Early Postnatal Neuroinflammation Produces Key Features of Diffuse Brain White Matter Injury in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
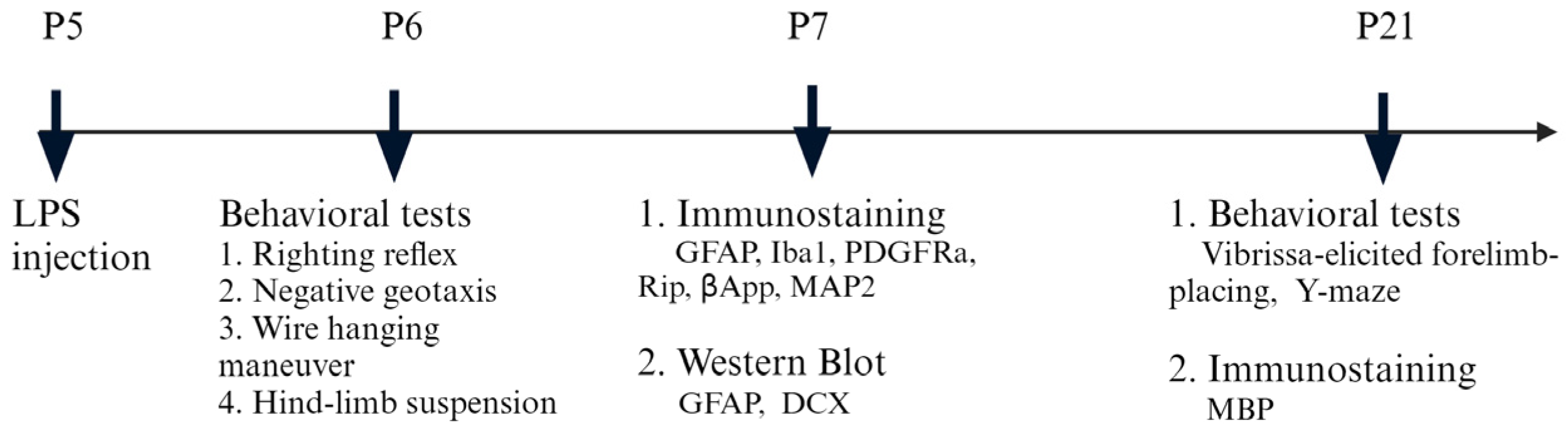
2.2. Animals and Treatments
2.3. Immunohistochemistry
2.4. Stereological Cell Counting
2.5. Neural Fiber Analysis by ImageJ
2.6. Western Blot
2.7. Behavioral Tests
2.7.1. Righting Reflex
2.7.2. Negative Geotaxis
2.7.3. Wire Hanging Maneuver
2.7.4. Hind-Limb Suspension Test
2.7.5. Vibrissa-Elicited Forelimb-Placing Test
2.7.6. Y-Maze
2.8. Data Analysis
3. Results
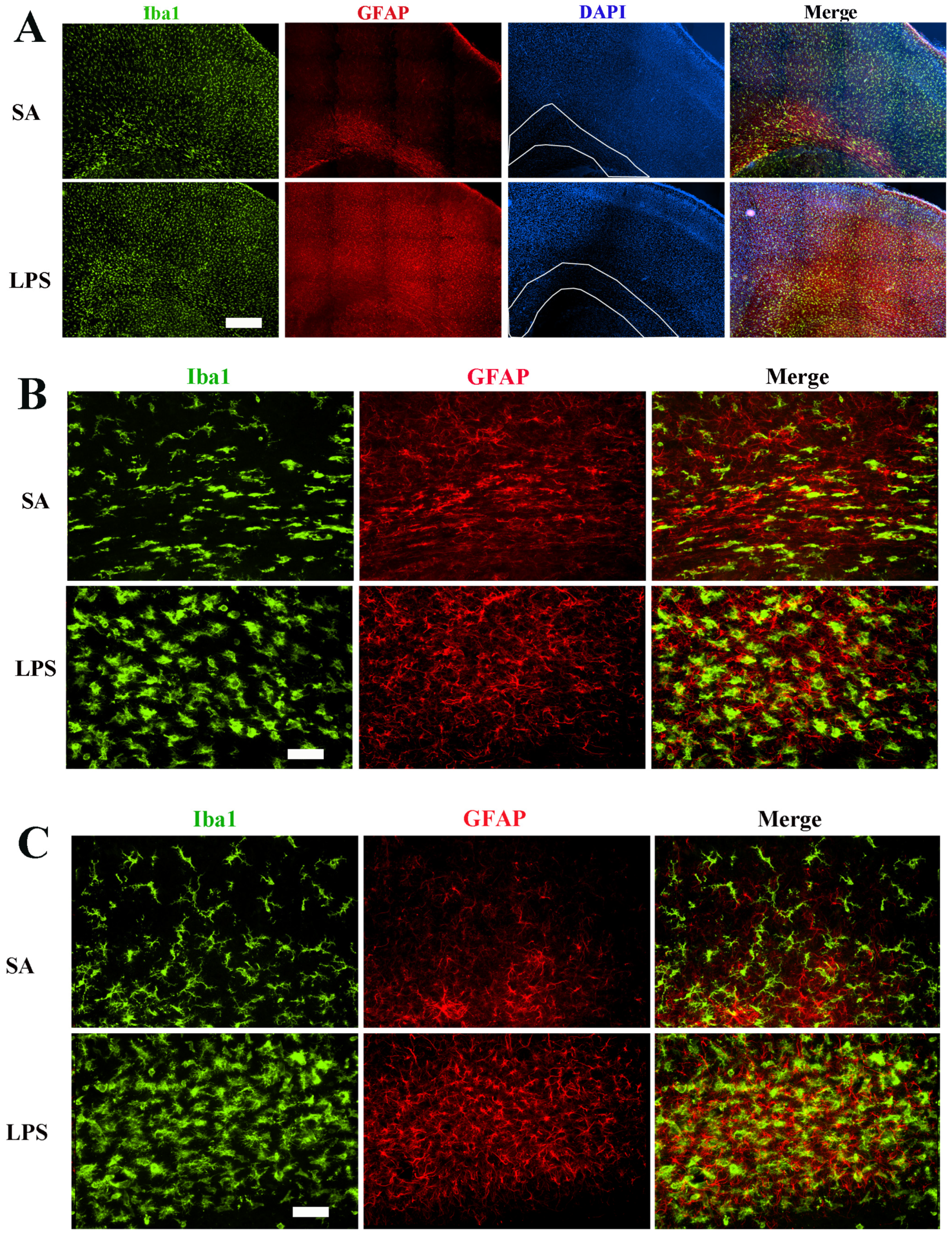
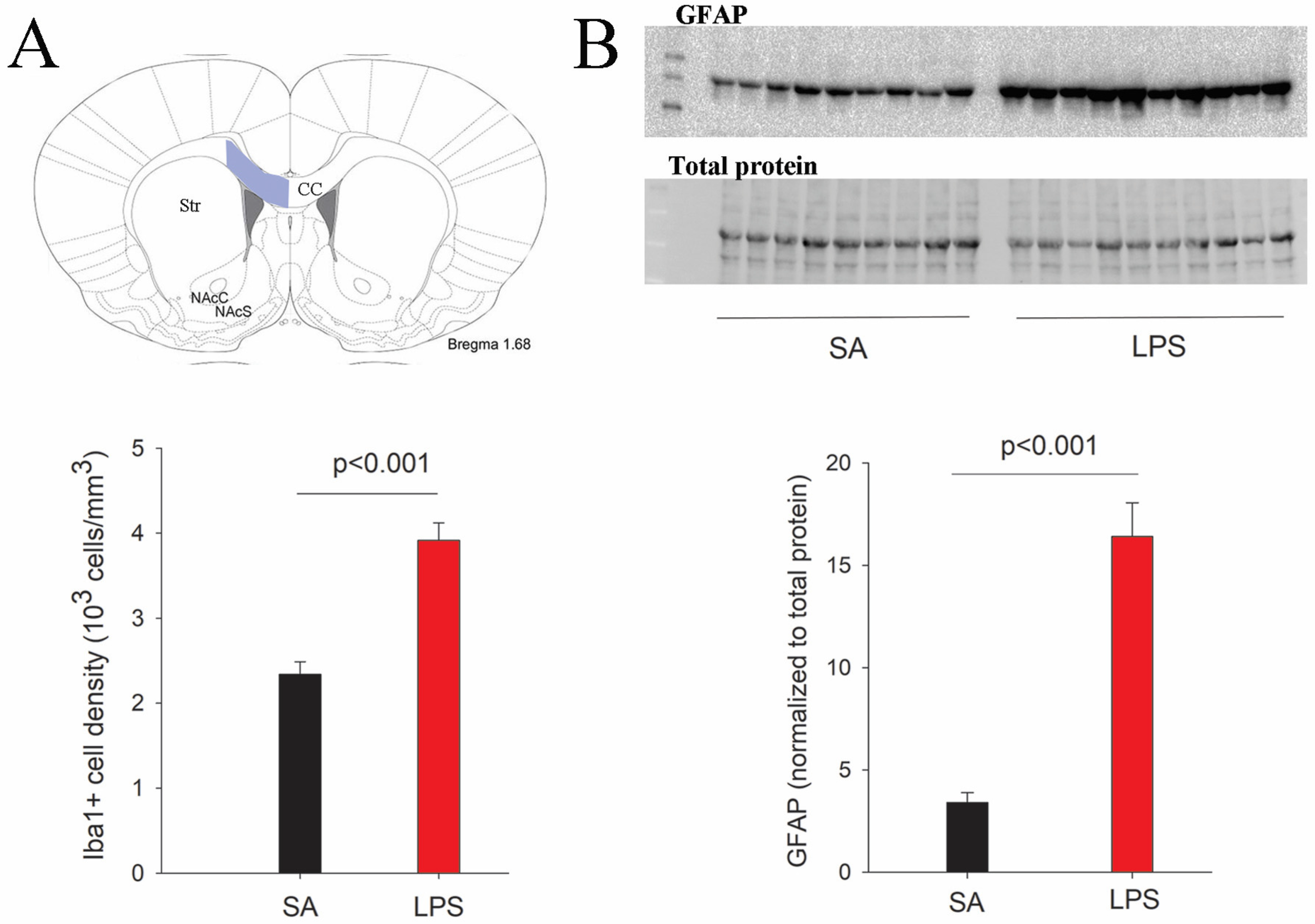
3.1. LPS Robustly Activates Microglia and Astrocytes in the Neonatal Rat Brain
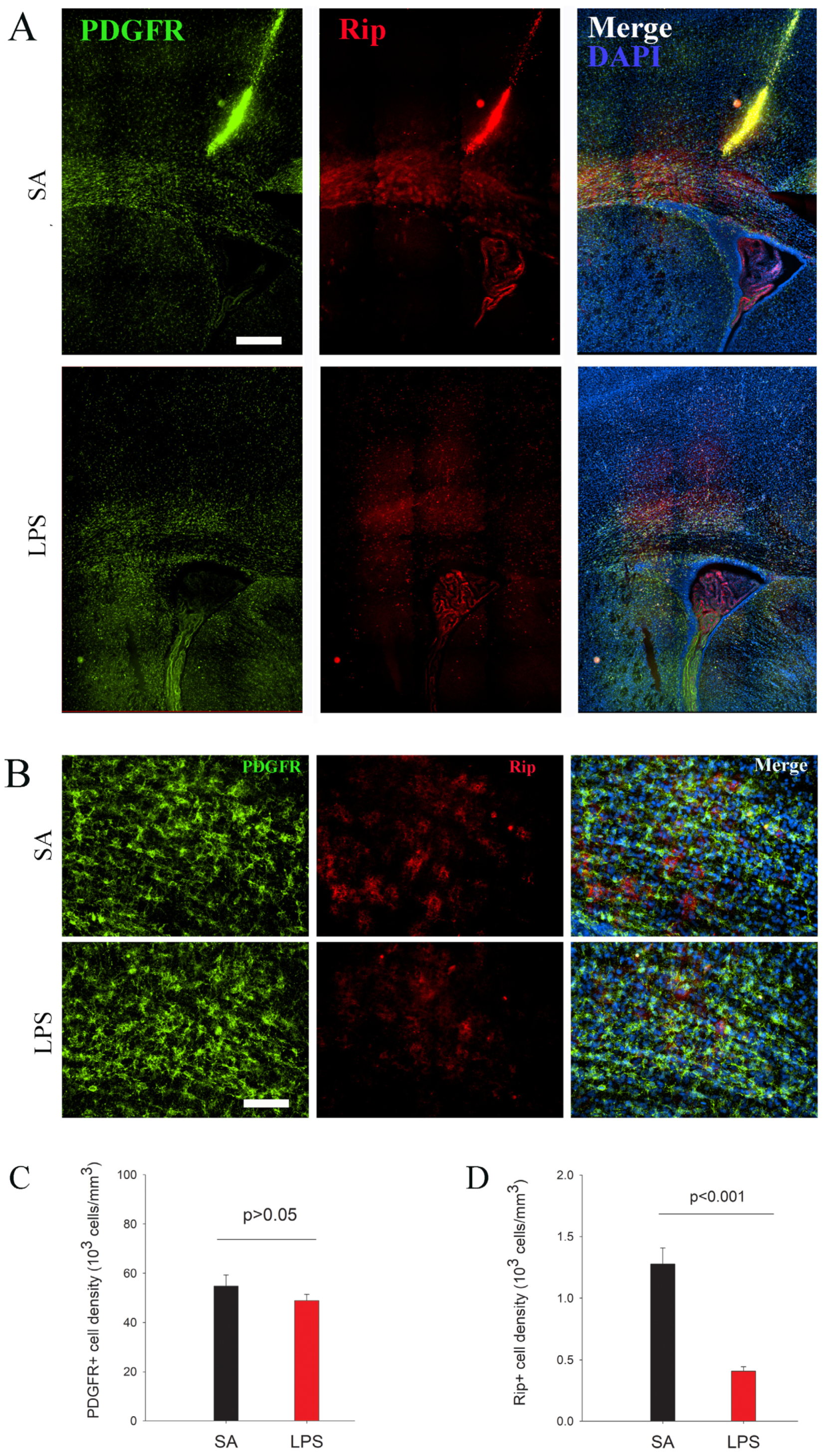
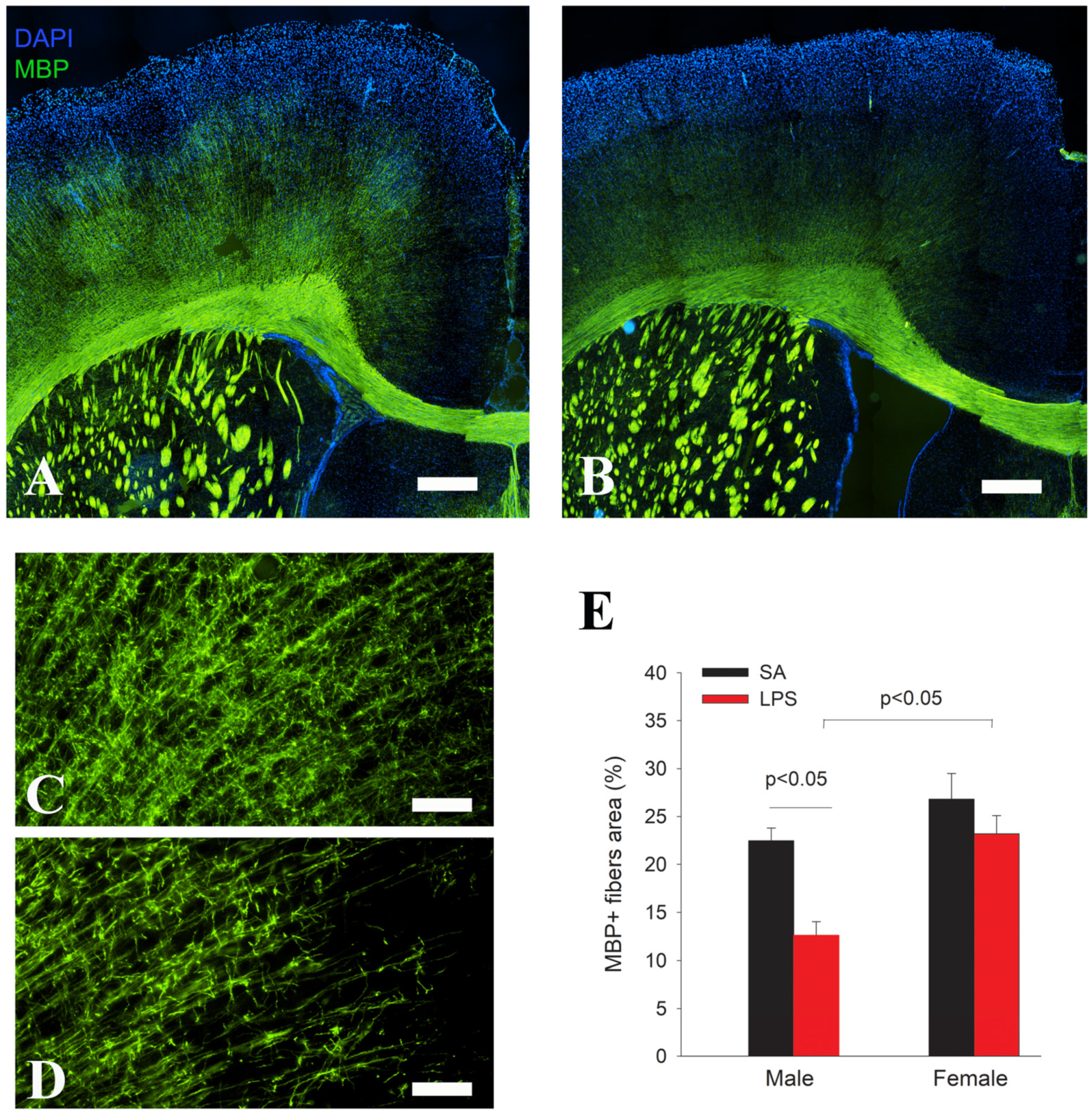
3.2. Impairment in OL Maturation and Myelination without Loss of OPCs Following LPS Exposure
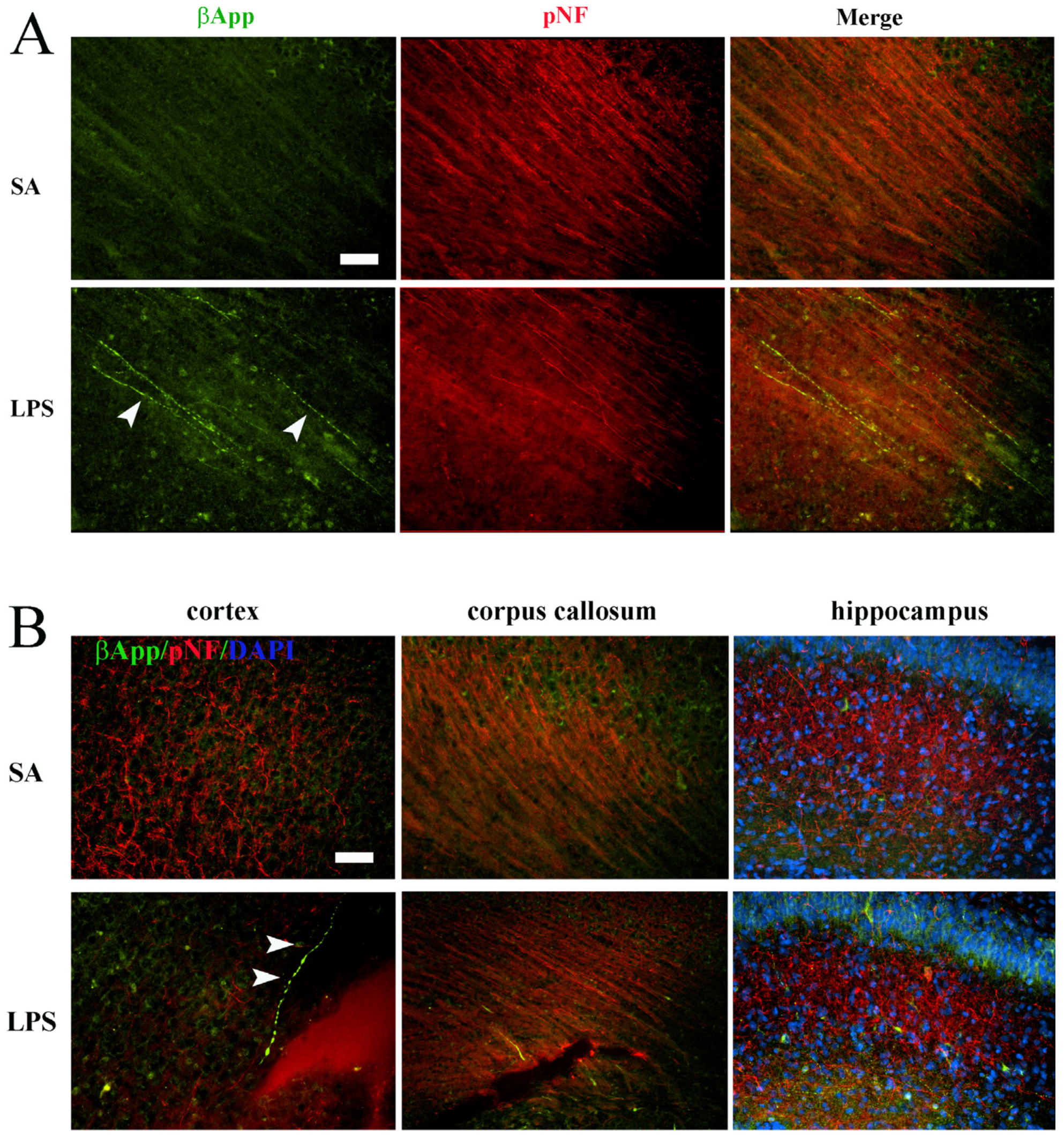
3.3. Neuroinflammation Leads to Acute Axonal Injury
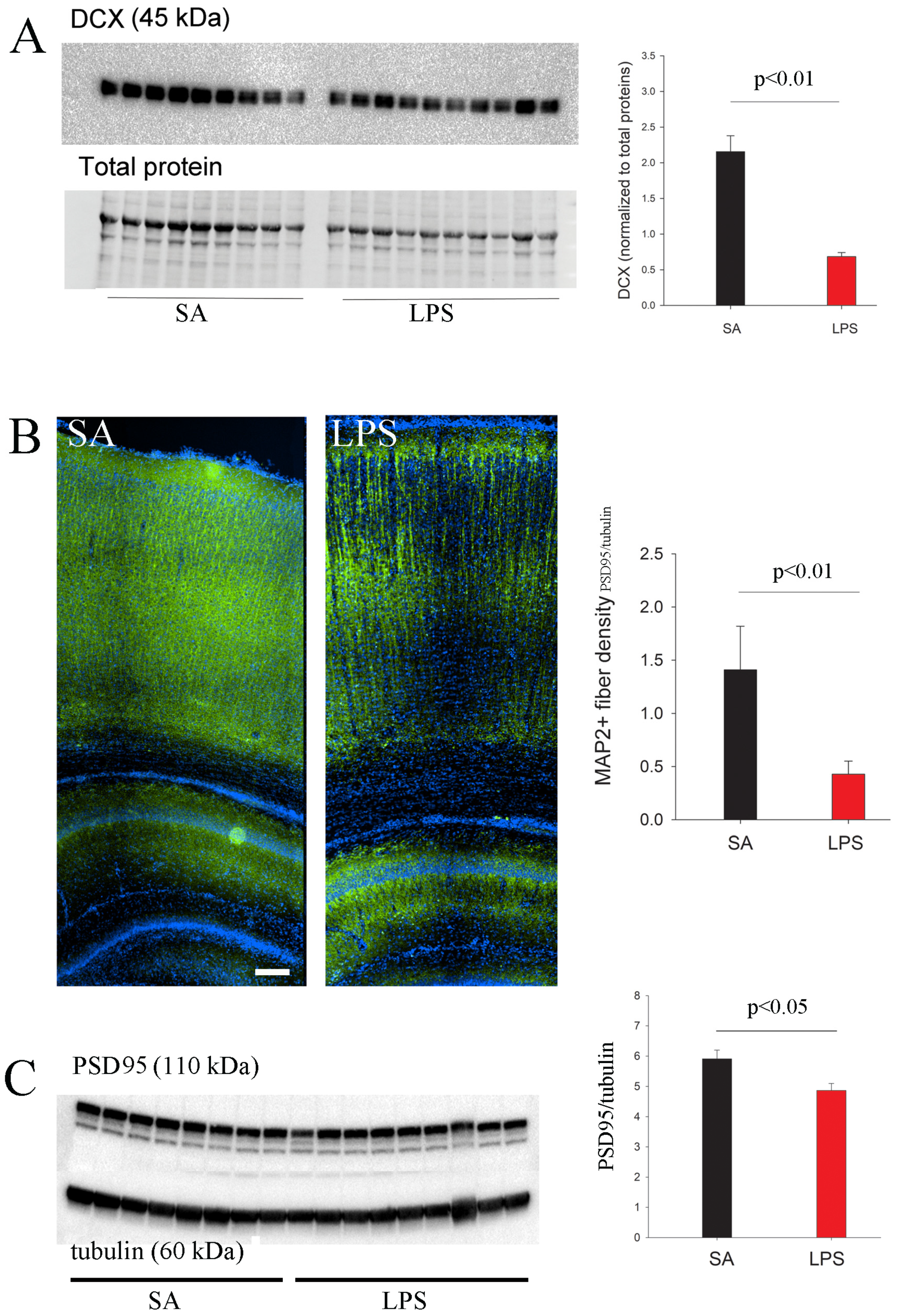
3.4. Neuroinflammation Impedes Neurogenesis, Dendritic and Synaptic Maturation
3.5. Neuroinflammation Leads to Functional Impairments
3.5.1. Righting Reflex
3.5.2. Negative Geotaxis
3.5.3. Hindlimb Suspension Test
3.5.4. Wire Hanging Maneuver Test
3.5.5. Y-Maze Test
3.5.6. Vibrissa-Elicited Forelimb-Placing Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barfield, W.D. Public Health Implications of Very Preterm Birth. Clin. Perinatol. 2018, 45, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Costello, D.; Friedman, H.; Minich, N.; Fanaroff, A.A.; Hack, M. Improved Survival Rates with Increased Neurodevelopmental Disability for Extremely Low Birth Weight Infants in the 1990s. Pediatrics 2005, 115, 997–1003. [Google Scholar] [CrossRef]
- Pascal, A.; Govaert, P.; Oostra, A.; Naulaers, G.; Ortibus, E.; Broeck, C.V.D. Neurodevelopmental Outcome in Very Preterm and Very-Low-Birthweight Infants Born over the Past Decade: A Meta-Analytic Review. Dev. Med. Child Neurol. 2018, 60, 342–355. [Google Scholar] [CrossRef]
- Volpe, J.J. Dysmaturation of Premature Brain: Importance, Cellular Mechanisms, and Potential Interventions. Pediatr. Neurol. 2019, 95, 42–66. [Google Scholar] [CrossRef]
- Ream, M.A.; Lehwald, L. Neurologic Consequences of Preterm Birth. Curr. Neurol. Neurosci. Rep. 2018, 18, 48. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Hansen, N.I.; Adams-Chapman, I.; Fanaroff, A.A.; Hintz, S.R.; Vohr, B.; Higgins, R.D.; National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and Growth Impairment among Extremely Low-Birth-Weight Infants with Neonatal Infection. JAMA 2004, 292, 2357–2365. [Google Scholar] [CrossRef]
- Volpe, J.J. Brain Injury in Premature Infants: A Complex Amalgam of Destructive and Developmental Disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Miller, S.P. Preterm Brain Injury: White Matter Injury. Handb. Clin. Neurol. 2019, 162, 155–172. [Google Scholar]
- Lee, E.S.; Kim, E.-K.; Shin, S.H.; Choi, Y.-H.; Jung, Y.H.; Kim, S.Y.; Koh, J.W.; Choi, E.K.; Cheon, J.-E.; Kim, H.-S. Factors Associated with Neurodevelopment in Preterm Infants with Systematic Inflammation. BMC Pediatr. 2021, 21, 114. [Google Scholar] [CrossRef]
- van Tilborg, E.; Heijnen, C.J.; Benders, M.J.; van Bel, F.; Fleiss, B.; Gressens, P.; Nijboer, C.H. Impaired Oligodendrocyte Maturation in Preterm Infants: Potential Therapeutic Targets. Prog. Neurobiol. 2016, 136, 28–49. [Google Scholar] [CrossRef]
- Buser, J.R.; Buser, J.R.; Maire, J.; Riddle, A.; Gong, X.; Nguyen, T.; Nelson, K.; Luo, N.L.; Ren, J.; Struve, J.; et al. Arrested Preoligodendrocyte Maturation Contributes to Myelination Failure in Premature Infants. Ann. Neurol. 2012, 71, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Cai, Z.; Rhodes, P.G. Disturbance of Oligodendrocyte Development, Hypomyelination and White Matter Injury in the Neonatal Rat Brain after Intracerebral Injection of Lipopolysaccharide. Dev. Brain Res. 2003, 140, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Lin, S.; Wright, C.; Shen, J.; Carter, K.; Bhatt, A.; Fan, L.-W. Intranasal Insulin Protects against Substantia Nigra Dopaminergic Neuronal Loss and Alleviates Motor Deficits Induced by 6-Ohda in Rats. Neuroscience 2016, 318, 157–165. [Google Scholar] [CrossRef]
- Altman, J.; Sudarshan, K.; Das, G.D.; McCormick, N.; Barnes, D. The Influence of Nutrition on Neural and Behavioral Development: III. Development of Some Motor, Particularly Locomotor Patterns during Infancy. Dev. Psychobiol. 1971, 4, 97–114. [Google Scholar] [CrossRef]
- Hermans, R.H.; Hunter, D.E.; McGivern, R.F.; Cain, C.D.; Longo, L.D. Behavioral Sequelae in Young Rats of Acute Intermittent Antenatal Hypoxia. Neurotoxicol. Teratol. 1992, 14, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.; Sudarshan, K. Postnatal Development of Locomotion in the Laboratory Rat. Anim. Behav. 1975, 23, 896–920. [Google Scholar] [CrossRef]
- El-Khodor, B.F.; Edgar, N.; Chen, A.; Winberg, M.L.; Joyce, C.; Brunner, D.; Suárez-Fariñas, M.; Heyes, M.P. Identification of a Battery of Tests for Drug Candidate Evaluation in the Smndelta7 Neonate Model of Spinal Muscular Atrophy. Exp. Neurol. 2008, 212, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Frost, S.B.; Barbay, S.; Mumert, M.L.; Stowe, A.M.; Nudo, R.J. An Animal Model of Capsular Infarct: Endothelin-1 Injections in the Rat. Behav. Brain Res. 2006, 169, 206–211. [Google Scholar] [CrossRef]
- Botanas, C.J.; Lee, H.; de la Peña, J.B.; Peña, I.J.D.; Woo, T.; Kim, H.J.; Han, D.H.; Kim, B.-N.; Cheong, J.H. Rearing in an Enriched Environment Attenuated Hyperactivity and Inattention in the Spontaneously Hypertensive Rats, an Animal Model of Attention-Deficit Hyperactivity Disorder. Physiol. Behav. 2016, 155, 30–37. [Google Scholar] [CrossRef]
- Sarter, M.; Bodewitz, G.; Stephens, D.N. Attenuation of Scopolamine-Induced Impairment of Spontaneous Alteration Behaviour by Antagonist but Not Inverse Agonist and Agonist Beta-Carbolines. Psychopharmacology 1988, 94, 491–495. [Google Scholar] [CrossRef]
- Farahani, R.M.; Xaymardan, M. Platelet-Derived Growth Factor Receptor Alpha as a Marker of Mesenchymal Stem Cells in Development and Stem Cell Biology. Stem Cells Int. 2015, 2015, 362753. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Campbell, L.; Zheng, B.; Fan, L.; Cai, Z.; Rhodes, P. Lipopolysaccharide-Activated Microglia Induce Death of Oligodendrocyte Progenitor Cells and Impede Their Development. Neuroscience 2010, 166, 464–475. [Google Scholar] [CrossRef]
- Ramarao, S.; Pang, Y.; Carter, K.; Bhatt, A. Azithromycin Protects Oligodendrocyte Progenitor Cells against Lipopolysaccharide-Activated Microglia-Induced Damage. Dev. Neurosci. 2022, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Fan, L.; Tien, L.; Dai, X.; Zheng, B.; Cai, Z.; Lin, R.C.S.; Bhatt, A. Differential Roles of Astrocyte and Microglia in Supporting Oligodendrocyte Development and Myelination in Vitro. Brain Behav. 2013, 3, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.L.; Barres, B.A. A Genetically Distinct Microglial Subset Promotes Myelination. EMBO J. 2017, 36, 3269–3271. [Google Scholar] [CrossRef]
- Pang, Y.; Cai, Z.; Rhodes, P.G. Analysis of Genes Differentially Expressed in Astrocytes Stimulated with Lipopolysaccharide Using Cdna Arrays. Brain Res. 2001, 914, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Yuan, Y.; Chen, J.; Zhu, Y.; Qiu, Y.; Zhu, F.; Huang, A.; He, C. Reactive Astrocytes Inhibit the Survival and Differentiation of Oligodendrocyte Precursor Cells by Secreted Tnf-α. J. Neurotrauma 2011, 28, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, X.; He, Q.; Zheng, Y.; Kim, D.H.; Whittemore, S.R.; Cao, Q.L. Astrocytes from the Contused Spinal Cord Inhibit Oligodendrocyte Differentiation of Adult Oligodendrocyte Precursor Cells by Increasing the Expression of Bone Morphogenetic Proteins. J. Neurosci. 2011, 31, 6053–6058. [Google Scholar] [CrossRef]
- Sloane, J.A.; Batt, C.; Ma, Y.; Harris, Z.M.; Trapp, B.; Vartanian, T. Hyaluronan Blocks Oligodendrocyte Progenitor Maturation and Remyelination through Tlr2. Proc. Natl. Acad. Sci. USA 2010, 107, 11555–11560. [Google Scholar] [CrossRef]
- Fan, L.W.; Pang, Y. Dysregulation of Neurogenesis by Neuroinflammation: Key Differences in Neurodevelopmental and Neurological Disorders. Neural Regen. Res. 2017, 12, 366–371. [Google Scholar]
- Elias, G.M.; Nicoll, R.A. Synaptic Trafficking of Glutamate Receptors by Maguk Scaffolding Proteins. Trends Cell Biol. 2007, 17, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Valtorta, F.; Pennuto, M.; Bonanomi, D.; Benfenati, F. Synaptophysin: Leading Actor or Walk-on Role in Synaptic Vesicle Exocytosis? Bioessays 2004, 26, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Coley, A.A.; Gao, W.J. Psd95: A Synaptic Protein Implicated in Schizophrenia or Autism? Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 82, 187–194. [Google Scholar] [CrossRef]
- Lin, L.; Chen, X.; Zhou, Q.; Huang, P.; Jiang, S.; Wang, H.; Deng, Y. Synaptic Structure and Alterations in the Hippocampus in Neonatal Rats Exposed to Lipopolysaccharide. Neurosci. Lett. 2019, 709, 134364. [Google Scholar] [CrossRef]
- Al-Haddad, B.J.S.; Oler, E.; Armistead, B.; Elsayed, N.A.; Weinberger, D.R.; Bernier, R.; Burd, I.; Kapur, R.; Jacobsson, B.; Wang, C.; et al. The Fetal Origins of Mental Illness. Am. J. Obs. Gynecol. 2019, 221, 549–562. [Google Scholar] [CrossRef]
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal Immune Activation and Neuroinflammation in Human Neurodevelopmental Disorders. Nat. Rev. Neurol. 2021, 17, 564–579. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Maternal Immune Activation: Implications for Neuropsychiatric Disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef]
- Skranes, J.; Vangberg, T.R.; Kulseng, S.; Indredavik, M.S.; Evensen, K.A.I.; Martinussen, M.; Dale, A.M.; Haraldseth, O.; Brubakk, A.-M. Clinical Findings and White Matter Abnormalities Seen on Diffusion Tensor Imaging in Adolescents with Very Low Birth Weight. Brain 2007, 130, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Taurines, R.; Schwenck, C.; Westerwald, E.; Sachse, M.; Siniatchkin, M.; Freitag, C. Adhd and Autism: Differential Diagnosis or Overlapping Traits? A Selective Review. Atten. Defic. Hyperact. Disord. 2012, 4, 115–139. [Google Scholar] [CrossRef]
- Jarlestedt, K.; Naylor, A.S.; Dean, J.; Hagberg, H.; Mallard, C. Decreased Survival of Newborn Neurons in the Dorsal Hippocampus after Neonatal Lps Exposure in Mice. Neuroscience 2013, 253, 21–28. [Google Scholar] [CrossRef]
- Mao, M.; Zhou, Z.; Sun, M.; Wang, C.; Sun, J. The Dysfunction of Parvalbumin Interneurons Mediated by Microglia Contributes to Cognitive Impairment Induced by Lipopolysaccharide Challenge. Neurosci. Lett. 2021, 762, 136133. [Google Scholar] [CrossRef] [PubMed]
- Lauber, E.; Filice, F.; Schwaller, B. Dysregulation of Parvalbumin Expression in the Cntnap2-/- Mouse Model of Autism Spectrum Disorder. Front. Mol. Neurosci. 2018, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Qin, H.; Chen, J.; Mou, L.; He, Y.; Yan, Y.; Zhou, H.; Lv, Y.; Chen, Z.; Wang, J.; et al. Postnatal Activation of Tlr4 in Astrocytes Promotes Excitatory Synaptogenesis in Hippocampal Neurons. J. Cell Biol. 2016, 215, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Hintz, S.R.; Kendrick, D.E.; Vohr, B.R.; Poole, W.K.; Higgins, R.D.; Nichd Neonatal Research Network. Gender Differences in Neurodevelopmental Outcomes among Extremely Preterm, Extremely-Low-Birthweight Infants. Acta Paediatr. 2006, 95, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, D.N.; McGovern, M.; Greene, C.M.; Molloy, E.J. Gender Disparities in Preterm Neonatal Outcomes. Acta Paediatr. 2018, 107, 1494–1499. [Google Scholar] [CrossRef]
- Stassart, R.M.; Mobius, W.; Nave, K.A.; Edgar, J.M. The Axon-Myelin Unit in Development and Degenerative Disease. Front. Neurosci. 2018, 12, 467. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waddell, J.; Lin, S.; Carter, K.; Truong, T.; Hebert, M.; Ojeda, N.; Fan, L.-W.; Bhatt, A.; Pang, Y. Early Postnatal Neuroinflammation Produces Key Features of Diffuse Brain White Matter Injury in Rats. Brain Sci. 2024, 14, 976. https://doi.org/10.3390/brainsci14100976
Waddell J, Lin S, Carter K, Truong T, Hebert M, Ojeda N, Fan L-W, Bhatt A, Pang Y. Early Postnatal Neuroinflammation Produces Key Features of Diffuse Brain White Matter Injury in Rats. Brain Sciences. 2024; 14(10):976. https://doi.org/10.3390/brainsci14100976
Chicago/Turabian StyleWaddell, John, Shuying Lin, Kathleen Carter, Tina Truong, May Hebert, Norma Ojeda, Lir-Wan Fan, Abhay Bhatt, and Yi Pang. 2024. "Early Postnatal Neuroinflammation Produces Key Features of Diffuse Brain White Matter Injury in Rats" Brain Sciences 14, no. 10: 976. https://doi.org/10.3390/brainsci14100976
APA StyleWaddell, J., Lin, S., Carter, K., Truong, T., Hebert, M., Ojeda, N., Fan, L.-W., Bhatt, A., & Pang, Y. (2024). Early Postnatal Neuroinflammation Produces Key Features of Diffuse Brain White Matter Injury in Rats. Brain Sciences, 14(10), 976. https://doi.org/10.3390/brainsci14100976






