Advancements in Pharmacological Treatment of Alzheimer’s Disease: The Advent of Disease-Modifying Therapies (DMTs)
Abstract
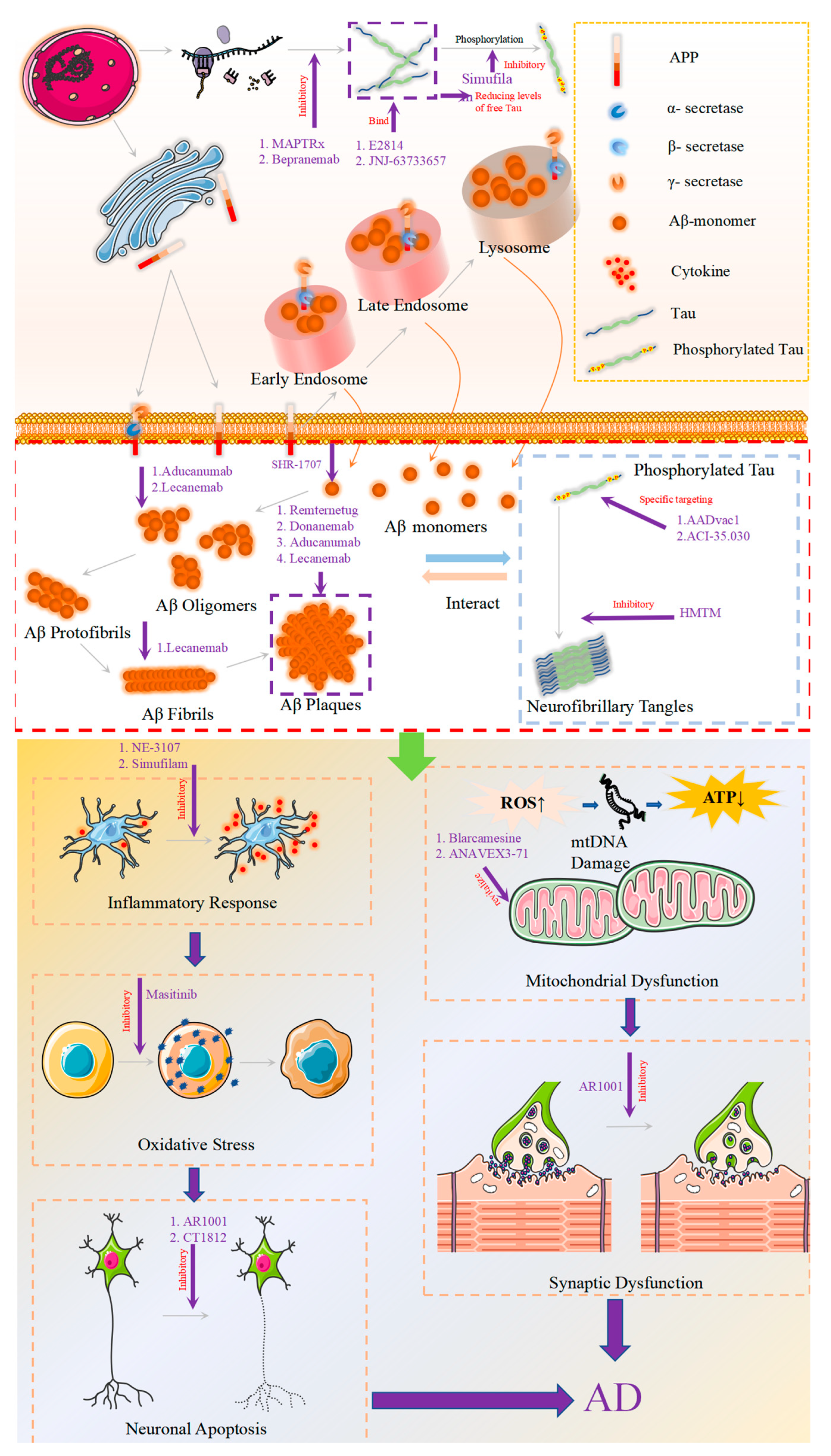
:1. Introduction
2. Novel Drugs Targeting Aβ
2.1. Approved Aβ Monoclonal Antibodies
2.1.1. Aducanumab
2.1.2. Lecanemab
2.1.3. Donanemab
2.2. Aβ Monoclonal Antibodies in Clinical Trials
2.2.1. Remternetug
2.2.2. SHR-1707
2.3. Characteristics of Aβ Monoclonal Antibody Treatment
2.3.1. Dosage of Treatment
2.3.2. Timing of Treatment
2.3.3. Form of Aβ Clearance
2.3.4. Monitoring of ARIA
3. New Drugs Targeting Tau Protein
3.1. Tau Aggregation Inhibition
3.2. Reducing the Expression of Tau Protein
3.3. Tau Immunotherapy
4. New Drugs: Beyond the Amyloid and Tau Proteins
4.1. Inflammation
4.1.1. NE-3107
4.1.2. Masitinib
4.1.3. Simufilam
4.2. Synaptic Plasticity/Neuroprotection
4.2.1. AR1001
4.2.2. Sigma-1 Receptor (S1R)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Jucker, M.; Walker, L.C. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann. Neurol. 2011, 70, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Birks, J.S.; Harvey, R.J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2018, 6, Cd001190. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef] [PubMed]
- Birks, J.; Grimley Evans, J.; Iakovidou, V.; Tsolaki, M.; Holt, F.E. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2009, 4, Cd001191. [Google Scholar]
- Reisberg, B.; Doody, R.; Stöffler, A.; Schmitt, F.; Ferris, S.; Möbius, H.J. Memantine in moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 2003, 348, 1333–1341. [Google Scholar] [CrossRef]
- Zou, B.; Li, J.; Ma, R.X.; Cheng, X.Y.; Ma, R.Y.; Zhou, T.Y.; Wu, Z.Q.; Yao, Y.; Li, J. Gut Microbiota is an Impact Factor based on the Brain-Gut Axis to Alzheimer’s Disease: A Systematic Review. Aging Dis. 2023, 14, 964–1678. [Google Scholar] [CrossRef]
- Xiao, G.; Tang, R.; Yang, N.; Chen, Y. Review on pharmacological effects of gastrodin. Arch. Pharmacal. Res. 2023, 46, 744–770. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, J.; Wang, B.; Sun, M.; Yang, H. Microglia in the Neuroinflammatory Pathogenesis of Alzheimer’s Disease and Related Therapeutic Targets. Front. Immunol. 2022, 13, 856376. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Y.; Ruan, S.; Hu, Y. Current Anti-Amyloid-β Therapy for Alzheimer’s Disease Treatment: From Clinical Research to Nanomedicine. Int. J. Nanomed. 2023, 18, 7825–7845. [Google Scholar] [CrossRef]
- Briggs, R.; Kennelly, S.P.; O’Neill, D. Drug treatments in Alzheimer’s disease. Clin. Med. 2016, 16, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Mangialasche, F.; Solomon, A.; Winblad, B.; Mecocci, P.; Kivipelto, M. Alzheimer’s disease: Clinical trials and drug development. Lancet Neurol. 2010, 9, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Tabor, G.T.; Holtzman, D.M. Current status of amyloid-targeting immunotherapies for Alzheimer’s disease. Sci. Transl. Med. 2023, 15, eadk9993. [Google Scholar] [CrossRef]
- Barakos, J.; Purcell, D.; Suhy, J.; Chalkias, S.; Burkett, P.; Marsica Grassi, C.; Castrillo-Viguera, C.; Rubino, I.; Vijverberg, E. Detection and Management of Amyloid-Related Imaging Abnormalities in Patients with Alzheimer’s Disease Treated with Anti-Amyloid Beta Therapy. J. Prev. Alzheimer’s Dis. 2022, 9, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.; Shakeri, A.; Rao, P.P. Amyloid cascade in Alzheimer’s disease: Recent advances in medicinal chemistry. Eur. J. Med. Chem. 2016, 113, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Wong, S.T.; Hanlon, E.; Morin, P. γ-Secretase modulator in Alzheimer’s disease: Shifting the end. J. Alzheimer’s Dis. 2012, 31, 685–696. [Google Scholar] [CrossRef]
- Coric, V.; van Dyck, C.H.; Salloway, S.; Andreasen, N.; Brody, M.; Richter, R.W.; Soininen, H.; Thein, S.; Shiovitz, T.; Pilcher, G.; et al. Safety and tolerability of the γ-secretase inhibitor avagacestat in a phase 2 study of mild to moderate Alzheimer disease. Arch. Neurol. 2012, 69, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.R.; Pacoma, R.; Watson, J.; Ou, W.; Alves, J.; Mason, D.E.; Peters, E.C.; Urbina, H.D.; Welzel, G.; Althage, A.; et al. Enhanced proteolytic clearance of plasma Aβ by peripherally administered neprilysin does not result in reduced levels of brain Aβ in mice. J. Neurosci. 2013, 33, 2457–2464. [Google Scholar] [CrossRef]
- Qian, C.; Yang, C.; Lu, M.; Bao, J.; Shen, H.; Deng, B.; Li, S.; Li, W.; Zhang, M.; Cao, C. Activating AhR alleviates cognitive deficits of Alzheimer’s disease model mice by upregulating endogenous Aβ catabolic enzyme Neprilysin. Theranostics 2021, 11, 8797–8812. [Google Scholar] [CrossRef]
- Jucker, M.; Walker, L.C. Alzheimer’s disease: From immunotherapy to immunoprevention. Cell 2023, 186, 4260–4270. [Google Scholar] [CrossRef]
- Song, C.; Shi, J.; Zhang, P.; Zhang, Y.; Xu, J.; Zhao, L.; Zhang, R.; Wang, H.; Chen, H. Immunotherapy for Alzheimer’s disease: Targeting β-amyloid and beyond. Transl. Neurodegener. 2022, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Salloway, S.; Sperling, R.; Fox, N.C.; Blennow, K.; Klunk, W.; Raskind, M.; Sabbagh, M.; Honig, L.S.; Porsteinsson, A.P.; Ferris, S.; et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014, 370, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.J.; Smith, J.; Donohue, M.C.; Delmar, P.; Abbas, R.; Salloway, S.; Wojtowicz, J.; Blennow, K.; Bittner, T.; Black, S.E.; et al. Two Phase 3 Trials of Gantenerumab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 389, 1862–1876. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Donohue, M.C.; Raman, R.; Rafii, M.S.; Johnson, K.; Masters, C.L.; van Dyck, C.H.; Iwatsubo, T.; Marshall, G.A.; Yaari, R.; et al. Trial of Solanezumab in Preclinical Alzheimer’s Disease. N. Engl. J. Med. 2023, 389, 1096–1107. [Google Scholar] [CrossRef]
- Ostrowitzki, S.; Bittner, T.; Sink, K.M.; Mackey, H.; Rabe, C.; Honig, L.S.; Cassetta, E.; Woodward, M.; Boada, M.; van Dyck, C.H.; et al. Evaluating the Safety and Efficacy of Crenezumab vs Placebo in Adults With Early Alzheimer Disease: Two Phase 3 Randomized Placebo-Controlled Trials. JAMA Neurol. 2022, 79, 1113–1121. [Google Scholar] [CrossRef]
- Söderberg, L.; Johannesson, M.; Nygren, P.; Laudon, H.; Eriksson, F.; Osswald, G.; Möller, C.; Lannfelt, L. Lecanemab, Aducanumab, and Gantenerumab—Binding Profiles to Different Forms of Amyloid-Beta Might Explain Efficacy and Side Effects in Clinical Trials for Alzheimer’s Disease. Neurotherapeutics 2023, 20, 195–206. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Salloway, S.; Chalkias, S.; Barkhof, F.; Burkett, P.; Barakos, J.; Purcell, D.; Suhy, J.; Forrestal, F.; Tian, Y.; Umans, K.; et al. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients with Early Alzheimer Disease. JAMA Neurol. 2022, 79, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Budd Haeberlein, S.; Aisen, P.S.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; von Hehn, C.; et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Cohen, S.; van Dyck, C.H.; Gee, M.; Doherty, T.; Kanekiyo, M.; Dhadda, S.; Li, D.; Hersch, S.; Irizarry, M.; Kramer, L.D. Lecanemab Clarity AD: Quality-of-Life Results from a Randomized, Double-Blind Phase 3 Trial in Early Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2023, 10, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.J.; Zhang, Y.; Dhadda, S.; Wang, J.; Kaplow, J.; Lai, R.Y.K.; Lannfelt, L.; Bradley, H.; Rabe, M.; Koyama, A.; et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimer’s Res. Ther. 2021, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- McDade, E.; Cummings, J.L.; Dhadda, S.; Swanson, C.J.; Reyderman, L.; Kanekiyo, M.; Koyama, A.; Irizarry, M.; Kramer, L.D.; Bateman, R.J. Lecanemab in patients with early Alzheimer’s disease: Detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimer’s Res. Ther. 2022, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, M.; Boncristiano, S.; Bondolfi, L.; Stalder, A.; Deller, T.; Staufenbiel, M.; Mathews, P.M.; Jucker, M. Cerebral hemorrhage after passive anti-Abeta immunotherapy. Science 2002, 298, 1379. [Google Scholar] [CrossRef]
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef]
- Maheshwari, S.; Singh, A.; Ansari, V.A.; Mahmood, T.; Wasim, R.; Akhtar, J.; Verma, A. Navigating the dementia landscape: Biomarkers and emerging therapies. Ageing Res. Rev. 2024, 94, 102193. [Google Scholar] [CrossRef]
- Hu, W.; Shakib, S.; Williams, J.; Fan, Y.; Zhang, Q.; Qin, H.; Wu, J.; Zhang, X.; Liu, Y.; Zhou, R.; et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of a single intravenous dose of SHR-1707 in healthy adult subjects: Two randomized, double-blind, phase 1 studies. J. Alzheimer’s Dis. 2023, 19 (Suppl. S21), e074758. [Google Scholar] [CrossRef]
- Cummings, J.; Osse, A.M.L.; Cammann, D.; Powell, J.; Chen, J. Anti-Amyloid Monoclonal Antibodies for the Treatment of Alzheimer’s Disease. BioDrugs 2023, 38, 5–22. [Google Scholar] [CrossRef]
- Rezai, A.R.; D’Haese, P.F.; Finomore, V.; Carpenter, J.; Ranjan, M.; Wilhelmsen, K.; Mehta, R.I.; Wang, P.; Najib, U.; Vieira Ligo Teixeira, C.; et al. Ultrasound Blood-Brain Barrier Opening and Aducanumab in Alzheimer’s Disease. N. Engl. J. Med. 2024, 390, 55–62. [Google Scholar] [CrossRef]
- Pardridge, W.M. Treatment of Alzheimer’s Disease and Blood-Brain Barrier Drug Delivery. Pharmaceuticals 2020, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Shcherbinin, S.; Evans, C.D.; Lu, M.; Andersen, S.W.; Pontecorvo, M.J.; Willis, B.A.; Gueorguieva, I.; Hauck, P.M.; Brooks, D.A.; Mintun, M.A.; et al. Association of Amyloid Reduction After Donanemab Treatment with Tau Pathology and Clinical Outcomes: The TRAILBLAZER-ALZ Randomized Clinical Trial. JAMA Neurol. 2022, 79, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Roytman, M.; Mashriqi, F.; Al-Tawil, K.; Schulz, P.E.; Zaharchuk, G.; Benzinger, T.L.S.; Franceschi, A.M. Amyloid-Related Imaging Abnormalities: An Update. AJR Am. J. Roentgenol. 2023, 220, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Barakos, J.; Sperling, R.; Salloway, S.; Jack, C.; Gass, A.; Fiebach, J.B.; Tampieri, D.; Melançon, D.; Miaux, Y.; Rippon, G.; et al. MR imaging features of amyloid-related imaging abnormalities. AJNR Am. J. Neuroradiol. 2013, 34, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Jouanne, M.; Rault, S.; Voisin-Chiret, A.S. Tau protein aggregation in Alzheimer’s disease: An attractive target for the development of novel therapeutic agents. Eur. J. Med. Chem. 2017, 139, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Mummery, C.J.; Börjesson-Hanson, A.; Blackburn, D.J.; Vijverberg, E.G.B.; De Deyn, P.P.; Ducharme, S.; Jonsson, M.; Schneider, A.; Rinne, J.O.; Ludolph, A.C.; et al. Tau-targeting antisense oligonucleotide MAPT(Rx) in mild Alzheimer’s disease: A phase 1b, randomized, placebo-controlled trial. Nat. Med. 2023, 29, 1437–1447. [Google Scholar] [CrossRef]
- Sinsky, J.; Pichlerova, K.; Hanes, J. Tau Protein Interaction Partners and Their Roles in Alzheimer’s Disease and Other Tauopathies. Int. J. Mol. Sci. 2021, 22, 9207. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.; Lucas, J.J.; Perez, M.; Hernandez, F. Role of tau protein in both physiological and pathological conditions. Physiol. Rev. 2004, 84, 361–384. [Google Scholar] [CrossRef]
- Wischik, C.M.; Bentham, P.; Gauthier, S.; Miller, S.; Kook, K.; Schelter, B.O. Oral Tau Aggregation Inhibitor for Alzheimer’s Disease: Design, Progress and Basis for Selection of the 16 mg/day Dose in a Phase 3, Randomized, Placebo-Controlled Trial of Hydromethylthionine Mesylate. J. Prev. Alzheimer’s Dis. 2022, 9, 780–790. [Google Scholar] [CrossRef]
- Self, W.K.; Holtzman, D.M. Emerging diagnostics and therapeutics for Alzheimer disease. Nat. Med. 2023, 29, 2187–2199. [Google Scholar] [CrossRef] [PubMed]
- Wiser, I.; Balicer, R.D.; Cohen, D. An update on smallpox vaccine candidates and their role in bioterrorism related vaccination strategies. Vaccine 2007, 25, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Novak, P.; Kovacech, B.; Katina, S.; Schmidt, R.; Scheltens, P.; Kontsekova, E.; Ropele, S.; Fialova, L.; Kramberger, M.; Paulenka-Ivanovova, N.; et al. ADAMANT: A placebo-controlled randomized phase 2 study of AADvac1, an active immunotherapy against pathological tau in Alzheimer’s disease. Nat. Aging 2021, 1, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Godyń, J.; Jończyk, J.; Panek, D.; Malawska, B. Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacol. Rep. 2016, 68, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Dibello, V.; Sardone, R.; Castellana, F.; Zupo, R.; Lampignano, L.; Bortone, I.; Stallone, R.; Cirillo, N.; Damiani, C.; et al. Clinical development of passive tau-based immunotherapeutics for treating primary and secondary tauopathies. Expert Opin. Investig. Drugs 2023, 32, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Congdon, E.E.; Ji, C.; Tetlow, A.M.; Jiang, Y.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease: Current status and future directions. Nat. Rev. Neurol. 2023, 19, 715–736. [Google Scholar] [CrossRef]
- Roberts, M.; Sevastou, I.; Imaizumi, Y.; Mistry, K.; Talma, S.; Dey, M.; Gartlon, J.; Ochiai, H.; Zhou, Z.; Akasofu, S.; et al. Pre-clinical characterisation of E2814, a high-affinity antibody targeting the microtubule-binding repeat domain of tau for passive immunotherapy in Alzheimer’s disease. Acta Neuropathol. Commun. 2020, 8, 13. [Google Scholar] [CrossRef]
- Lövestam, S.; Li, D.; Wagstaff, J.L.; Kotecha, A.; Kimanius, D.; McLaughlin, S.H.; Murzin, A.G.; Freund, S.M.V.; Goedert, M.; Scheres, S.H.W. Disease-specific tau filaments assemble via polymorphic intermediates. Nature 2024, 625, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 5–21. [Google Scholar] [CrossRef]
- The, L. Lecanemab for Alzheimer’s disease: Tempering hype and hope. Lancet 2022, 400, 1899. [Google Scholar]
- Mead, S.; Fox, N.C. Lecanemab slows Alzheimer’s disease: Hope and challenges. Lancet Neurol. 2023, 22, 106–108. [Google Scholar] [CrossRef]
- Twarowski, B.; Herbet, M. Inflammatory Processes in Alzheimer’s Disease-Pathomechanism, Diagnosis and Treatment: A Review. Int. J. Mol. Sci. 2023, 24, 6518. [Google Scholar] [CrossRef] [PubMed]
- Reading, C.L.; Ahlem, C.N.; Murphy, M.F. NM101 Phase III study of NE3107 in Alzheimer’s disease: Rationale, design and therapeutic modulation of neuroinflammation and insulin resistance. Neurodegener. Dis. Manag. 2021, 11, 289–298. [Google Scholar] [CrossRef]
- McShane, R.; Westby, M.J.; Roberts, E.; Minakaran, N.; Schneider, L.; Farrimond, L.E.; Maayan, N.; Ware, J.; Debarros, J. Memantine for dementia. Cochrane Database Syst. Rev. 2019, 3, Cd003154. [Google Scholar] [CrossRef] [PubMed]
- Ettcheto, M.; Cano, A.; Sanchez-López, E.; Verdaguer, E.; Folch, J.; Auladell, C.; Camins, A. Masitinib for the treatment of Alzheimer’s disease. Neurodegener. Dis. Manag. 2021, 11, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; López-Arrieta, J.; Lipschitz, S.; Doskas, T.; Spiru, L.; Moroz, S.; Venger, O.; Vermersch, P.; Moussy, A.; Mansfield, C.D.; et al. Masitinib for mild-to-moderate Alzheimer’s disease: Results from a randomized, placebo-controlled, phase 3, clinical trial. Alzheimer’s Res. Ther. 2023, 15, 39. [Google Scholar] [CrossRef]
- Wang, H.Y.; Cecon, E.; Dam, J.; Pei, Z.; Jockers, R.; Burns, L.H. Simufilam Reverses Aberrant Receptor Interactions of Filamin A in Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 13927. [Google Scholar] [CrossRef]
- Wang, H.Y.; Pei, Z.; Lee, K.C.; Nikolov, B.; Doehner, T.; Puente, J.; Friedmann, N.; Burns, L.H. Simufilam suppresses overactive mTOR and restores its sensitivity to insulin in Alzheimer’s disease patient lymphocytes. Front. Aging 2023, 4, 1175601. [Google Scholar] [CrossRef]
- Kang, B.W.; Kumar, A.; Song, D.-K.; Ha, J.-Y.; Choung, J.J. Protective Effects of AR1001 in Alzheimer’s Disease Models: Polypharmacological Mechanisms. J. Alzheimer’s Dis. 2023, 19 (Suppl. S24), e082892. [Google Scholar]
- Ryskamp, D.A.; Korban, S.; Zhemkov, V.; Kraskovskaya, N.; Bezprozvanny, I. Neuronal Sigma-1 Receptors: Signaling Functions and Protective Roles in Neurodegenerative Diseases. Front. Neurosci. 2019, 13, 862. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Pokrass, M.J.; Klauer, N.R.; Nohara, H.; Su, T.P. Sigma-1 receptor regulates Tau phosphorylation and axon extension by shaping p35 turnover via myristic acid. Proc. Natl. Acad. Sci. USA 2015, 112, 6742–6747. [Google Scholar] [CrossRef]
- Christ, M.G.; Huesmann, H.; Nagel, H.; Kern, A.; Behl, C. Sigma-1 Receptor Activation Induces Autophagy and Increases Proteostasis Capacity In Vitro and In Vivo. Cells 2019, 8, 211. [Google Scholar] [CrossRef]
- Delprat, B.; Crouzier, L.; Su, T.P.; Maurice, T. At the Crossing of ER Stress and MAMs: A Key Role of Sigma-1 Receptor? Adv. Exp. Med. Biol. 2020, 1131, 699–718. [Google Scholar] [PubMed]
- Pannuzzo, M. On the physiological/pathological link between Aβ peptide, cholesterol, calcium ions and membrane deformation: A molecular dynamics study. Biochim. Biophys. Acta 2016, 1858, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Caraci, F.; Cuello, A.C.; Caruso, G.; Nisticò, R.; Corbo, M.; Baldacci, F.; Toschi, N.; Garaci, F.; Chiesa, P.A.; et al. A Path Toward Precision Medicine for Neuroinflammatory Mechanisms in Alzheimer’s Disease. Front. Immunol. 2020, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Orciani, C.; Do Carmo, S.; Foret, M.K.; Hall, H.; Bonomo, Q.; Lavagna, A.; Huang, C.; Cuello, A.C. Early treatment with an M1 and sigma-1 receptor agonist prevents cognitive decline in a transgenic rat model displaying Alzheimer-like amyloid pathology. Neurobiol. Aging 2023, 132, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Grundman, M.; Morgan, R.; Lickliter, J.D.; Schneider, L.S.; DeKosky, S.; Izzo, N.J.; Guttendorf, R.; Higgin, M.; Pribyl, J.; Mozzoni, K.; et al. A phase 1 clinical trial of the sigma-2 receptor complex allosteric antagonist CT1812, a novel therapeutic candidate for Alzheimer’s disease. Alzheimer’s Dement. 2019, 5, 20–26. [Google Scholar] [CrossRef]
- LaBarbera, K.M.; Sheline, Y.I.; Izzo, N.J.; Yuede, C.M.; Waybright, L.; Yurko, R.; Edwards, H.M.; Gardiner, W.D.; Blennow, K.; Zetterberg, H.; et al. A phase 1b randomized clinical trial of CT1812 to measure Aβ oligomer displacement in Alzheimer’s disease using an indwelling CSF catheter. Transl. Neurodegener. 2023, 12, 24. [Google Scholar] [CrossRef]
| Mechanism of Action | Drug Name | Targets of Drug Action | Effectiveness | Safety | Research Enterprise | Present Stage |
|---|---|---|---|---|---|---|
| Aβ Monoclonal Antibodies | Aducanumab | oligomers, fibrils, plaques | yes | low | Biogen/Eisai | Approvable for marketing (2021) |
| Lecanemab | oligomers, fibrils, plaques | yes | high | Biogen/Eisai | Full approval for marketing (2023) | |
| Donanemab | plaques | yes | moderate | Eli Lilly | Full approval for marketing (2024) | |
| Remternetug | N3pG Aβ | yes | moderate | Eli Lilly | Phase III | |
| SHR-1707 | Aβ monomer | yes | / | Hengrui | Phase III | |
| Targeting Tau Protein | HMTM (TRx0237) | tau, TDP-43, and synuclein | uncertain | uncertain | Taurx Therapeutics | Phase III |
| MAPTRx | antisense oligonucleotide | uncertain | uncertain | Biogen | Phase II | |
| ACI-35.030 | p-tau | uncertain | uncertain | AC Immune | Phase II | |
| AADvac-1 | tau | uncertain | uncertain | Axon Neuroscience SE | Phase II | |
| JNJ-63733657 | tau | uncertain | uncertain | Janssen | Phase II | |
| Bepranemab | tau | uncertain | uncertain | Hoffmann-La Roche | Phase II | |
| E2814 | tau | uncertain | uncertain | Eisai Co | Phase I b/IIa | |
| Inflammation | NE3107 | anti-inflammatory | uncertain | uncertain | BioVie Pharma | Phase III |
| Masitinib | innate immune cells | uncertain | uncertain | AB Science | Phase III | |
| Simufilam | filamin A | uncertain | uncertain | Cassava Sciences | Phase III | |
| Synaptic Plasticity/Neuroprotection | Anavex 2-73 | M1 and σ1 receptor | uncertain | uncertain | Anavex Life Science Corp | Phase IIb/III |
| Anavex 3-71 | M1 receptor | uncertain | uncertain | Anavex Life Sciences Corp | Phase II | |
| CT1812 | Sigma-2 Receptor | uncertain | uncertain | Cognition Therapeutics Inc | Phase II | |
| AR1001 | Phosphodiesterase-5 | uncertain | uncertain | Aribio Co | Phase III | |
| Exosomes | ahaMSC-Exos | exosomes | uncertain | uncertain | Shanghai Jiaotong University | Phase I b/IIa |
| Psychiatric Abnormalities Associated with AD | Rexulti | agitation | yes | high | Otsuka Pharmaceutical/Lundbeck | Full approval for marketing (2023) |
| KarXT | hallucinations and delusions | yes | high | Karuna Therapeutics | Phase III |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Chen, S.; Wang, J.; Shang, H.; Chen, X. Advancements in Pharmacological Treatment of Alzheimer’s Disease: The Advent of Disease-Modifying Therapies (DMTs). Brain Sci. 2024, 14, 990. https://doi.org/10.3390/brainsci14100990
Wang Q, Chen S, Wang J, Shang H, Chen X. Advancements in Pharmacological Treatment of Alzheimer’s Disease: The Advent of Disease-Modifying Therapies (DMTs). Brain Sciences. 2024; 14(10):990. https://doi.org/10.3390/brainsci14100990
Chicago/Turabian StyleWang, Qiong, Sihui Chen, Junhui Wang, Huifang Shang, and Xueping Chen. 2024. "Advancements in Pharmacological Treatment of Alzheimer’s Disease: The Advent of Disease-Modifying Therapies (DMTs)" Brain Sciences 14, no. 10: 990. https://doi.org/10.3390/brainsci14100990
APA StyleWang, Q., Chen, S., Wang, J., Shang, H., & Chen, X. (2024). Advancements in Pharmacological Treatment of Alzheimer’s Disease: The Advent of Disease-Modifying Therapies (DMTs). Brain Sciences, 14(10), 990. https://doi.org/10.3390/brainsci14100990









