Subcortical Change and Neurohabilitation Treatment Adherence Effects in Extremely Preterm Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Prenatal and Perinatal Risk Factors
2.3. Katona Neurohabilitation Treatment
2.4. Brain MRI
2.4.1. MRI Acquisition
2.4.2. MRI Individual Analyses
2.5. Neuropsychological Screening
2.6. Statistical Analyses
3. Results
3.1. Prenatal and Perinatal Risk Factors
3.2. Subcortical Volume Change
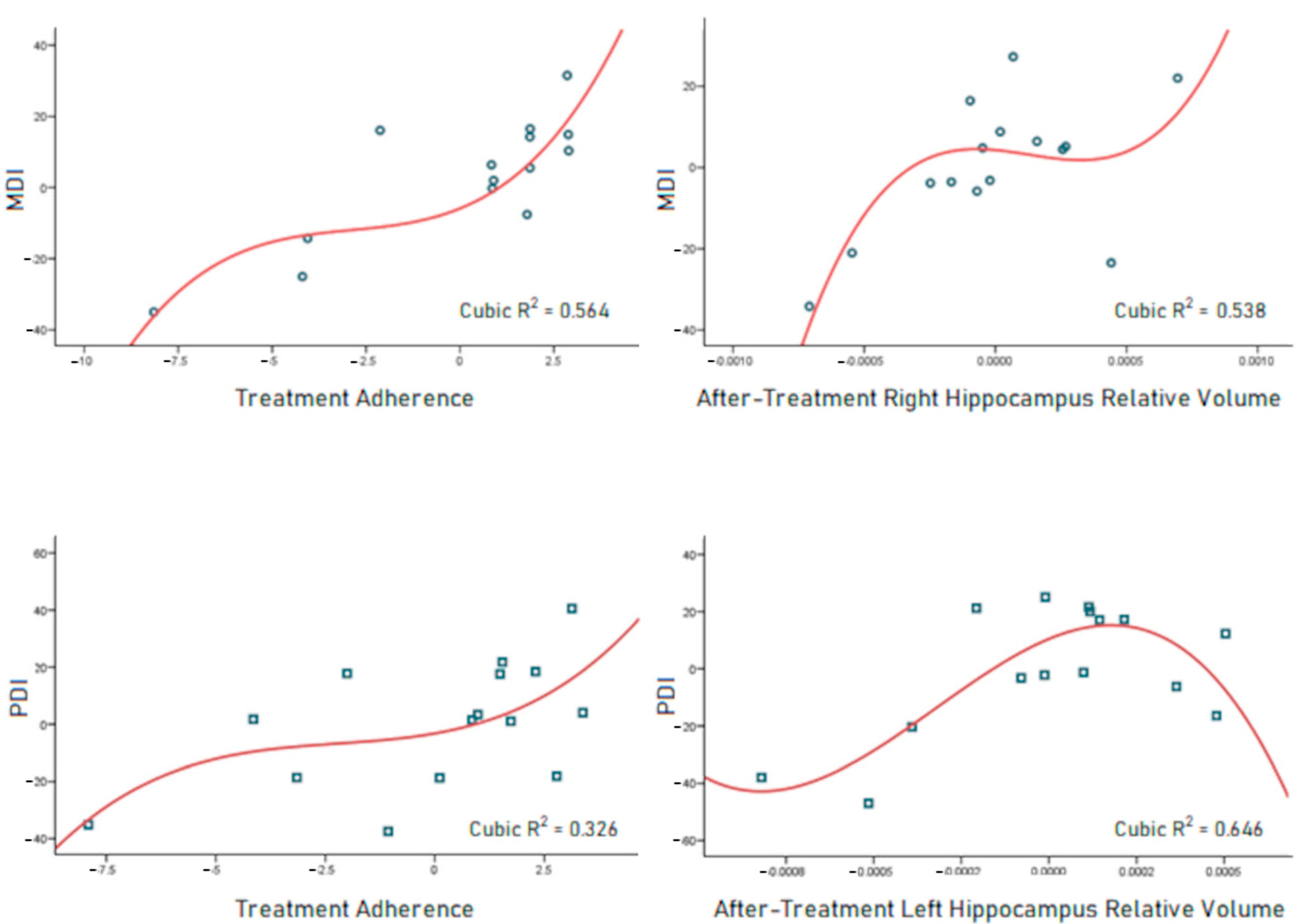
3.3. Correlations between Subcortical Volumes, Treatment Adherence, and Neurodevelopment Outcomes
3.4. Neurodevelopment Outcome Predictors
4. Discussion
Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barfield, W.D. Public Health Implications of Very Preterm Birth. Clin. Perinatol. 2018, 45, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Felderhoff-Mueser, U.; Rutherford, M.A.; Squier, W.V.; Cox, P.; Maalouf, E.F.; Counsell, S.J.; Bydder, G.M.; David Edwards, A. Relationship between MR Imaging and Histopathologic Findings of the Brain in Extremely Sick Preterm Infants. AJNR Am. J. Neuroradiol. 1999, 20, 1349–1357. [Google Scholar] [PubMed]
- Harrison, M.S.; Goldenberg, R.L. Global Burden of Prematurity. Semin. Fetal Neonatal Med. 2016, 21, 74–79. [Google Scholar] [CrossRef]
- Taylor, G.L.; O’Shea, T.M. Extreme Prematurity: Risk and Resiliency. Curr. Probl. Pediatr. Adolesc. Health Care 2022, 52, 101132. [Google Scholar] [CrossRef]
- Sølsnes, A.E.; Sripada, K.; Yendiki, A.; Bjuland, K.J.; Østgård, H.F.; Aanes, S.; Grunewaldt, K.H.; Løhaugen, G.C.; Eikenes, L.; Håberg, A.K.; et al. Limited Microstructural and Connectivity Deficits despite Subcortical Volume Reductions in School-Aged Children Born Preterm with Very Low Birth Weight. Neuroimage 2016, 130, 24–34. [Google Scholar] [CrossRef]
- Bjuland, K.J.; Rimol, L.M.; Løhaugen, G.C.C.; Skranes, J. Brain Volumes and Cognitive Function in Very-Low-Birth-Weight (VLBW) Young Adults. Eur. J. Paediatr. Neurol. 2014, 18, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Boardman, J.P.; Craven, C.; Valappil, S.; Counsell, S.J.; Dyet, L.E.; Rueckert, D.; Aljabar, P.; Rutherford, M.A.; Chew, A.T.M.; Allsop, J.M.; et al. A Common Neonatal Image Phenotype Predicts Adverse Neurodevelopmental Outcome in Children Born Preterm. Neuroimage 2010, 52, 409–414. [Google Scholar] [CrossRef]
- Volpe, J.J. Brain Injury in Premature Infants: A Complex Amalgam of Destructive and Developmental Disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef]
- Nosarti, C.; Nam, K.W.; Walshe, M.; Murray, R.M.; Cuddy, M.; Rifkin, L.; Allin, M.P.G. Preterm Birth and Structural Brain Alterations in Early Adulthood. Neuroimage Clin. 2014, 6, 180–191. [Google Scholar] [CrossRef]
- Ream, M.A.; Lehwald, L. Neurologic Consequences of Preterm Birth. Curr. Neurol. Neurosci. Rep. 2018, 18, 48. [Google Scholar] [CrossRef]
- Macpherson, T.; Hikida, T. Role of Basal Ganglia Neurocircuitry in the Pathology of Psychiatric Disorders. Psychiatry Clin. Neurosci. 2019, 73, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Lecciso, F.; Colombo, B. Beyond the Cortico-Centric Models of Cognition: The Role of Subcortical Functioning in Neurodevelopmental Disorders. Front. Psychol. 2019, 10, 2809. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Y.; Ceschin, R.; An, X.; Lao, Y.; Vanderbilt, D.; Nelson, M.D.; Thompson, P.M.; Panigrahy, A.; Leporé, N. A Multivariate Surface-Based Analysis of the Putamen in Premature Newborns: Regional Differences within the Ventral Striatum. PLoS ONE 2013, 8, e66736. [Google Scholar] [CrossRef] [PubMed]
- Spittle, A.; Orton, J.; Anderson, P.J.; Boyd, R.; Doyle, L.W. Early Developmental Intervention Programmes Provided Post Hospital Discharge to Prevent Motor and Cognitive Impairment in Preterm Infants. Cochrane Database Syst. Rev. 2015, 2015, CD005495. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Katona, F. Clinical Neurodevelopment Diagnosis and Treatment. In Challenges to Developmental Paradigms: Implications for Theory and Treatment; Zelazo, P.R., Barr, R.G., Eds.; Lawrence Erlbaum: Mahwah, NJ, USA; Hillsdale: Hillsdale, NSW, Australian, 1989; pp. 167–187. [Google Scholar]
- Porras-Kattz, E.; Harmony, T. Neurohabilitación: Un Método Diagnóstico y Terapéutico Para Prevenir Secuelas por Lesión Cerebral en el Recién Nacido y el Lactante. Bol. Med. Hosp. Infant. Mex. 2007, 64, 125–135. [Google Scholar]
- Alvarado-Guerrero, I.; Poblano, A.; Marosi, E.; Corsi-Cabrera, M.; Otero-Ojeda, G.A. Early Intervention in the Neurodevelopment of Premature Infants during the First Six Months of Life. Neurosci. Med. 2011, 2, 104–109. [Google Scholar] [CrossRef]
- Garófalo-Gómez, N.; Barrera-Reséndiz, J.; Juárez-Colín, M.E.; Pedraza-Aguilar, M.d.C.; Carrillo-Prado, C.; Martínez-Chávez, J.; Hinojosa-Rodríguez, M.; Fernández, T.; Harmony, T. Outcome at Age Five Years or Older for Children with Perinatal Brain Injury Treated with Neurohabilitation or Neurodevelopmental Therapy. EC Paediatr. 2019, 8, 1090–1098. [Google Scholar]
- Gonzalez-Moreira, E.; Harmony, T.; Hinojosa-Rodríguez, M.; Carrillo-Prado, C.; Juárez-Colín, M.E.; Gutiérrez-Hernández, C.C.; Carlier, M.E.M.; Cubero-Rego, L.; Castro-Chavira, S.A.; Fernández, T. Prevention of Neurological Sequelae in Preterm Infants. Brain Sci. 2023, 13, 753. [Google Scholar] [CrossRef]
- Harmony, T. Early Diagnosis and Treatment of Infants with Prenatal and Perinatal Risk Factors for Brain Damage at the Neurodevelopmental Research Unit in Mexico. Neuroimage 2021, 235, 117984. [Google Scholar] [CrossRef]
- Harmony, T. Outcome of Infants at Risk of Brain Damage after Katona Neurohabilitation Therapy. Int. J. Neurorehabil. 2017, 4, 1000277. [Google Scholar] [CrossRef]
- Harmony, T.; Barrera-Reséndiz, J.; Juárez-Colín, M.E.; Carrillo-Prado, C.; del Consuelo Pedraza-Aguilar, M.; Asprón Ramírez, A.; Hinojosa-Rodríguez, M.; Fernández, T.; Ricardo-Garcell, J. Longitudinal Study of Children with Perinatal Brain Damage in Whom Early Neurohabilitation Was Applied: Preliminary Report. Neurosci. Lett. 2016, 611, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa-Rodríguez, M.; De Leo-Jiménez, J.O.; Juárez-Colín, M.E.; Gonzalez-Moreira, E.; Flores-Bautista, C.S.; Harmony, T. Long-Term Therapeutic Effects of Katona Therapy in Moderate-to-Severe Perinatal Brain Damage. Neurosci. Lett. 2020, 738, 135345. [Google Scholar] [CrossRef] [PubMed]
- Barrera Reséndiz, J.E. Terapia Neurohabilitatoria; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2015; ISBN 9786070269387. [Google Scholar]
- Bedetti, C.; Arnaudbore; Carlin, J.; Nick; Guay, S.; Joseph, M.; Routier, A.; Kastman, E.; Stojic, H.; Isla; et al. Dcm2bids. 2021. Available online: https://zenodo.org/records/6596007 (accessed on 8 September 2024).
- Li, X.; Morgan, P.S.; Ashburner, J.; Smith, J.; Rorden, C. The First Step for Neuroimaging Data Analysis: DICOM to NIfTI Conversion. J. Neurosci. Methods 2016, 264, 47–56. [Google Scholar] [CrossRef]
- Coupé, P.; Manjón, J.V.; Robles, M.; Collins, D.L. Adaptive Multiresolution Non-Local Means Filter for Three-Dimensional Magnetic Resonance Image Denoising. IET Image Process 2012, 6, 558–568. [Google Scholar] [CrossRef]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; Van Der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef]
- Zöllei, L.; Iglesias, J.E.; Ou, Y.; Grant, P.E.; Fischl, B. Infant FreeSurfer: An Automated Segmentation and Surface Extraction Pipeline for T1-Weighted Neuroimaging Data of Infants 0–2 Years. Neuroimage 2020, 218, 116946. [Google Scholar] [CrossRef]
- Bayley, N. Bayley Scales of Infant Development; The Psychological Corporation: New York, NY, USA, 1993. [Google Scholar]
- Secretaría de Salud. Gobierno de Mexico. Cada Año Nacen En México 200 Mil Bebés Prematuros. Available online: https://www.gob.mx/salud/prensa/558-cada-ano-nacen-en-mexico-200-mil-bebes-prematuros-secretaria-de-salud?idiom=es (accessed on 4 June 2023).
- López-García, B.; Ávalos Antonio, N.; Díaz Gómez, N.B.; López-García, B.; Ávalos Antonio, N.; Díaz Gómez, N.B. Incidencia de Prematuros En El Hospital General Naval de Alta Especialidad 2015–2017. Rev. Sanid. Milit. 2018, 72, 19–23. [Google Scholar]
- Lear, B.A.; Lear, C.A.; Dhillon, S.K.; Davidson, J.O.; Bennet, L.; Gunn, A.J. Is Late Prevention of Cerebral Palsy in Extremely Preterm Infants Plausible? Dev. Neurosci. 2022, 44, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Padilla, N.; Alexandrou, G.; Blennow, M.; Lagercrantz, H.; Ådén, U. Brain Growth Gains and Losses in Extremely Preterm Infants at Term. Cereb. Cortex 2015, 25, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Yates, N.; Gunn, A.J.; Bennet, L.; Dhillon, S.K.; Davidson, J.O. Molecular Sciences Preventing Brain Injury in the Preterm Infant-Current Controversies and Potential Therapies. Int. J. Mol. Sci. 2021, 22, 1671. [Google Scholar] [CrossRef]
- Sewell, E.; Roberts, J.; Mukhopadhyay, S. Association of Infection in Neonates and Long-Term Neurodevelopmental Outcome. Clin. Perinatol. 2021, 48, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Alex, A.M.; Aguate, F.; Botteron, K.; Buss, C.; Chong, Y.S.; Dager, S.R.; Donald, K.A.; Entringer, S.; Fair, D.A.; Fortier, M.V.; et al. A Global Multicohort Study to Map Subcortical Brain Development and Cognition in Infancy and Early Childhood. Nat. Neurosci. 2024, 27, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Choe, M.-S.; Ortiz-Mantilla, S.; Makris, N.; Gregas, M.; Bacic, J.; Haehn, D.; Kennedy, D.; Pienaar, R.; Caviness, V.S.; Benasich, A.A.; et al. Regional Infant Brain Development: An MRI-Based Morphometric Analysis in 3 to 13 Month Olds. Cereb. Cortex 2013, 23, 2100–2117. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. Dysmaturation of Premature Brain: Importance, Cellular Mechanisms, and Potential Interventions. Pediatr. Neurol. 2019, 95, 42–66. [Google Scholar] [CrossRef] [PubMed]
- Dima, D.; Modabbernia, A.; Papachristou, E.; Doucet, G.E.; Agartz, I.; Aghajani, M.; Akudjedu, T.N.; Albajes-Eizagirre, A.; Alnæs, D.; Alpert, K.I.; et al. Subcortical Volumes across the Lifespan: Data from 18,605 Healthy Individuals Aged 3–90 Years. Hum. Brain Mapp. 2022, 43, 452–469. [Google Scholar] [CrossRef]
- Østby, Y.; Tamnes, C.K.; Fjell, A.M.; Westlye, L.T.; Due-Tønnessen, P.; Walhovd, K.B. Heterogeneity in Subcortical Brain Development: A Structural Magnetic Resonance Imaging Study of Brain Maturation from 8 to 30 Years. J. Neurosci. 2009, 29, 11772–11782. [Google Scholar] [CrossRef]
- Ocklenburg, S.; Peterburs, J.; Mundorf, A. Hemispheric Asymmetries in the Amygdala: A Comparative Primer. Prog. Neurobiol. 2022, 214, 102283. [Google Scholar] [CrossRef]
- Russell, J.D.; Marsee, M.A.; Weems, C.F. Developmental Variation in Amygdala Volumes: Modeling Differences Across Time, Age, and Puberty. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 117–125. [Google Scholar] [CrossRef]
- Novak, C.M.; Ozen, M.; Burd, I. Perinatal Brain Injury: Mechanisms, Prevention, and Outcomes. Clin Perinatol. 2018, 45, 357–375. [Google Scholar] [CrossRef]
- Piper, M.C.; Darrah, J. Motor Assessment of the Developing Infant: Alberta Infant Motor Scale (AIMS); Elsevier: Amsterdam, The Netherlands, 2021; p. 190. [Google Scholar]
- Chen, L.; Wang, Y.; Wu, Z.; Shan, Y.; Li, T.; Hung, S.C.; Xing, L.; Zhu, H.; Wang, L.; Lin, W.; et al. Four-Dimensional Mapping of Dynamic Longitudinal Brain Subcortical Development and Early Learning Functions in Infants. Nat. Commun. 2023, 14, 3727. [Google Scholar] [CrossRef]
- Serenius, F.; Källén, K.; Blennow, M.; Ewald, U.; Fellman, V.; Holmström, G.; Lindberg, E.; Lundqvist, P.; Maršál, K.; Norman, M.; et al. Neurodevelopmental Outcome in Extremely Preterm Infants at 2.5 Years after Active Perinatal Care in Sweden. JAMA 2013, 309, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.J.; De Luca, C.R.; Hutchinson, E.; Roberts, G.; Doyle, L.W.; Callanan, C.; Davis, N.; Duff, J.; Kelly, E.; McDonald, M.; et al. Underestimation of Developmental Delay by the New Bayley-III Scale. Arch. Pediatr. Adolesc. Med. 2010, 164, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.K.; Wood, S.J.; Doyle, L.W.; Warfield, S.K.; Lodygensky, G.A.; Anderson, P.J.; Egan, G.F.; Inder, T.E. Neonate Hippocampal Volumes: Prematurity, Perinatal Predictors, and 2-Year Outcome. Ann. Neurol. 2008, 63, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Díaz Nieto, D.M. Factores Psicosocioculturales Que Propician En Personas Cuidadoras Primarias La No Adherencia al Proceso Terapéutico-Neurohabilitatorio Que Reciben Niñas y Niños Atendidos En La Unidad de Investigación En Neurodesarrollo; Del Instituto de Neurobiología de La UNAM, Campus Juriquilla. Licenciatura, Universidad Nacional Autónoma de México: Mexico City, Mexico, 2022. [Google Scholar]
| Measure | Values | |
|---|---|---|
| Maternal Characteristics | ||
| Infections | n (%) | 10 (66.7) |
| Early membrane rupture | n (%) | 8 (53.3) |
| Pre-eclampsia | n (%) | 4 (26.7) |
| Placental alterations | n (%) | 3 (20.0) |
| Intrauterine growth restriction | n (%) | 2 (13.3) |
| Diabetes | n (%) | 1 (6.7) |
| Infant Characteristics | ||
| Number of days in hospital | Mean (SD) | 67.1 (21.3) |
| Number of days on ventilation | Mean (SD) | 40.9 (37.4) |
| Neonatal sepsis | n (%) | 13 (86.7) |
| Hypoxic–ischemic encephalopathy | n (%) | 9 (60.0) |
| Intraventricular hemorrhage | n (%) | 9 (60.0) |
| Congenital heart disease | n (%) | 8 (53.3) |
| Retinopathy of prematurity | n (%) | 6 (40.0) |
| Anemia | n (%) | 5 (33.3) |
| Bronchopulmonary dysplasia | n (%) | 5 (33.3) |
| Necrotizing enterocolitis | n (%) | 4 (26.7) |
| Seizures | n (%) | 3 (20.0) |
| Structure | Hemisphere | Treatment Beginning | After Treatment | z | p | Change |
|---|---|---|---|---|---|---|
| Median (Range) | Median (Range) | |||||
| Age at Scan (weeks) | 27 (79) | 157 (367) | ||||
| Amygdala | Left | 717 (726) | 1181 (848) | −3.408 | 0.001 | ↑ |
| Right | 570 (843) | 1351 (1432) | −3.408 | 0.001 | ↑ | |
| Caudate | Left | 1947 (2146) | 2949 (2037) | −3.408 | 0.001 | ↑ |
| Right | 2017 (2222) | 3053 (2997) | −3.408 | 0.001 | ↑ | |
| Hippocampus | Left | 1599 (1990) | 3126 (2244) | −3.408 | 0.001 | ↑ |
| Right | 1698 (1683) | 3206 (2129) | −3.408 | 0.001 | ↑ | |
| Pallidum | Left | 1749 (2101) | 1394 (1024) | −1.647 | 0.100 | ↓ |
| Right | 1535 (1482) | 1384 (927) | −1.590 | 0.112 | ↓ | |
| Putamen | Left | 3140 (2276) | 3999 (2777) | −3.408 | 0.001 | ↑ |
| Right | 3175 (2467) | 4110 (1824) | −3.408 | 0.001 | ↑ | |
| Thalamus | Left | 5831 (3266) | 5477 (5081) | −0.511 | 0.609 | ↓ |
| Right | 5775 (3428) | 5584 (4845) | −0.341 | 0.733 | ↓ | |
| Subcortical Gray Matter | 33,402 (19,621) | 44,741 (31,878) | −3.408 | 0.001 | ↑ | |
| Cortex | 289,075 (357,519) | 493,793 (217,370) | −3.408 | 0.001 | ↑ | |
| Cerebral White Matter | 118,670 (221,699) | 260,553 (227,569) | −3.408 | 0.001 | ↑ | |
| Intracranial * | 470,838 (792,745) | 1,052,688 (644,737) | −3.408 | 0.001 | ↑ |
| Structure | Hemisphere | Beginning of Treatment | After Treatment | z | p | Change |
|---|---|---|---|---|---|---|
| Median (Range) | Median (Range) | |||||
| Amygdala | Left | 0.00153 (0.00206) | 0.00116 (0.00051) | −2.045 | 0.041 | ↓ |
| Right | 0.00117 (0.00197) | 0.00128 (0.00104) | −1.022 | 0.307 | ↑ | |
| Caudate | Left | 0.00329 (0.00204) | 0.00254 (0.00165) | −3.181 | 0.001 | ↓ |
| Right | 0.00376 (0.00271) | 0.00272 (0.00188) | −2.726 | 0.006 | ↓ | |
| Hippocampus | Left | 0.00335 (0.00297) | 0.00281 (0.00135) | −1.590 | 0.112 | ↓ |
| Right | 0.00277 (0.00258) | 0.00287 (0.00141) | −1.079 | 0.281 | ↑ | |
| Pallidum | Left | 0.00443 (0.00626) | 0.00130 (0.00068) | −3.010 | 0.003 | ↓ |
| Right | 0.00363 (0.0460) | 0.00129 (0.00047) | −3.294 | 0.001 | ↓ | |
| Putamen | Left | 0.00635 (0.00622) | 0.00388 (0.00192) | −2.953 | 0.003 | ↓ |
| Right | 0.00628 (0.00536) | 0.00405 (0.00146) | −2.840 | 0.005 | ↓ | |
| Thalamus | Left | 0.01261 (0.01432) | 0.00549 (0.00266) | −3.408 | 0.001 | ↓ |
| Right | 0.01097 (0.01515) | 0.00507 (0.00244) | −3.408 | 0.001 | ↓ | |
| Subcortical Gray Matter | 0.07292 (0.06170) | 0.04187 (0.01173) | −2.442 | 0.015 | ↓ | |
| Cortex | 0.51719 (0.25186) | 0.40221 (0.11938) | −3.408 | 0.001 | ↓ | |
| Cerebral White Matter | 0.21410 (0.19096) | 0.24257 (0.09671) | −1.931 | 0.053 | ↑ |
| Evaluation | Models | R2 | p | Predictor | Beta | p |
|---|---|---|---|---|---|---|
| MDI | 1 | 0.415 | 0.010 | Treatment adherence | 0.644 | 0.010 |
| 2 | 0.598 | 0.004 | Treatment adherence | 0.649 | 0.004 | |
| Right hippocampus relative volume | 0.428 | 0.037 | ||||
| PDI | 1 | 0.319 | 0.028 | Treatment adherence | 0.565 | 0.028 |
| 2 | 0.543 | 0.009 | Treatment adherence | 0.454 | 0.043 | |
| Left hippocampus relative volume | 0.486 | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Chavira, S.A.; Gutiérrez-Hernández, C.C.; Carrillo-Prado, C.; Harmony, T. Subcortical Change and Neurohabilitation Treatment Adherence Effects in Extremely Preterm Children. Brain Sci. 2024, 14, 957. https://doi.org/10.3390/brainsci14100957
Castro-Chavira SA, Gutiérrez-Hernández CC, Carrillo-Prado C, Harmony T. Subcortical Change and Neurohabilitation Treatment Adherence Effects in Extremely Preterm Children. Brain Sciences. 2024; 14(10):957. https://doi.org/10.3390/brainsci14100957
Chicago/Turabian StyleCastro-Chavira, Susana A., Claudia C. Gutiérrez-Hernández, Cristina Carrillo-Prado, and Thalía Harmony. 2024. "Subcortical Change and Neurohabilitation Treatment Adherence Effects in Extremely Preterm Children" Brain Sciences 14, no. 10: 957. https://doi.org/10.3390/brainsci14100957
APA StyleCastro-Chavira, S. A., Gutiérrez-Hernández, C. C., Carrillo-Prado, C., & Harmony, T. (2024). Subcortical Change and Neurohabilitation Treatment Adherence Effects in Extremely Preterm Children. Brain Sciences, 14(10), 957. https://doi.org/10.3390/brainsci14100957







