRef]
- Desai, S.V.; Law, T.J.; Needham, D.M. Long-term complications of critical care. Crit. Care Med. 2011, 39, 371–379. [Google Scholar] [CrossRef]
- Pandharipande, P.P.; Girard, T.D.; Jackson, J.C.; Morandi, A.; Thompson, J.L.; Pun, B.T.; Brummel, N.E.; Hughes, C.G.; Vasilevskis, E.E.; Shintani, A.K.; et al. Long-term cognitive impairment after critical illness. N. Engl. J. Med. 2013, 369, 1306–1316. [Google Scholar] [CrossRef]
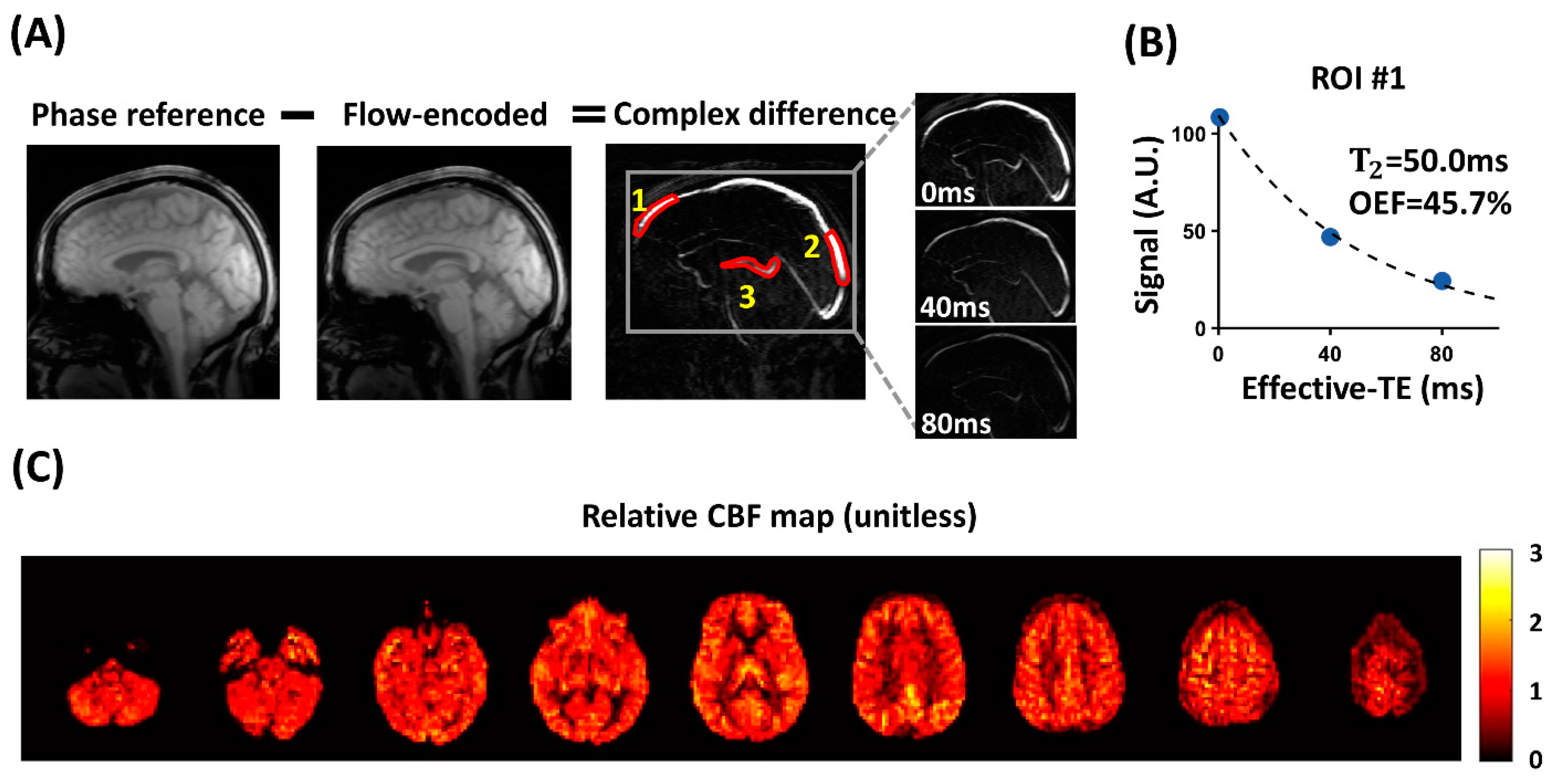
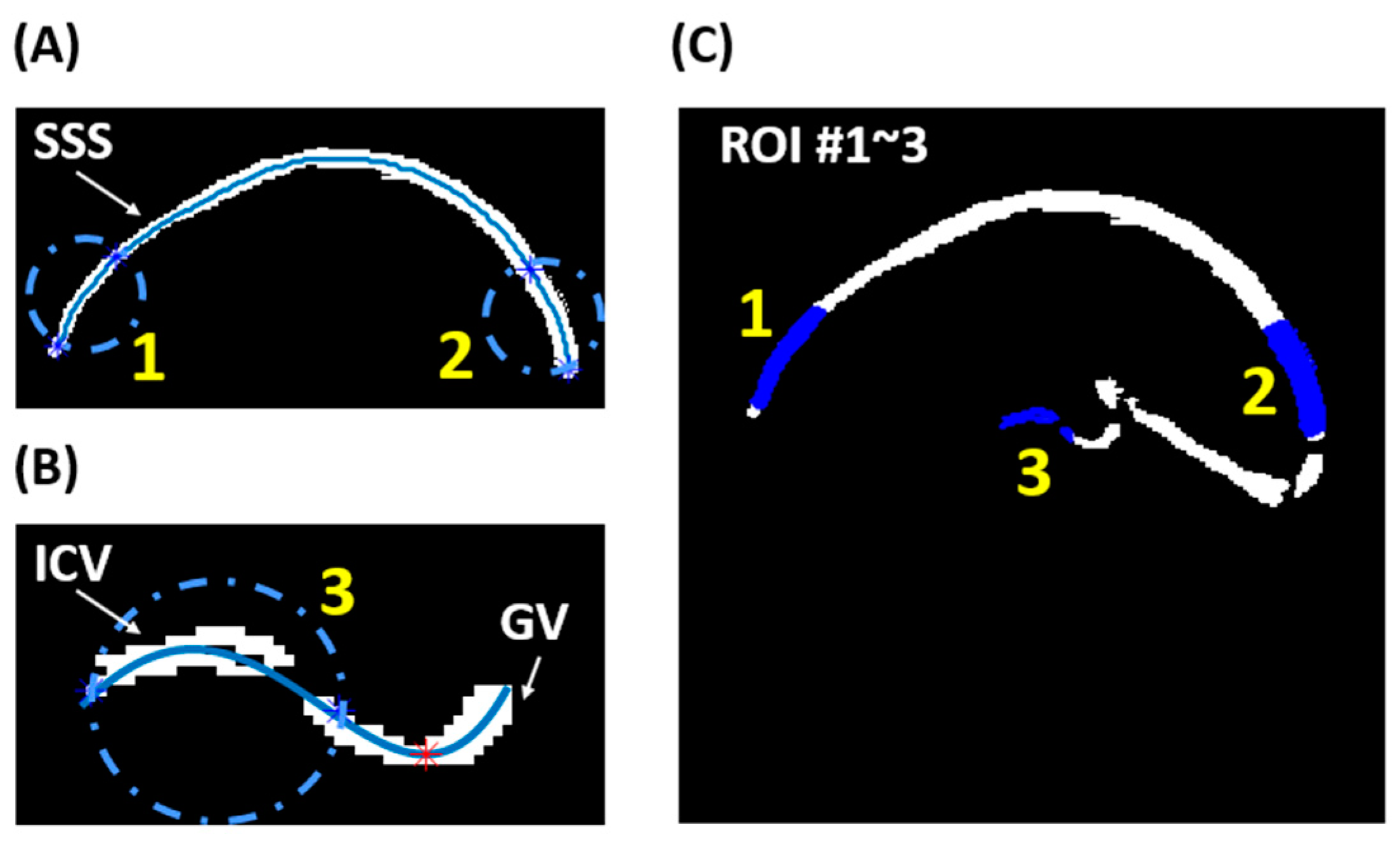
| COVID-19 ICU Survivors | Control | p-Value | |
|---|---|---|---|
| N | 7 | 19 | N/A |
| Sex (Female) * | 4 (57.1%) | 12 (63.2%) | 1.00 a |
| Age (years) † | 50 (20–77) | 64 (22–77) | 0.71 b |
| Ya (%) ‡ | 97.7 ± 1.5 | 97.5 ± 1.0 | 0.72 c |
| Length of hospital stay ‡ | 29.1 ± 25.1 | NA | NA |
| Time from symptom onset to ICU admission (days) † | 1 (1–8) | NA | NA |
| Duration of stay in ICU (days) ‡ | 20.2 ± 18.2 | NA | NA |
| Time from ICU admission to MRI (days) ‡ | 118.6 ± 30.3 | NA | NA |
| Intubation * | 2 (28.6%) | NA | NA |
| Post-acute COVID-19 symptoms | |||
| Coughing * | 3 (42.9%) | NA | NA |
| Shortness of breath * | 4 (57.1%) | NA | NA |
| Fatigue * | 3 (42.9%) | NA | NA |
| Variable | ROI | COVID-19 ICU Survivors | Control |
|---|---|---|---|
| OEF (%) | frontal SSS | 38.3 ± 9.0 | 33.5 ± 4.7 |
| posterior SSS | 38.2 ± 8.4 | 35.4 ± 5.1 | |
| ICV | 32.5 ± 11.5 | 27.9 ± 7.7 | |
| rCBF (unitless) | frontal GM | 1.20 ± 0.07 | 1.27 ± 0.07 |
| GM of all lobes | 1.22 ± 0.04 | 1.23 ± 0.03 | |
| BGT | 0.96 ± 0.15 | 0.95 ± 0.08 |
| Model | Dependent Variable | Independent Variables | Coefficient ± Standard Error | p-Value |
|---|---|---|---|---|
| OEF1 | OEF in frontal SSS | Group | 5.21 ± 2.48% | 0.047 |
| Age | 0.11 ± 0.050%/year | 0.03 | ||
| OEF2 | OEF in posterior SSS | Group | 3.06 ± 2.65% | 0.26 |
| Age | 0.078 ± 0.053%/year | 0.16 | ||
| OEF3 | OEF in ICV | Group | 4.90 ± 3.93% | 0.23 |
| Age | 0.068 ± 0.079%/year | 0.40 | ||
| rCBF1 | rCBF in frontal GM | Group | −0.083 ± 0.025 | 0.003 |
| Age | −0.0018 ± 0.00051/year | 0.002 | ||
| rCBF2 | rCBF in GM of all lobes | Group | −0.018 ± 0.015 | 0.24 |
| Age | −0.00056 ± 0.00030/year | 0.08 | ||
| rCBF3 | rCBF in BGT | Group | 0.027 ± 0.032 | 0.41 |
| Age | 0.0031 ± 0.00064/year | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Khanduja, S.; Rando, H.; Shi, W.; Hazel, K.; Pottanat, G.P.; Jones, E.; Xu, C.; Hu, Z.; Lin, D.; et al. Brain Frontal-Lobe Misery Perfusion in COVID-19 ICU Survivors: An MRI Pilot Study. Brain Sci. 2024, 14, 94. https://doi.org/10.3390/brainsci14010094
Song J, Khanduja S, Rando H, Shi W, Hazel K, Pottanat GP, Jones E, Xu C, Hu Z, Lin D, et al. Brain Frontal-Lobe Misery Perfusion in COVID-19 ICU Survivors: An MRI Pilot Study. Brain Sciences. 2024; 14(1):94. https://doi.org/10.3390/brainsci14010094
Chicago/Turabian StyleSong, Jie, Shivalika Khanduja, Hannah Rando, Wen Shi, Kaisha Hazel, George Paul Pottanat, Ebony Jones, Cuimei Xu, Zhiyi Hu, Doris Lin, and et al. 2024. "Brain Frontal-Lobe Misery Perfusion in COVID-19 ICU Survivors: An MRI Pilot Study" Brain Sciences 14, no. 1: 94. https://doi.org/10.3390/brainsci14010094
APA StyleSong, J., Khanduja, S., Rando, H., Shi, W., Hazel, K., Pottanat, G. P., Jones, E., Xu, C., Hu, Z., Lin, D., Yasar, S., Lu, H., Cho, S.-M., & Jiang, D. (2024). Brain Frontal-Lobe Misery Perfusion in COVID-19 ICU Survivors: An MRI Pilot Study. Brain Sciences, 14(1), 94. https://doi.org/10.3390/brainsci14010094







