Role of the Dorsal Raphe Nucleus in Pain Processing
Abstract
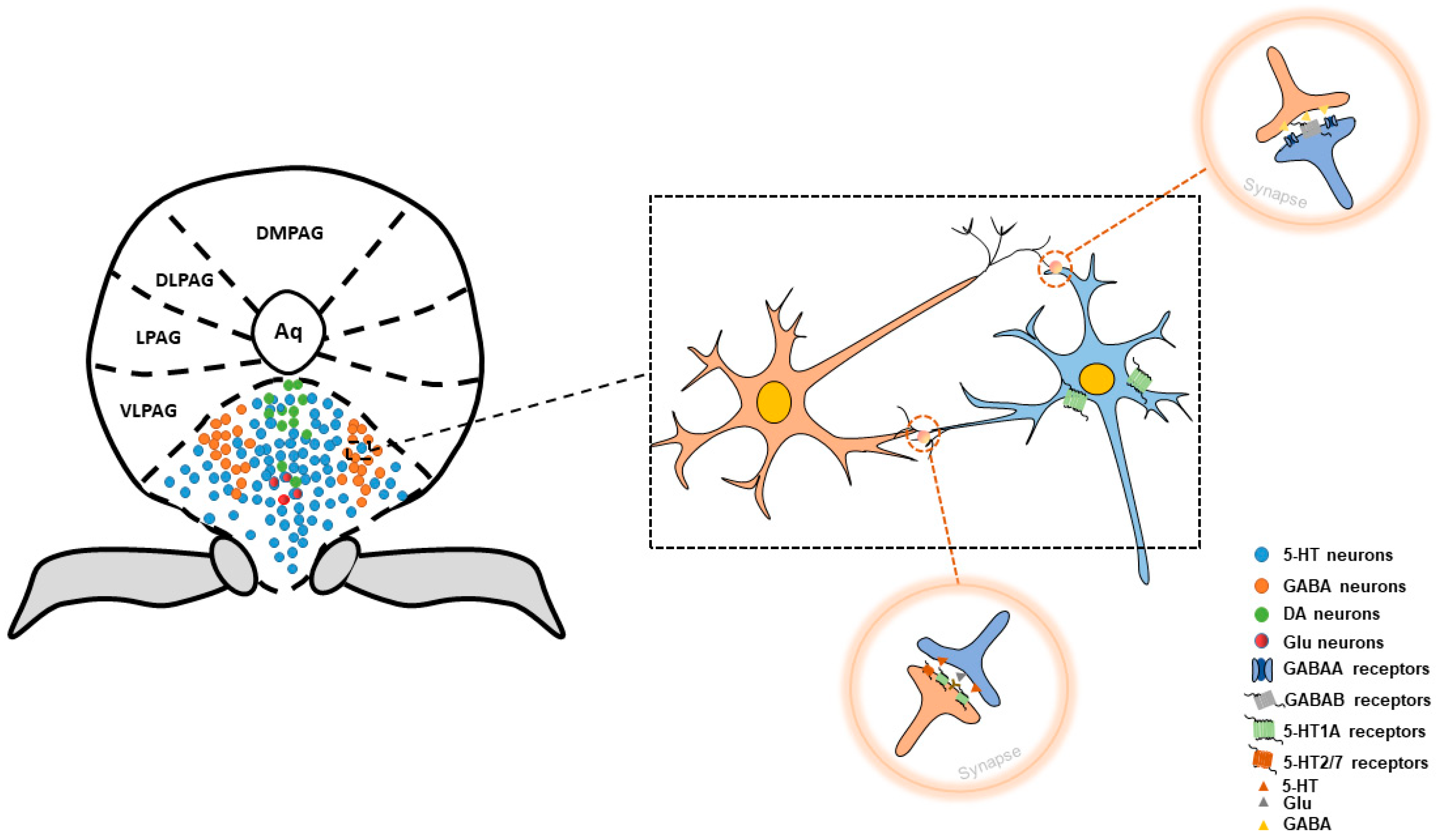
:1. Structure of Dorsal Raphe Nucleus
2. Neurons within DRN Involved in Pain Control
2.1. 5-HT Neurons
2.2. GABA Neurons
2.3. DA Neurons
2.4. Glutamatergic Neurons
2.5. Other Types of Neurons
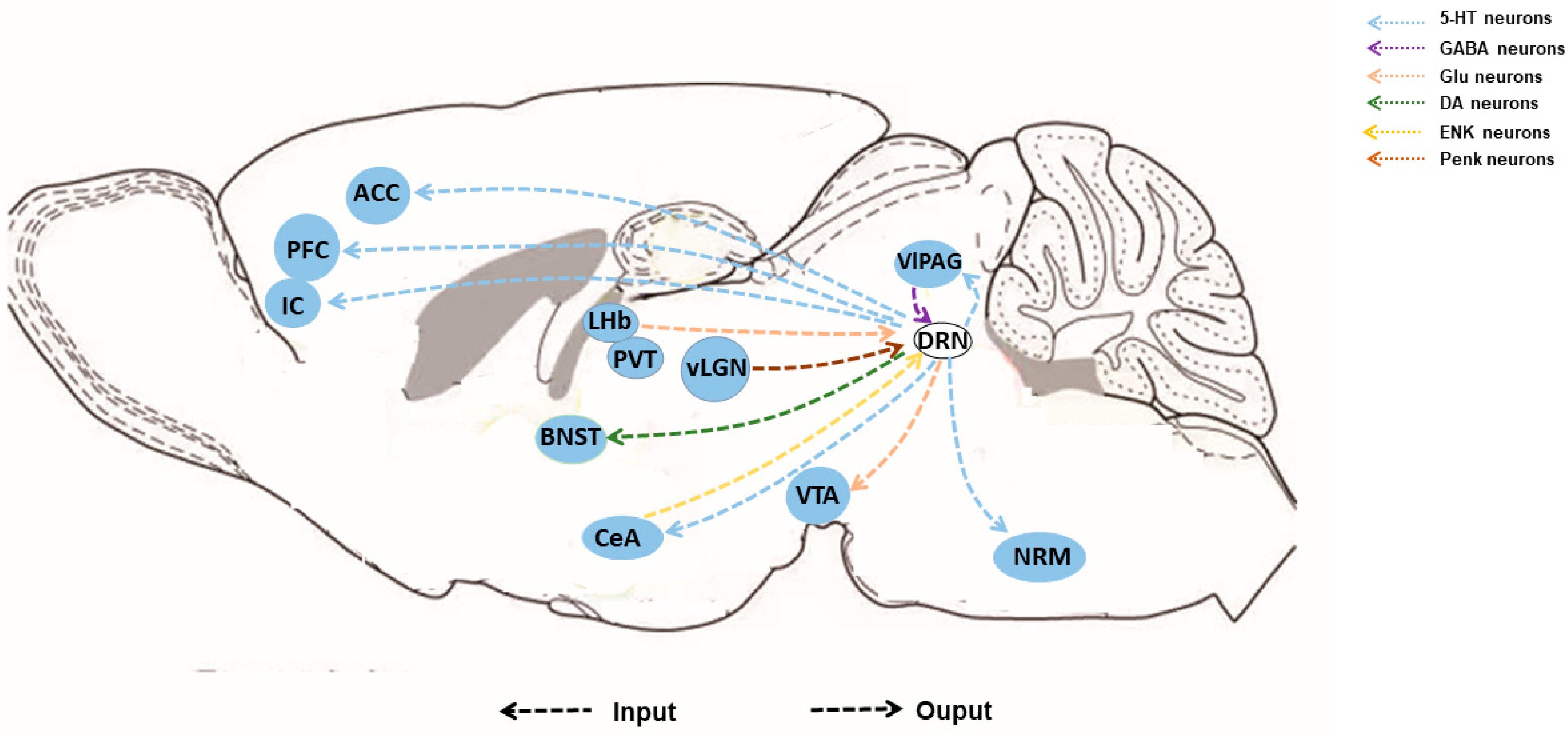
3. Afferent and Efferent Connections
3.1. Afferent Connections
3.2. Efferent Connections
4. Interaction between GABA Neurons and 5-HT Neurons
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, K.W.; Ochandarena, N.E.; Philson, A.C.; Hyun, M.; Birnbaum, J.E.; Cicconet, M.; Sabatini, B.L. Molecular and anatomical organization of the dorsal raphe nucleus. eLife 2019, 8, e46464. [Google Scholar] [CrossRef] [PubMed]
- Monti, J.M. The structure of the dorsal raphe nucleus and its relevance to the regulation of sleep and wakefulness. Sleep Med. Rev. 2010, 14, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Dorocic, I.P.; Fürth, D.; Xuan, Y.; Johansson, Y.; Pozzi, L.; Silberberg, G.; Carlén, M.; Meletis, K. A Whole-Brain Atlas of Inputs to Serotonergic Neurons of the Dorsal and Median Raphe Nuclei. Neuron 2014, 83, 663–678. [Google Scholar] [CrossRef]
- Luo, M.M.; Li, Y.; Zhong, W.X. Do dorsal raphe 5-HT neurons encode “beneficialness”? Neurobiol. Learn Mem. 2016, 135, 40–49. [Google Scholar] [CrossRef]
- Xu, Z.C.; Feng, Z.; Zhao, M.T.; Sun, Q.T.; Deng, L.; Jia, X.Y.; Jiang, T.; Luo, P.; Chen, W.; Tudi, A.; et al. Whole-brain connectivity atlas of glutamatergic and GABAergic neurons in the mouse dorsal and median raphe nuclei. eLife 2021, 10, e65502. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhang, S.L.; Qi, J.; Wang, H.K.; Cachope, R.; Mejias-Aponte, C.A.; Gomez, J.A.; Mateo-Semidey, G.E.; Beaudoin, G.M.J.; Paladini, C.A.; et al. Dorsal Raphe Dual Serotonin-Glutamate Neurons Drive Reward by Establishing Excitatory Synapses on VTA Mesoaccumbens Dopamine Neurons. Cell Rep. 2019, 26, 1128–1142. [Google Scholar] [CrossRef]
- Paquelet, G.E.; Carrion, K.; Lacefield, C.O.; Zhou, P.C.; Hen, R.; Miller, B.R. Single-cell activity and network properties of dorsal raphe nucleus serotonin neurons during emotionally salient behaviors. Neuron 2022, 110, 2664–2679. [Google Scholar] [CrossRef]
- Wang, Q.P.; Nakai, Y. The Dorsal Raphe—An Important Nucleus in Pain Modulation. Brain Res. Bull. 1994, 34, 575–585. [Google Scholar]
- Forni, M.; Thorbergsson, P.T.; Thelin, J.; Schouenborg, J. 3D microelectrode cluster and stimulation paradigm yield powerful analgesia without noticeable adverse effects. Sci. Adv. 2021, 7, eabj2847. [Google Scholar] [CrossRef]
- Forni, M.; Thorbergsson, P.T.; Gällentoft, L.; Thelin, J.; Schouenborg, J. Sustained and potent analgesia with negligible side effects enabled by adaptive individualized granular stimulation in rat brainstem. J. Neural Eng. 2023, 20, 036014. [Google Scholar] [CrossRef]
- Sommer, C. Serotonin in pain and analgesia. Mol. Neurobiol. 2004, 30, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; Chiechio, S.; Sun, Y.G.; Zhang, K.H.; Zhao, C.S.; Scott, M.; Johnson, R.L.; Deneris, E.S.; Renner, K.J.; Gereau, R.W.; et al. Mice lacking central serotonergic neurons show enhanced inflammatory pain and an impaired analgesic response to antidepressant drugs. J. Neurosci. 2007, 27, 6045–6053. [Google Scholar] [CrossRef] [PubMed]
- Perrin, F.E.; Noristani, H.N. Serotonergic mechanisms in spinal cord injury. Exp. Neurol. 2019, 318, 174–191. [Google Scholar] [CrossRef] [PubMed]
- Moriya, S.; Yamashita, A.; Nishi, R.; Ikoma, Y.; Yamanaka, A.; Kuwaki, T. Acute nociceptive stimuli rapidly induce the activity of serotonin and noradrenalin neurons in the brain stem of awake mice. IBRO Rep. 2019, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.N.; Wang, Y.; Zhang, Y.; Yang, C.X. Duloxetine for pain in fibromyalgia in adults: A systematic review and a meta-analysis. Int. J. Neurosci. 2020, 130, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Jing, J.J.; Fu, S.Y.; Zhong, Y.L.; Su, X.Z.; Shi, Z.M.; Wu, X.Z.; Yang, F.; Chen, G.Z. Ropivacaine-induced seizures evoked pain sensitization in rats: Participation of 5-HT/5-HT3R br. Neurotoxicology 2022, 93, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Q.; Yao, X.X.; Gao, S.H.; Li, R.; Li, B.J.; Yang, W.; Cui, R.J. Role of 5-HT receptors in neuropathic pain: Potential therapeutic implications. Pharmacol. Res. 2020, 159, 104949. [Google Scholar] [CrossRef]
- Cortes-Altamirano, J.L.; Olmos-Hernández, A.; Jaime, H.B.; Carrillo-Mora, P.; Bandala, C.; Reyes-Long, S.; Alfaro-Rodríguez, A. Review: 5-HT1, 5-HT2, 5-HT3 and 5-HT7 Receptors and their Role in the Modulation of Pain Response in the Central Nervous System. Curr. Neuropharmacol. 2018, 16, 210–221. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Zhou, Y.Q.; Li, J.Y.; Sun, J.; Zhang, S.; Wu, J.Y.; Gao, S.J.; Tian, X.B.; Mei, W. 5-HT1F Receptor Agonist Ameliorates Mechanical Allodynia in Neuropathic Pain via Induction of Mitochondrial Biogenesis and Suppression of Neuroinflammation. Front. Pharmacol. 2022, 13, 834570. [Google Scholar] [CrossRef]
- Okumura, T.; Nozu, T.; Ishioh, M.; Igarashi, S.; Kumei, S.; Ohhira, M. 5-HT receptors but not cannabinoid receptors in the central nervous system mediate levodopa-induced visceral antinociception in conscious rats. Naunyn-Schmiedeberg Arch. Pharmacol. 2020, 393, 1419–1425. [Google Scholar] [CrossRef]
- Yang, J.; Bae, H.B.; Ki, H.G.; Oh, J.M.; Kim, W.M.; Lee, H.G.; Yoon, M.H.; Choi, J.I. Different role of spinal 5-HT(hydroxytryptamine)7 receptors and descending serotonergic modulation in inflammatory pain induced in formalin and carrageenan rat models. Br. J. Anaesth. 2014, 113, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, J.; Zheng, Q.; Qian, Z.; Zhang, L.; Wei, C.; Han, J.; Liu, Z.; Ren, W. 5-HT1A autoreceptor in dorsal raphe nucleus mediates sensitization of conditioned place preference to cocaine in mice experienced with chronic pain. Neuroreport 2019, 30, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, J.; Tuominen, L.; Någren, K.; Hietala, J. Neuroticism and serotonin 5-HT1A receptors in healthy subjects. Psychiatry Res. Neuroimaging 2015, 234, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cornelison, L.E.; Woodman, S.E.; Durham, P.L. 5-HT3/7 and GABA receptors mediate inhibition of trigeminal nociception by dietary supplementation of grape seed extract. Nutr. Neurosci. 2022, 25, 1565–1576. [Google Scholar] [CrossRef]
- Khan, K.M.; de la Rosa, G.B.; Biggerstaff, N.; Selvakumar, G.P.; Wang, R.X.; Mason, S.; Dailey, M.E.; Marcinkiewcz, C.A. Adolescent ethanol drinking promotes hyperalgesia, neuroinflammation and serotonergic deficits in mice that persist into adulthood. Brain Behav. Immun. 2023, 107, 419–431. [Google Scholar] [CrossRef]
- Ito, H.; Yanase, M.; Yamashita, A.; Kitabatake, C.; Hamada, A.; Suhara, Y.; Narita, M.; Ikegami, D.; Sakai, H.; Yamazaki, M.; et al. Analysis of sleep disorders under pain using an optogenetic tool: Possible involvement of the activation of dorsal raphe nucleus-serotonergic neurons. Mol. Brain 2013, 6, 59. [Google Scholar] [CrossRef]
- Wu, Z.M.; Shen, Z.; Xu, Y.L.; Chen, S.Z.; Xiao, S.Q.; Ye, J.Y.; Zhang, H.Y.; Ma, X.Y.; Zhu, Y.C.; Zhu, X.X.; et al. A neural circuit associated with anxiety-like behaviors induced by chronic inflammatory pain and the anxiolytic effects of electroacupuncture. CNS Neurosci. Ther. 2024, 30, e14520. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.-Z.; He, Z.-X.; Ma, H.; Zhang, Y.-T.; Xun, Y.-F.; Yuan, W.; Hou, W.-J.; Li, Y.-T.; Lv, Z.-J.; et al. Dorsal raphe nucleus to anterior cingulate cortex 5-HTergic neural circuit modulates consolation and sociability. eLife 2021, 10, e67638. [Google Scholar] [CrossRef]
- Akbar, L.; Castillo, V.C.G.; Olorocisimo, J.P.; Ohta, Y.; Kawahara, M.; Takehara, H.; Haruta, M.; Tashiro, H.; Sasagawa, K.; Ohsawa, M.; et al. Multi-Region Microdialysis Imaging Platform Revealed Dorsal Raphe Nucleus Calcium Signaling and Serotonin Dynamics during Nociceptive Pain. Int. J. Mol. Sci. 2023, 24, 6654. [Google Scholar] [CrossRef]
- Zhou, W.J.; Jin, Y.; Meng, Q.; Zhu, X.; Bai, T.J.; Tian, Y.H.; Mao, Y.; Wang, L.K.; Xie, W.; Zhong, H.; et al. A neural circuit for comorbid depressive symptoms in chronic pain. Nat. Neurosci. 2019, 22, 1649–1658, Erratum in Nat. Neurosci. 2019, 22, 1945. [Google Scholar] [CrossRef]
- Li, Y.H.; Wang, Y.M.; Xuan, C.L.; Li, Y.; Piao, L.H.; Li, J.C.; Zhao, H. Role of the Lateral Habenula in Pain-Associated Depression. Front. Behav. Neurosci. 2017, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Bacqué-Cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdère, P. Serotonin in Animal Cognition and Behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef] [PubMed]
- Saffarpour, S.; Nasirinezhad, F. The CA1 hippocampal serotonin alterations involved in anxiety-like behavior induced by sciatic nerve injury in rats. Scand. J. Pain 2021, 21, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.S.; Meng, F.C.; Cui, Y.; Xiong, Y.L.; Li, X.Y.; Meng, F.B.; Niu, Z.X.; Zheng, J.X.; Quan, Y.Q.; Wu, S.X.; et al. Regular Aerobic Exercise Attenuates Pain and Anxiety in Mice by Restoring Serotonin-Modulated Synaptic Plasticity in the Anterior Cingulate Cortex. Med. Sci. Sports Exerc. 2022, 54, 566–581. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Zajdel, J.; Mirrasekhian, E.; Almoosawi, N.; Frisch, I.; Klawonn, A.M.; Jaarola, M.; Fritz, M.; Engblom, D. Prostaglandin-mediated inhibition of serotonin signaling controls the affective component of inflammatory pain. J. Clin. Investig. 2017, 127, 1370–1374. [Google Scholar] [CrossRef]
- Li, C.; Meng, F.T.; Garza, J.C.; Liu, J.; Lei, Y.; Kirov, S.A.; Guo, M.; Lu, X.Y. Modulation of depression-related behaviors by adiponectin AdipoR1 receptors in 5-HT neurons. Mol. Psychiatr. 2021, 26, 4205–4220. [Google Scholar] [CrossRef]
- Colangeli, R.; Teskey, G.C.; Di Giovanni, G. Endocannabinoid-serotonin systems interaction in health and disease. In Progress in Brain Research; 5-Ht Interaction with Other Neurotransmitters: Experimental Evidence and Therapeutic Relevance, Part A; Elsevier: Amsterdam, The Netherlands, 2021; Volume 259, pp. 83–134. [Google Scholar] [CrossRef]
- De Gregorio, D.; McLaughlin, R.J.; Posa, L.; Ochoa-Sanchez, R.; Enns, J.; Lopez-Canul, M.; Aboud, M.; Maione, S.; Comai, S.; Gobbi, G. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain 2019, 160, 136–150. [Google Scholar] [CrossRef]
- Toikumo, S.; Vickers-Smith, R.; Jinwala, Z.; Xu, H.; Saini, D.; Hartwell, E.E.; Pavicic, M.; Sullivan, K.A.; Xu, K.; Jacobson, D.A.; et al. A multi-ancestry genetic study of pain intensity in 598,339 veterans. Nat. Med. 2024, 30, 1075–1084. [Google Scholar] [CrossRef]
- Xie, L.H.; Wu, H.; Chen, Q.; Xu, F.; Li, H.; Xu, Q.; Jiao, C.C.; Sun, L.H.; Ullah, R.; Chen, X.Z. Divergent modulation of pain and anxiety by GABAergic neurons in the ventrolateral periaqueductal gray and dorsal raphe. Neuropsychopharmacology 2023, 48, 1509–1519. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, C.; Yue, F.; Tang, J.; Zhang, W.; Zheng, Y.; Fang, Y.; Wang, N.; Song, Z.; Zhang, Z.; et al. A midbrain GABAergic circuit constrains wakefulness in a mouse model of stress. Nat. Commun. 2024, 15, 2722. [Google Scholar] [CrossRef]
- Wu, H.; Xie, L.; Chen, Q.; Xu, F.; Dai, A.; Ma, X.; Xie, S.; Li, H.; Zhu, F.; Jiao, C.; et al. Activation of GABAergic neurons in the dorsal raphe nucleus alleviates hyperalgesia induced by ovarian hormone withdrawal. Pain 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Yao, J.Q.; Lu, C.; Wang, B.; Li, Z.Q.; Huang, M.; Tian, T.; Peng, H.; Liu, S.J. Dorsal Raphe Nucleus Serotoninergic Neurons Mediate Morphine Rewarding Effect and Conditioned Place Preference. Neuroscience 2022, 480, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, M.Z.; Li, Q.; Deng, J.; Mu, D.; Sun, Y.G. Organization of Functional Long-Range Circuits Controlling the Activity of Serotonergic Neurons in the Dorsal Raphe Nucleus. Cell Rep. 2017, 20, 1991–1993. [Google Scholar] [CrossRef]
- Cai, P.; Wang, F.D.; Yao, J.; Wang, W.F.; Hu, Y.D.; Liu, R.F.; Li, Z.S.; Zhu, Z.H.; Cai, Y.T.; Lin, Z.H.; et al. Regulation of wakefulness by GABAergic dorsal raphe nucleus-ventral tegmental area pathway. Sleep 2022, 45, zsac235. [Google Scholar] [CrossRef]
- Paretkar, T.; Dimitrov, E. Activation of enkephalinergic (Enk) interneurons in the central amygdala (CeA) buffers the behavioral effects of persistent pain. Neurobiol. Dis. 2019, 124, 364–372. [Google Scholar] [CrossRef]
- Choi, J.E.; Choi, D.I.; Lee, J.; Kim, J.; Kim, M.J.; Hong, I.; Jung, H.; Sung, Y.; Kim, J.I.; Kim, T.; et al. Synaptic ensembles between raphe and D1R-containing accumbens shell neurons underlie postisolation sociability in males. Sci. Adv. 2022, 8, eabo7527. [Google Scholar] [CrossRef]
- Cai, X.; Liu, H.L.; Feng, B.; Yu, M.; He, Y.; Liu, H.S.; Liang, C.; Yang, Y.J.; Tu, L.L.; Zhang, N.; et al. A D2 to D1 shift in dopaminergic inputs to midbrain 5-HT neurons causes anorexia in mice. Nat. Neurosci. 2022, 25, 646–658. [Google Scholar] [CrossRef]
- Lin, R.; Liang, J.W.; Wang, R.Y.; Yan, T.; Zhou, Y.T.; Liu, Y.; Feng, Q.R.; Sun, F.M.; Li, Y.L.; Li, A.A.; et al. The Raphe Dopamine System Controls the Expression of Incentive Memory. Neuron 2021, 106, 498–514.e8. [Google Scholar] [CrossRef]
- Lin, R.; Liang, J.W.; Luo, M.M. The Raphe Dopamine System: Roles in Salience Encoding, Memory Expression, and Addiction. Trends Neurosci. 2021, 44, 366–377. [Google Scholar] [CrossRef]
- Yu, W.; Pati, D.; Pina, M.M.; Schmidt, K.T.; Boyt, K.M.; Hunker, A.C.; Zweifel, L.S.; McElligott, Z.A.; Kash, T.L. Periaqueductal gray/dorsal raphe dopamine neurons contribute to sex differences in pain-related behaviors. Neuron 2021, 109, 1365–1380.e5. [Google Scholar] [CrossRef]
- Taylor, N.E.; Pei, J.Z.; Zhang, J.; Vlasov, K.Y.; Davis, T.; Taylor, E.; Weng, F.J.; Van Dort, C.J.; Solt, K.; Brown, E.N. The Role of Glutamatergic and Dopaminergic Neurons in the Periaqueductal Gray/Dorsal Raphe: Separating Analgesia and Anxiety. Eneuro 2019, 6, ENEURO.0018-0018. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.W.; Becker, S.; Schweinhardt, P.; Cahill, C. Mesolimbic dopamine signaling in acute and chronic pain: Implications for motivation, analgesia, and addiction. Pain 2016, 157, 1194–1198. [Google Scholar] [CrossRef]
- Li, C.; Kash, T.L. κ-Opioid Receptor Modulation of GABAergic Inputs onto Ventrolateral Periaqueductal Gray Dopamine Neurons. Complex Psychiatry 2019, 5, 190–199. [Google Scholar] [CrossRef]
- Flores, J.A.; El Banoua, F.; Galán-Rodríguez, B.; Fernandez-Espejo, E. Opiate anti-nociception is attenuated following lesion of large dopamine neurons of the periaqueductal grey: Critical role for D1 (not D2) dopamine receptors. Pain 2004, 110, 205–214. [Google Scholar] [CrossRef]
- Li, C.; Sugam, J.A.; Lowery-Gionta, E.G.; McElligott, Z.A.; McCall, N.M.; Lopez, A.J.; McKlveen, J.M.; Pleil, K.E.; Kash, T.L. Mu Opioid Receptor Modulation of Dopamine Neurons in the Periaqueductal Gray/Dorsal Raphe: A Role in Regulation of Pain. Neuropsychopharmacology 2016, 41, 2122–2132. [Google Scholar] [CrossRef]
- Poulin, J.F.; Zou, J.; Drouin-Ouellet, J.; Kim, K.Y.A.; Cicchetti, F.; Awatramani, R.B. Defining Midbrain Dopaminergic Neuron Diversity by Single-Cell Gene Expression Profiling. Cell Rep. 2014, 9, 930–943. [Google Scholar] [CrossRef]
- Yu, X.D.; Zhu, Y.; Sun, Q.X.; Deng, F.; Wan, J.X.; Zheng, D.; Gong, W.K.; Xie, S.Z.; Shen, C.J.; Fu, J.Y.; et al. Distinct serotonergic pathways to the amygdala underlie separate behavioral features of anxiety. Nat. Neurosci. 2022, 25, 1651. [Google Scholar] [CrossRef]
- McDevitt, R.A.; Tiran-Cappello, A.; Shen, H.; Balderas, I.; Britt, J.P.; Marino, R.A.M.; Chung, S.L.; Richie, C.T.; Harvey, B.K.; Bonci, A. Serotonergic versus Nonserotonergic Dorsal Raphe Projection Neurons: Differential Participation in Reward Circuitry. Cell Rep. 2014, 8, 1857–1869. [Google Scholar] [CrossRef]
- Liu, D.; Hu, S.-W.; Wang, D.; Zhang, Q.; Zhang, X.; Ding, H.-L.; Cao, J.-L. An Ascending Excitatory Circuit from the Dorsal Raphe for Sensory Modulation of Pain. J. Neurosci. 2024, 44, e0869232023. [Google Scholar] [CrossRef]
- Wang, X.Y.; Jia, W.B.; Xu, X.; Chen, R.; Wang, L.B.; Su, X.J.; Xu, P.F.; Liu, X.Q.; Wen, J.; Song, X.Y.; et al. A glutamatergic DRN-VTA pathway modulates neuropathic pain and comorbid anhedonia-like behavior in mice. Nat. Commun. 2023, 14, 5124. [Google Scholar] [CrossRef]
- Ma, Q.P.; Bleasdale, C. Modulation of brain stem monoamines and γ-aminobutyric acid by NKI receptors in rats. Neuroreport 2002, 13, 1809–1812. [Google Scholar] [CrossRef] [PubMed]
- Klamt, J.G.; Prado, W.A. Antinociception and Behavioral-Changes Induced by Carbachol Microinjected into Identified Sites of the Rat-Brain. Brain Res. 1991, 549, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Shi, H.P.; Hu, S.; Zha, B.X.; Yuan, A.H.; Shu, J.H.; Fan, Y.Q.; Bai, J.; Xie, H.Y.; Cui, J.C.; et al. Acupuncture Enhances Dorsal Raphe Functional Connectivity in Knee Osteoarthritis With Chronic Pain. Front. Neurol. 2022, 12, 813723. [Google Scholar] [CrossRef] [PubMed]
- Weissbourd, B.; Ren, J.; DeLoach, K.E.; Guenthner, C.J.; Miyamichi, K.; Luo, L.Q. Presynaptic Partners of Dorsal Raphe Serotonergic and GABAergic Neurons. Neuron 2014, 83, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Broadbelt, K.G.; Paterson, D.S.; Rivera, K.D.; Trachtenberg, F.L.; Kinney, H.C. Neuroanatomic relationships between the GABAergic and serotonergic systems in the developing human medulla. Auton. Neurosci. 2010, 154, 30–41. [Google Scholar] [CrossRef]
- Peyron, C.; Rampon, C.; Petit, J.M.; Luppi, P.H. Sub-regions of the dorsal raphe nucleus receive different inputs from the brainstem. Sleep Med. 2018, 49, 53–63. [Google Scholar] [CrossRef]
- Commons, K.G. Ascending serotonin neuron diversity under two umbrellas. Brain Struct. Funct. 2016, 221, 3347–3360. [Google Scholar] [CrossRef]
- Vasudeva, R.K.; Lin, R.C.S.; Simpson, K.L.; Waterhouse, B.D. Functional organization of the dorsal raphe efferent system with special consideration of nitrergic cell groups. J. Chem. Neuroanat. 2011, 41, 281–293. [Google Scholar] [CrossRef]
- Hernandez-Vazquez, F.; Garduno, J.; Hernandez-Lopez, S. GABAergic modulation of serotonergic neurons in the dorsal raphe nucleus. Rev. Neurosci. 2019, 30, 289–303. [Google Scholar] [CrossRef]
- Satija, R.; Farrell, J.A.; Gennert, D.; Schier, A.F.; Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 2015, 33, 495–502. [Google Scholar] [CrossRef]
- Santello, M.; Nevian, T. Dysfunction of cortical dendritic integration in neuropathic pain reversed by serotoninergic neuromodulation. Neuron 2015, 86, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.J.; Vincelette, L.K.; Li, J.Y.; Brady, B.H.; Christianson, J.P. Serotonin modulates social responses to stressed conspecifics via insular 5-HT2C receptors in rat. Neuropharmacology 2023, 236, 529065. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Qiu, H.B.; Xu, H.H.; Wei, K.; Zhao, L.; Zhu, C.C.; Li, C.J.; Lu, Z.J. Nicotine withdrawal induces hyperalgesia via downregulation of descending serotonergic pathway in the nucleus raphe magnus. Neuropharmacology 2021, 189, 108515. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Liu, A.L.; Lv, S.S.; Zhou, Z.R.; Cao, H.; Weng, S.J.; Zhang, Y.Q. Green light analgesia in mice is mediated by visual activation of enkephalinergic neurons in the ventrolateral geniculate nucleus. Sci. Transl. Med. 2022, 14, eabq6474. [Google Scholar] [CrossRef]
- Suzuki, T.; Nurrochmad, A.; Ozaki, M.; Khotib, J.; Nakamura, A.; Imai, S.; Shibasaki, M.; Yajima, Y.; Narita, M. Effect of a selective GABA(B) receptor agonist baclofen on the μ-opioid receptor agonist-induced antinociceptive, emetic and rewarding effects. Neuropharmacology 2005, 49, 1121–1131. [Google Scholar] [CrossRef]
- Zhou, L.C.; Liu, D.; Xie, Z.D.; Deng, D.; Shi, G.Q.; Zhao, J.L.; Bai, S.S.; Yang, L.; Zhang, R.; Shi, Y.F. Electrophysiological Characteristics of Dorsal Raphe Nucleus in Tail Suspension Test. Front. Behav. Neurosci. 2022, 16, 893465. [Google Scholar] [CrossRef]
- Li, C.; Mcelroy, B.D.; Phillips, J.; Mccloskey, N.S.; Shi, X.D.; Unterwald, E.M.; Kirby, L.G. Role of α1-GABA receptors in the serotonergic dorsal raphe nucleus in models of opioid reward, anxiety, and depression. J. Psychopharmacol. 2024, 38, 188–199. [Google Scholar] [CrossRef]
- Liu, X.J.; Wang, H.J.; Wang, X.Y.; Ning, Y.X.; Gao, J. GABABR1 in DRN mediated GABA to regulate 5-HT expression in multiple brain regions in male rats with high and low aggressive behavior. Neurochem. Int. 2021, 150, 105180. [Google Scholar] [CrossRef]
- Liu, X.J.; Wang, H.J.; Wang, X.Y.; Ning, Y.X.; Liu, W.; Gao, J. Baixiangdan capsule and Shuyu capsule regulate anger-out and anger-in, respectively: GB1-mediated GABA can regulate 5-HT levels in multiple brain regions. Aging 2023, 15, 2046–2065. [Google Scholar] [CrossRef]
- Sun, N.; Qin, Y.J.; Xu, C.; Xia, T.; Du, Z.W.; Zheng, L.P.; Li, A.A.; Meng, F.; Zhang, Y.; Zhang, J.; et al. Design of fast-onset antidepressant by dissociating SERT from nNOS in the DRN. Science 2022, 378, 390–398. [Google Scholar] [CrossRef]
- Yaman, B.; Bal, R. Pindolol potentiates the antidepressant effect of venlafaxine by inhibiting 5-HT1A receptor in DRN neurons of mice. Int. J. Neurosci. 2022, 132, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Mazzocco, M.T.; Guarnieri, F.C.; Monzani, E.; Benfenati, F.; Valtorta, F.; Comai, S. Dysfunction of the serotonergic system in the brain of synapsin triple knockout mice is associated with behavioral abnormalities resembling synapsin-related human pathologies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 105, 110135. [Google Scholar] [CrossRef]
- Li, C.; McCloskey, N.; Phillips, J.; Simmons, S.J.; Kirby, L.G. CRF-5-HT interactions in the dorsal raphe nucleus and motivation for stress-induced opioid reinstatement. Psychopharmacology 2021, 238, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Kijima, T.; Muroi, Y.; Ishii, T. Regulation of Maternal Care by Corticotropin-Releasing Factor Receptors in the Dorsal Raphe Nucleus in Mice. Behav. Neurosci. 2021, 135, 359–368. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.Z.; Du, X.A.; Zhang, H.L. Potassium Channel Conductance Is Involved in Phenylephrine-Induced Spontaneous Firing of Serotonergic Neurons in the Dorsal Raphe Nucleus. Front. Cell Neurosci. 2022, 16, 891912. [Google Scholar] [CrossRef]
- Devroye, C.; Haddjeri, N.; Cathala, A.; Rovera, R.; Drago, F.; Piazza, P.V.; Artigas, F.; Spampinato, U. Opposite control of mesocortical and mesoaccumbal dopamine pathways by serotonin receptor blockade: Involvement of medial prefrontal cortex serotonin receptors. Neuropharmacology 2017, 119, 91–99. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Gao, J.; Guo, Y.; Wang, X.; Huo, J.; Wei, P.; Cao, J. In Vivo Effect of a 5-HT7 Receptor Agonist on 5-HT Neurons and GABA Interneurons in the Dorsal Raphe Nuclei of Sham and PD Rats: An Electrophysiological Study. Am. J. Alzheimers Dis. 2017, 32, 73–81. [Google Scholar] [CrossRef]
- Rahaman, S.M.; Chowdhury, S.; Mukai, Y.; Ono, D.; Yamaguchi, H.; Yamanaka, A. Functional Interaction Between GABAergic Neurons in the Ventral Tegmental Area and Serotonergic Neurons in the Dorsal Raphe Nucleus. Front. Neurosci. 2022, 16, 87705. [Google Scholar] [CrossRef]
- Lin, S.; Huang, L.; Luo, Z.C.; Li, X.; Jin, S.Y.; Du, Z.J.; Wu, D.Y.; Xiong, W.C.; Huang, L.; Luo, Z.Y.; et al. The ATP Level in the Medial Prefrontal Cortex Regulates Depressive-like Behavior via the Medial Prefrontal Cortex-Lateral Habenula Pathway. Biol. Psychiatry 2022, 92, 179–192. [Google Scholar] [CrossRef]
- Sellnow, R.C.; Newman, J.H.; Chambers, N.; West, A.R.; Steece-Collier, K.; Sandoval, I.M.; Benskey, M.J.; Bishop, C.; Manfredsson, F.P. Regulation of dopamine neurotransmission from serotonergic neurons by ectopic expression of the dopamine D2 autoreceptor blocks levodopa-induced dyskinesia. Acta Neuropathol. Com. 2019, 7, 8. [Google Scholar] [CrossRef]
- Guiard, B.P.; El Mansari, M.; Merali, Z.; Blier, P. Functional interactions between dopamine, serotonin and norepinephrine neurons: An in-vivo electrophysiological study in rats with monoaminergic lesions. Int. J. Neuropsychoph. 2008, 11, 625–639. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, L.; Zhang, X.; Ru, G.; Zang, W. Role of the Dorsal Raphe Nucleus in Pain Processing. Brain Sci. 2024, 14, 982. https://doi.org/10.3390/brainsci14100982
Zhang H, Li L, Zhang X, Ru G, Zang W. Role of the Dorsal Raphe Nucleus in Pain Processing. Brain Sciences. 2024; 14(10):982. https://doi.org/10.3390/brainsci14100982
Chicago/Turabian StyleZhang, Huijie, Lei Li, Xujie Zhang, Guanqi Ru, and Weidong Zang. 2024. "Role of the Dorsal Raphe Nucleus in Pain Processing" Brain Sciences 14, no. 10: 982. https://doi.org/10.3390/brainsci14100982





