Awake Craniotomy in Epilepsy Surgery: A Case Series and Proposal for Three Different Scenarios
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Diagnostic and Treatment Flow (Figure 1)

2.3. Evaluations
3. Results
3.1. Clinical Course of Patients (Figure 1)
3.2. Patients Who Underwent AWC (Table 1)
| No. | Age, Sex | Etiology | Side | Location | Seizure Type | Indication of AWC | Neurological Deficit | Seizure Outcome (Engel) | Additional Surgery |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 14, F | Cavernous malformation | R | Frontal | FAS, FBTCS | #1 | - | IIIA -> IA | Craniotomy |
| 2 | 24, M | Foral cortical dysplasia | R | Frontal | FAS, FBTCS | #1 | - | IIIA -> IA | Craniotomy |
| 3 | 41, M | LEAT | L | Insula | FIAS, FBTCS | #1 | - | IA | - |
| 4 | 29, F | Unknown | L | Frontal | FAS, FBTCS | #2 | - | IIA | - |
| 5 | 35, F | Unknown | L | Frontal | FIAS, FBTCS | #2 | - | IA | - |
| 6 | 21, M | Ulegyria | R | Fronto-Parietal | FAS | #3 | Left hand numbness | IIA | - |
| 7 | 24, F | LEAT | L | Temporal | FIAS | #3 | - | IVA -> IIIA | VNS |
| 8 | 25, M | Unknown | L | Frontal | FIAS | #3 | - | IIIA | - |
| 9 | 17, F | Unknown | L | Frontal | FIAS, FBTCS | #3 | Cognitive decline | IIIA | - |
| 10 | 32, M | Unknown | L | Temporal | FIAS, FBTCS | #3 | - | IA | - |
3.3. Case Illustrations
3.3.1. Patient #1 (AWC#1) (Figure 2)

3.3.2. Patient #5 (AWC#2) (Figure 3)
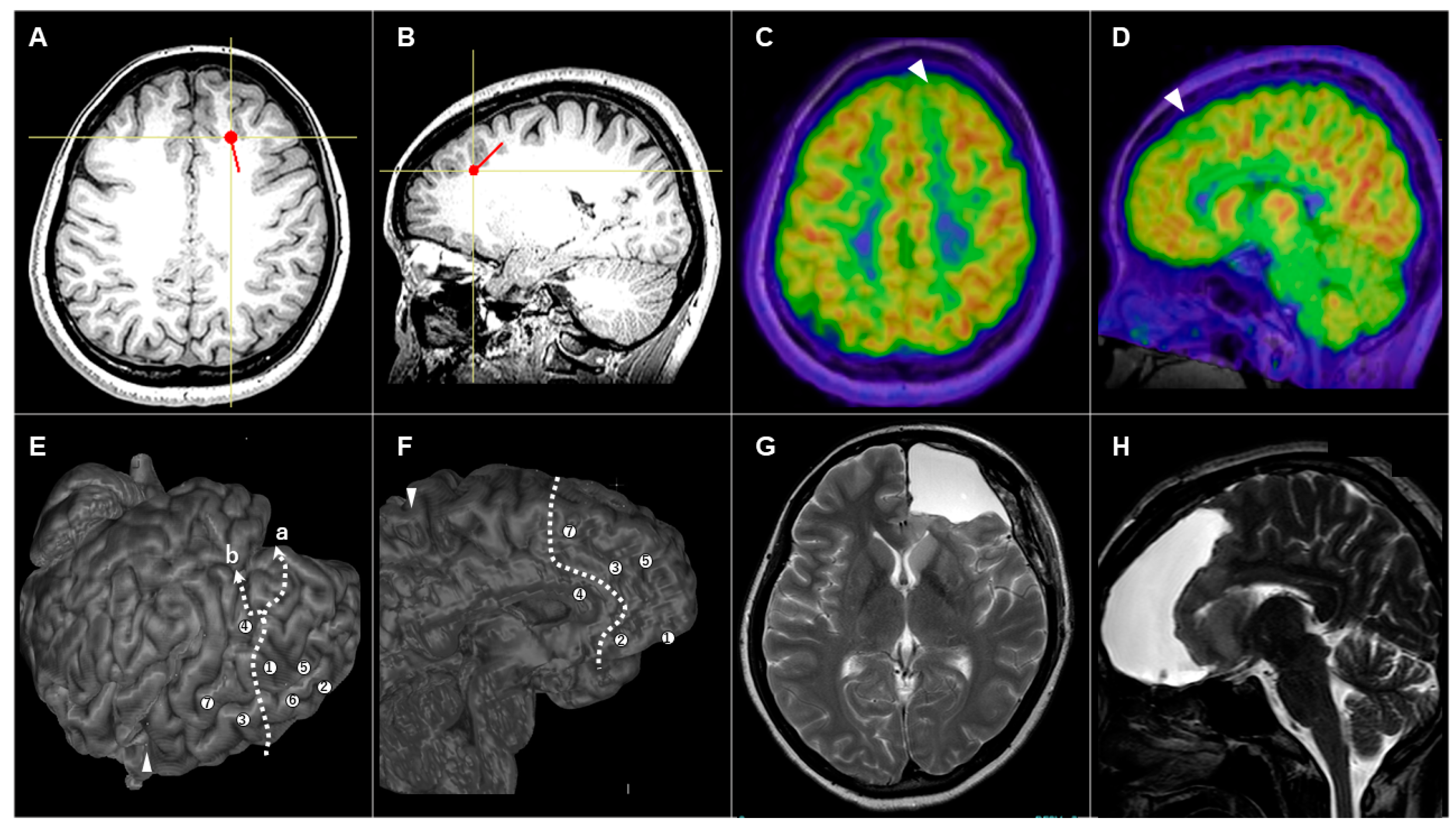
3.3.3. Patient #10 (AWC#3) (Figure 4)
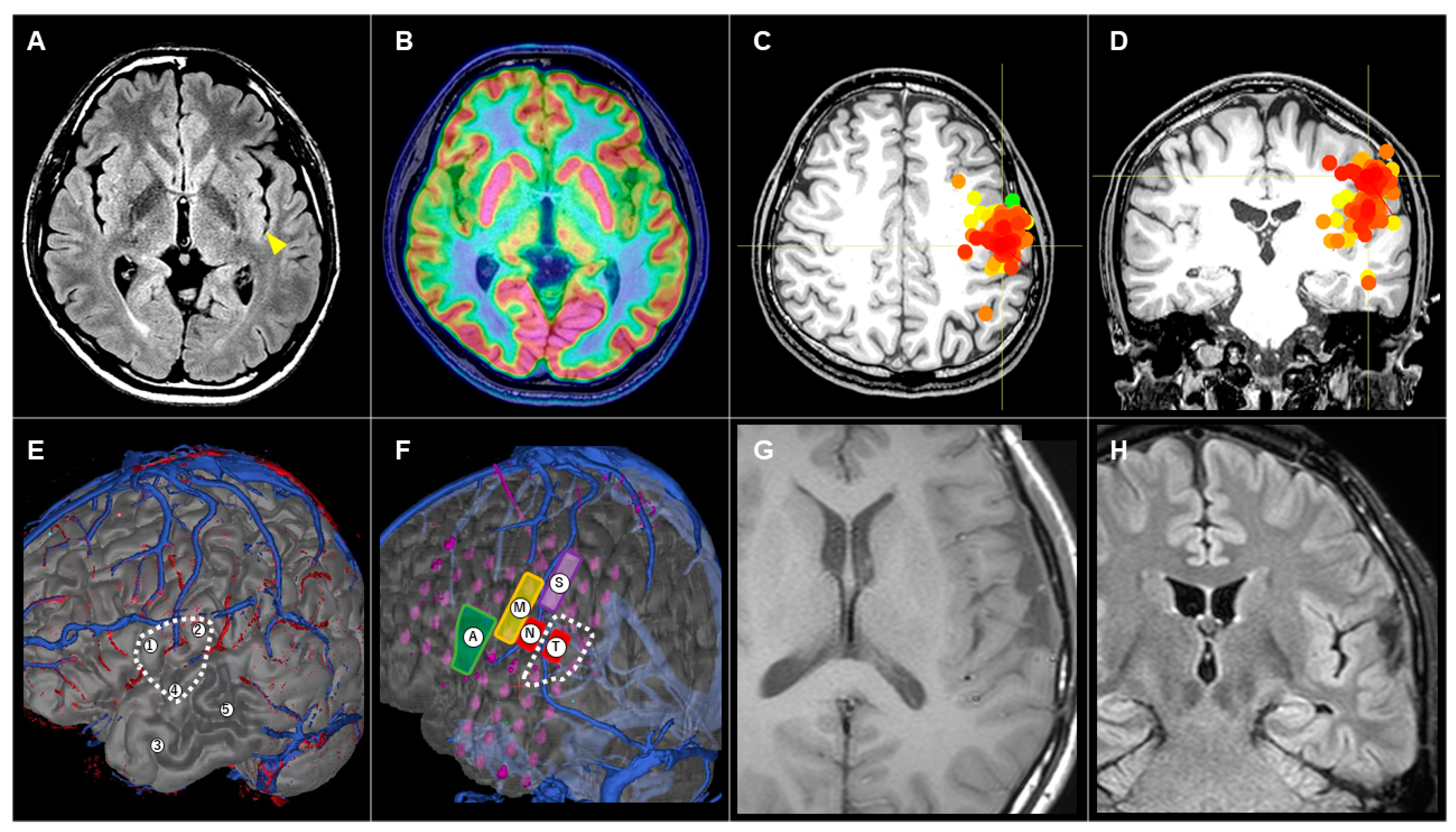
4. Discussion
4.1. Summary of the Present Study
4.2. Three Different Scenarios for Applying AWC in Epilepsy Surgery
4.2.1. AWC#1
4.2.2. AWC#2
4.2.3. AWC#3
4.3. Future Directions of AWC in Epilepsy Surgery
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fiore, G.; Abete-Fornara, G.; Forgione, A.; Tariciotti, L.; Pluderi, M.; Borsa, S.; Bana, C.; Cogiamanian, F.; Vergari, M.; Conte, V.; et al. Indication and eligibility of glioma patients for awake surgery: A scoping review by a multidisciplinary perspective. Front. Oncol. 2022, 12, 951246. [Google Scholar] [CrossRef] [PubMed]
- Guidelines Committee of the Japan Awake Surgery Conference. Guidelines for Awake Surgery. Neurol. Med. Chir. 2024, 64, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Vigren, P.; Eriksson, M.; Duffau, H.; Wretman, A.; Lindehammar, H.; Milos, P.; Richter, J.; Karlsson, T.; Gauffin, H. Experiences of awake surgery in non-tumoural epilepsy in eloquent localizations. Clin. Neurol. Neurosurg. 2020, 199, 106251. [Google Scholar] [CrossRef] [PubMed]
- Minkin, K.; Gabrovski, K.; Karazapryanov, P.; Milenova, Y.; Sirakov, S.; Karakostov, V.; Romanski, K.; Dimova, P. Awake Epilepsy Surgery in Patients with Focal Cortical Dysplasia. World Neurosurg. 2021, 151, e257–e264. [Google Scholar] [CrossRef] [PubMed]
- Sahjpaul, R.L. Awake craniotomy: Controversies, indications and techniques in the surgical treatment of temporal lobe epilepsy. Can. J. Neurol. Sci. 2000, 27 (Suppl. S1), S55–S63; discussion S92–S56. [Google Scholar] [CrossRef] [PubMed]
- Korkar, G.H.; Isnard, J.; Montavont, A.; Catenoix, H.; Rheims, S.; Guénot, M. Awake craniotomy for epilepsy surgery on eloquent speech areas: A single-centre experience. Epileptic Disord. 2021, 23, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Maesawa, S.; Nakatsubo, D.; Fujii, M.; Iijima, K.; Kato, S.; Ishizaki, T.; Shibata, M.; Wakabayashi, T. Application of Awake Surgery for Epilepsy in Clinical Practice. Neurol. Med. Chir. 2018, 58, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.M.; Hall, J.A.; Dubeau, F.; Tani, N.; Oshino, S.; Fujita, Y.; Gotman, J.; Kishima, H. Technical Aspects of SEEG and Its Interpretation in the Delineation of the Epileptogenic Zone. Neurol. Med. Chir. 2020, 60, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.V.N.P., Jr.; Rasmussen, T.B.; Ojemann, L.M. (Eds.) Outcome with Respect to Epileptic Seizures; Raven Press: New York, NY, USA, 1993; pp. 609–621. [Google Scholar]
- Chong, S.; Phi, J.H.; Lee, J.Y.; Kim, S.K. Surgical Treatment of Lesional Mesial Temporal Lobe Epilepsy. J. Epilepsy Res. 2018, 8, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T. Recent Advancement of Technologies and the Transition to New Concepts in Epilepsy Surgery. Neurol. Med. Chir. 2020, 60, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Maesawa, S.; Ishizaki, T.; Mutoh, M.; Ito, Y.; Torii, J.; Tanei, T.; Nakatsubo, D.; Saito, R. Clinical Impacts of Stereotactic Electroencephalography on Epilepsy Surgery and Associated Issues in the Current Situation in Japan. Neurol. Med. Chir. 2023, 63, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Abou-Al-Shaar, H.; Brock, A.A.; Kundu, B.; Englot, D.J.; Rolston, J.D. Increased nationwide use of stereoencephalography for intracranial epilepsy electroencephalography recordings. J. Clin. Neurosci. 2018, 53, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Burneo, J.G.; Diosy, D.C.; Joswig, H.; MacDougall, K.W.; McLachlan, R.S.; Mirsattari, S.M.; Parrent, A.G.; Steven, D.A. Intracranial Electroencephalographic Monitoring: From Subdural to Depth Electrodes. Can. J. Neurol. Sci./J. Can. Sci. Neurol. 2018, 45, 336–338. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uda, T.; Tanoue, Y.; Kawashima, T.; Yindeedej, V.; Nishijima, S.; Kunihiro, N.; Umaba, R.; Ishimoto, K.; Goto, T. Awake Craniotomy in Epilepsy Surgery: A Case Series and Proposal for Three Different Scenarios. Brain Sci. 2024, 14, 958. https://doi.org/10.3390/brainsci14100958
Uda T, Tanoue Y, Kawashima T, Yindeedej V, Nishijima S, Kunihiro N, Umaba R, Ishimoto K, Goto T. Awake Craniotomy in Epilepsy Surgery: A Case Series and Proposal for Three Different Scenarios. Brain Sciences. 2024; 14(10):958. https://doi.org/10.3390/brainsci14100958
Chicago/Turabian StyleUda, Takehiro, Yuta Tanoue, Toshiyuki Kawashima, Vich Yindeedej, Shugo Nishijima, Noritsugu Kunihiro, Ryoko Umaba, Kotaro Ishimoto, and Takeo Goto. 2024. "Awake Craniotomy in Epilepsy Surgery: A Case Series and Proposal for Three Different Scenarios" Brain Sciences 14, no. 10: 958. https://doi.org/10.3390/brainsci14100958
APA StyleUda, T., Tanoue, Y., Kawashima, T., Yindeedej, V., Nishijima, S., Kunihiro, N., Umaba, R., Ishimoto, K., & Goto, T. (2024). Awake Craniotomy in Epilepsy Surgery: A Case Series and Proposal for Three Different Scenarios. Brain Sciences, 14(10), 958. https://doi.org/10.3390/brainsci14100958







