Metacontrol Regulates Creative Thinking: An EEG Complexity Analysis Based on Multiscale Entropy
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Experimental Materials
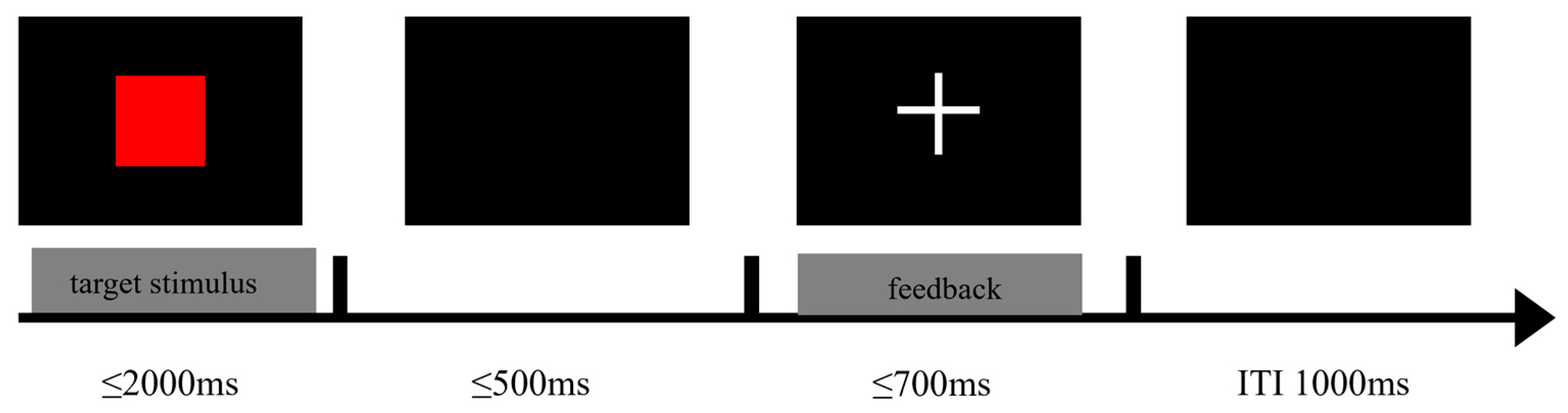
2.2.1. Metacontrol Task
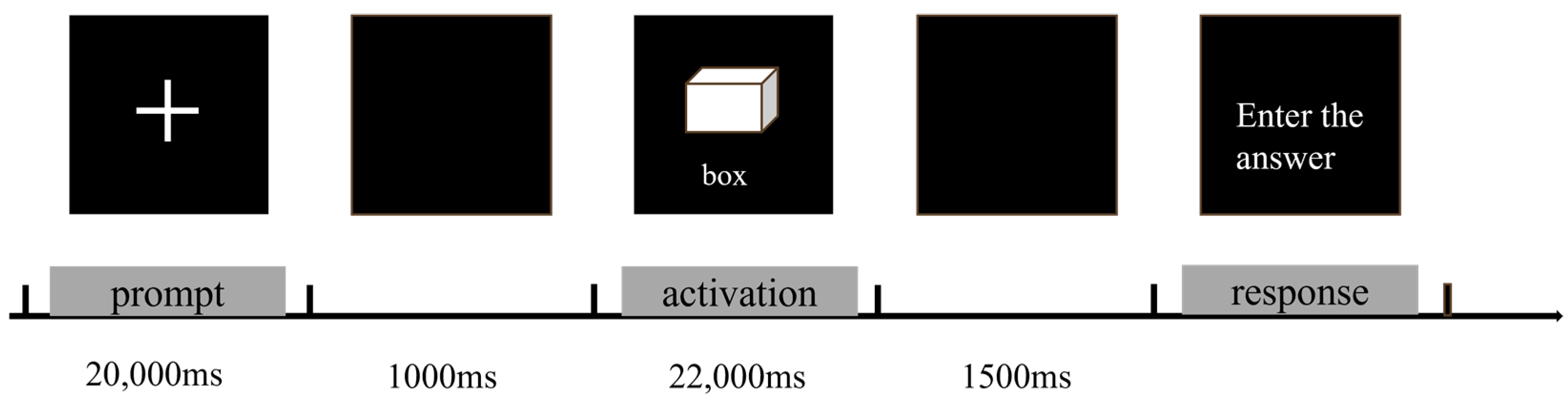
2.2.2. The Alternative Uses Task (AUT)
2.3. Recording and Analysis of EEG Data
2.4. Multiscale Entropy Calculation
2.5. Power Analysis
2.6. Functional Network Analysis
2.7. Statistical Analysis
3. Experimental Results
3.1. Behavioural Data
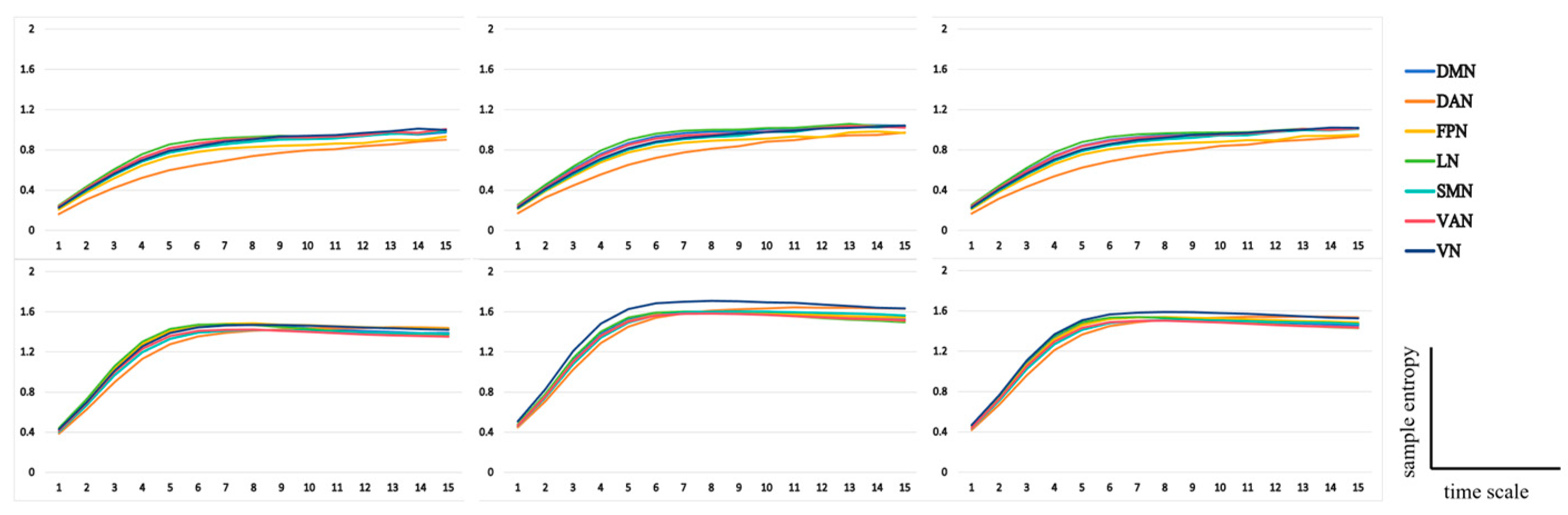
3.2. Electroencephalographic Data
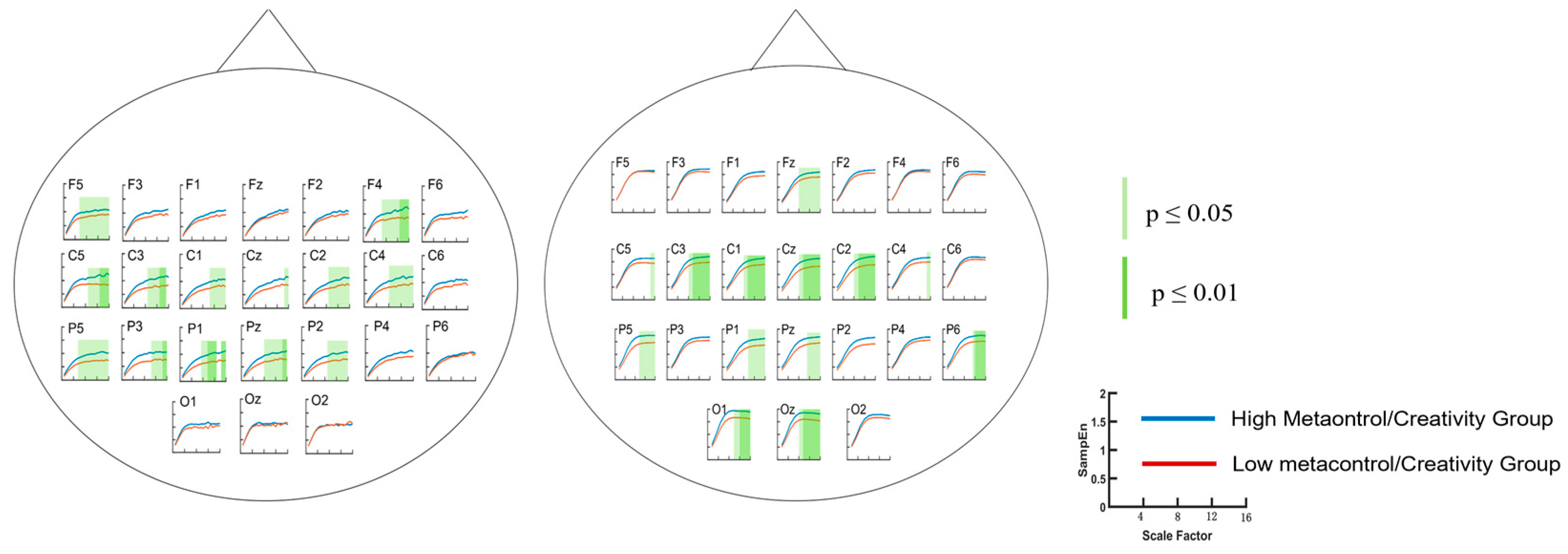
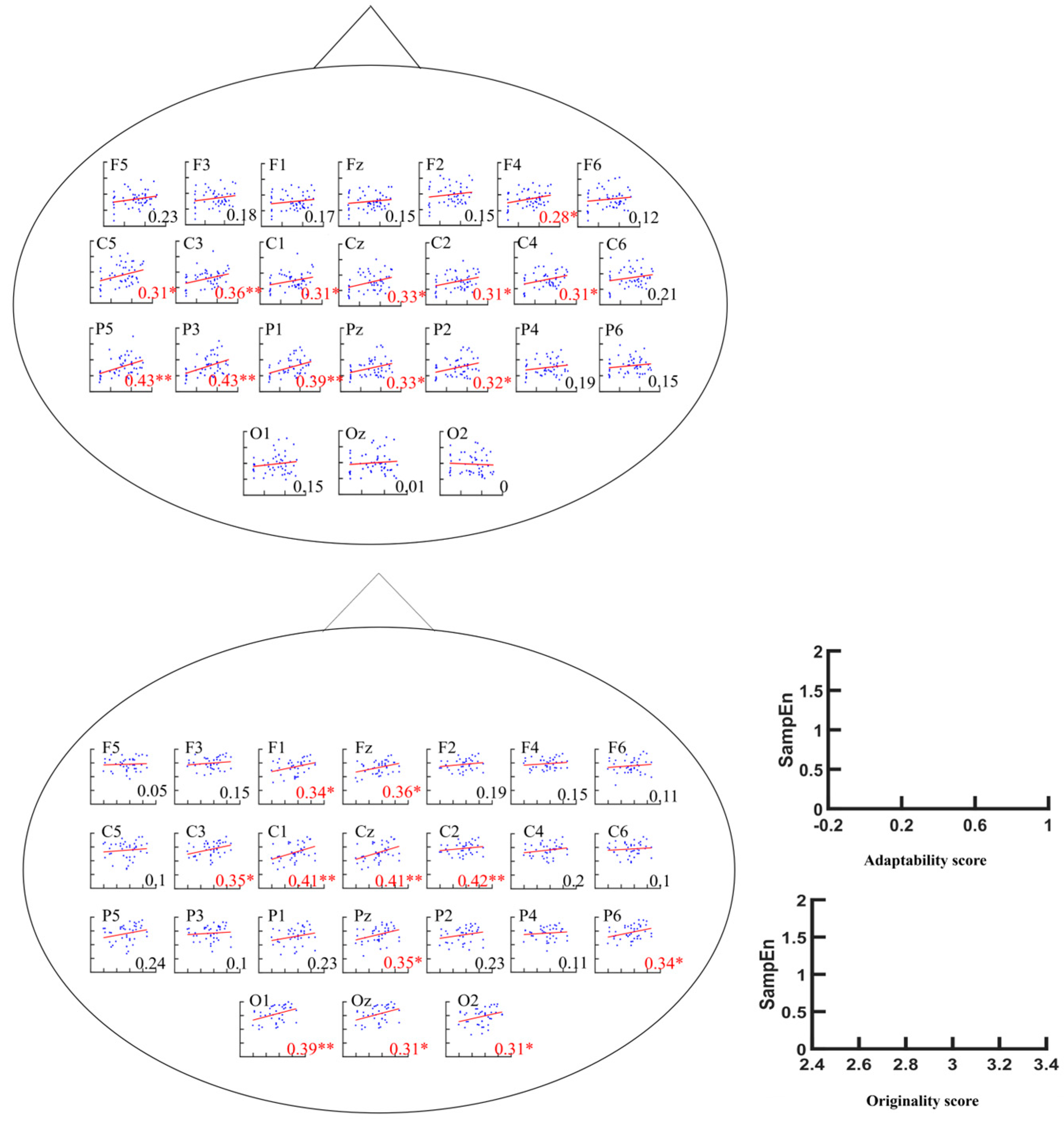
3.2.1. Metacontrol Task
3.2.2. AUT Task
3.2.3. Functional Network Analysis
4. Discussion
5. Conclusions
6. Limitation and Preference
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beaty, R.E.; Benedek, M.; Barry, K.S.; Silvia, P.J. Default and executive network coupling supports creative idea production. Sci. Rep. 2015, 5, 10964. [Google Scholar] [CrossRef] [PubMed]
- Goschke, T.; Bolte, A. Emotional modulation of control dilemmas: The role of positive affect, reward, and dopamine in cognitive stability and flexibility. Neuropsychologia 2014, 62, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, M.; Crespi, B.J. Basic functional trade-offs in cognition: An integrative framework. Cognition 2018, 179, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Musslick, S.; Cohen, J.D. Rationalizing constraints on the capacity for cognitive control. Trends Cogn. Sci. 2020, 25, 757–775. [Google Scholar] [CrossRef]
- Akbari, C.S.; Hommel, B. Creative mood swings: Divergent and convergent thinking affect mood in opposite ways. Psychol. Res. 2012, 76, 634–640. [Google Scholar] [CrossRef]
- Carsten, K.W.; Dreu, D.; Bernard, A.; Nijstad, M.B.; Inge, W.; Marieke, R. Working Memory Benefits Creative Insight, Musical Improvisation, and Original Ideation Through Maintained Task-Focused Attention. Personal. Soc. Psychol. Bull. 2012, 38, e5. [Google Scholar]
- Hommel, B. Between persistence and flexibility: The Yin and Yang of action control. Adv. Motiv. Sci. 2015, 2, 33–67. [Google Scholar]
- Hsieh, Y.; Kirschner, K.; Copland, M. Improving outcomes in chronic myeloid leukemia through harnessing the immunological landscape. Leukemia 2021, 35, 1229–1242. [Google Scholar] [CrossRef]
- Eppinger, B.; Goschke, T.; Musslick, S. Meta-control: From psychology to computational neuroscience. Cogn. Affect. Behav. Neurosci. 2021, 21, 447–452. [Google Scholar] [CrossRef]
- Arden, R.; Chavez, R.S.; Grazioplene, R.; Jung, R.E. Neuroimaging creativity: A psychometric view. Behav. Brain Res. 2010, 214, 143–156. [Google Scholar] [CrossRef]
- Dietrich, A.; Kanso, R. A review of EEG ERP and neuroimaging studies of creativity and insight. Psychol. Bull. 2010, 136, 822–848. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Mead, B.S.; Carrasco, J.; Flores, R.A. The structure of creative cognition in the human brain. Front. Hum. Neurosci. 2013, 7, 330. [Google Scholar] [CrossRef] [PubMed]
- Tononi, G.; Edelman, G.M.; Sporns, O. Complexity and coherency: Integrating information in the brain. Trends Cogn. Sci. 1998, 2, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Abarbanel, H.D.; Rabinovich, M.I. Neurodynamics: Nonlinear dynamics and neurobiology. Curr. Opin. Neurobiol. 2001, 11, 423–430. [Google Scholar] [CrossRef]
- Vakorin, V.A.; Lippe, S.; McIntosh, A.R. Variability of brain signals processed locally transforms into higher connectivity with brain development. J. Neurosci. 2011, 31, 6405–6413. [Google Scholar] [CrossRef]
- McDonough, I.M.; Nashiro, K. Network complexity as a measure of information processing across resting-state networks: Evidence from the Human Connectome Project. Front. Hum. Neurosci. 2014, 8, 00409. [Google Scholar] [CrossRef]
- Garrett, D.D.; Samanez-Larkin, G.R.; MacDonald, S.W.S.; Lindenberger, U.; McIntosh, A.R.; Grady, C.L. Moment-to-moment brain signal variability:a next frontier in human brain mapping? Neurosci. Biobehav. Rev. 2013, 37, 610–624. [Google Scholar] [CrossRef]
- Ghanbari, Y.; Bloy, L.; Edgar, J.C.; Blaskey, L.; Verma, R.; Roberts, T.P.L. Joint analysis of band-specific functional connectivity and signal complexity in autism. J. Autism Dev. Disord. 2015, 45, 444–460. [Google Scholar] [CrossRef]
- Faisal, A.A.; Selen, L.P.J.; Wolpert, D.M. Noise in the nervous system. Nat. Rev. Neurosci. 2008, 9, 292–303. [Google Scholar] [CrossRef]
- Richman, J.S.; Lake, D.E.; Moorman, J.R.; Michael, L.J.; Ludwig, B. Sample entropy. Methods Enzymol. 2004, 384, 172–184. [Google Scholar]
- Takahashi, T. Complexity of spontaneous brain activity in mental disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Bocanegra, B.B.; Hommel, B. When Cognitive Control Is Not Adaptive. Psychol. Sci. 2014, 25, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Von, S.A.; Sarnthein, J. Different frequencies for different scales of cortical integration: From local gamma to long range alpha/theta synchronization. Int. J. Psychophysiol. 2000, 38, 301–313. [Google Scholar]
- Kowatari, Y.; Lee, S.H.; Yamamura, H.; Nagamori, Y.; Levy, P.; Yamane, S. Neural networks involved in artistic creativity. Hum. Brain Mapp. 2009, 30, 1678–1690. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of biological signals. Phys. Rev. E Stat. Nonlinear Soft Matter Physic. 2005, 71, 021906. [Google Scholar] [CrossRef]
- Takeuchi, H.; Taki, Y.; Sassa, Y.; Hashizume, H.; Sekiguchi, A.; Fukushima, A. Regional gray matter volume of dopaminergic system associate with creativity: Evidence from voxel-based morphometry. Neuroimage 2010, 51, 578–585. [Google Scholar] [CrossRef]
- Clayson, P.E.; Carbine, K.A.; Baldwin, S.A.; Larson, M.L. Methodological reporting behavior, sample sizes, and statistical power in studies of event-related potentials: Barriers to reproducibility and replicability. Psychophysiology 2019, 56, e13437. [Google Scholar] [CrossRef]
- Zink, N.; Bensmann, W.; Arning, L.; Colzato, L.S.; Stock, A.K.; Beste, C. The role of DRD1 and DRD2 receptors for response selection under varying complexity levels: Implications for metacontrol processes. Int. J. Neuropsychopharmacol. 2019, 22, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Zink, N.; Stock, A.K.; Vahid, A.; Beste, C. On the neurophysiological mechanisms underlying the adaptability to varying cognitive control demands. Front. Hum. Neurosci. 2018, 12, 411. [Google Scholar] [CrossRef]
- Runco, M.A.; Acar, S. Divergent thinking as an indicator of creative potential. Creat. Res. J. 2012, 24, 66–75. [Google Scholar] [CrossRef]
- Rominger, C.; Papousek, I.; Perchtold, C.M.; Benedek, M.; Weiss, E.M.; Schwerdtfeger, A.; Fink, A. Creativity is associated with a characteristic U-shaped function of alpha power changes accompanied by an early increase in functional coupling. Cogn. Affect. Behav. Neurosci. 2019, 19, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol.-Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Raymond, Y.C.; Mizunoa, T.; Kikuchic, M.; Murata, T.; Takahashi, K.; Wada, Y. Antipsychotics reverse abnormal EEG complexity in drug-naive schizophrenia: A multiscale entropy analysis. NeuroImage 2010, 51, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Takahashi, T.; Takahashi, K.; Mizukami, K.; Tanaka, Y.; Wada, Y. Neurophysiological basis of creativity in healthy elderly people: A multiscale entropy approach. Clin. Neurophysiol. 2015, 126, 520–531. [Google Scholar] [CrossRef]
- Takahashia, T.; Raymond, Y.C.; Murataa, T.; Mizuno, T.; Kikuchi, M.; Mizukami, K.; Kosaka, H.; Takahashi, K.; Wada, Y. Age-related variation in EEG complexity to photic stimulation: A multiscale entropy analysis. Clin. Neurophysiol. 2009, 120, 476–483. [Google Scholar] [CrossRef]
- Lewandowska, M.; Tołpa, K.; Rogala, J.; Piotrowski, T.; Dreszer, J. Multivariate multiscale entropy (mMSE) as a tool for understanding the resting-state EEG signal dynamics: The spatial distribution and sex/gender-related differences. Behav. Brain Funct. 2023, 19, 18. [Google Scholar] [CrossRef]
- Giacometti, P.; Perdue, K.L.; Diamond, S.G. Algorithm to find high density EEG scalp coordinates and analysis of their correspondence to structural and functional regions of the brain. J. Neurosci. Methods 2014, 229, 84–96. [Google Scholar] [CrossRef]
- Jung, R.E.; Grazioplene, R.; Caprihan, A.; Chavez, R.S.; Haier, R.J. White matter integrity creativity and psychopathology: Disentangling constructs with diffusion tensor imaging. Public Libr. Sci. ONE 2010, 5, e9818. [Google Scholar] [CrossRef]
- Levine, M.S.; Chen, J.Y.; Wang, E.A.; Cepeda, C. Dopamine imbalance in Huntington’s disease: A mechanism for the lack of behavioral flexibility. Front. Neurosci. 2013, 4, 114. [Google Scholar]
- Takeuchi, H.; Taki, Y.; Hashizume, H.; Sassa, Y.; Nagase, T.; Nouchi, R. The association between resting functional connectivity and creativity. Cereb. Cortex 2012, 22, 2921–2929. [Google Scholar] [CrossRef]
- Beaty, R.E.; Seli, P.; Schacter, D.L. Network neuroscience of creative cognition: Mapping cognitive mechanisms and individual differences in the creative brain. Curr. Opin. Behav. Sci. 2019, 27, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, A.; Gross, J. Normal and pathological oscillatory communication in the brain. Nat. Rev. Neurosci. 2005, 6, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Breedlove, J.L.; St-Yves, G.; Olman, C.A.; Naselaris, T. Generative Feedback Explains Distinct Brain Activity Codes for Seen and Mental Images. Curr. Biol. 2020, 30, 2211–2224. [Google Scholar] [CrossRef]
- Andreasen, N.C. Creativity and mental illness: Prevalence rates in writers and their first-degree relatives. Am. J. Psychiatry 1987, 144, 1288–1292. [Google Scholar]
- Kyaga, S.; Landen, M.; Boman, M.; Hultman, C.M.; Langstrom, N.; Lichtenstein, P. Mental illness suicide and creativity: 40-year prospective total population study. J. Psychiatr. Res. 2013, 47, 83–90. [Google Scholar] [CrossRef]
- Boot, N.; Baas, M.; van Gaal, S.; Cools, R.; De Dreu, C.K. Creative cognition and dopaminergic modulation of fronto-striatal networks: Integrative review and research agenda. Neurosci. Biobehav. Rev. 2017, 78, 13–23. [Google Scholar] [CrossRef]
- Mizuno, T.; Takahashi, T.; Cho, R.Y.; Kikuchi, M.; Murata, T.; Takahashi, K. Assessment of EEG dynamical complexity in Alzheimer’s disease using multiscale entropy. Clin. Neurophysiol. 2010, 121, 1438–1446. [Google Scholar] [CrossRef]
- Carson, S.H.; Peterson, J.B.; Higgins, D.M. Decreased latent inhibition is associated with increased creative achievement in high-functioning individuals. J. Personal. Soc. Psychol. 2003, 85, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, R.J. Handbook of Creativity; Cambridge University Press: New York, NY, USA, 1999. [Google Scholar]
- Runco, M.A.; Jaeger, G.J. The standard definition of creativity. Creat. Res. J. 2012, 24, 92–96. [Google Scholar] [CrossRef]
- Happé, F.G.E. Communicative competence and theory of mind in autism: A test of relevance theory. Cognitive 1993, 48, 101–119. [Google Scholar] [CrossRef]
- Ursino, M.; Serra, M.; Tarasi, L.; Ricci, G.; Magosso, E.; Romei, V. Bottom-up vs. top-down connectivity imbalance in individuals with high-autistic traits: An electroencephalographic study. Front. Syst. Neurosci. 2022, 16, e932128. [Google Scholar] [CrossRef] [PubMed]
- Santosa, C.M.; Strong, C.M.; Nowakowska, C.; Wang, P.W. Enhanced creativity in bipolar disorder patients: A controlled study. J. Affect. Disord. 2007, 100, 31–39. [Google Scholar] [CrossRef] [PubMed]


| Network | t | p | Cohen’s d |
|---|---|---|---|
| DMN | 1.56 | 0.062 | |
| DAN | 1.85 | 0.035 | 0.363 |
| FMN | 1.31 | 0.097 | |
| LN | 1.39 | 0.085 | |
| SMN | 1.31 | 0.098 | |
| VAN | 1.14 | 0.13 | |
| VN | 1.05 | 0.149 |
| Network | t | p | Cohen’s d |
|---|---|---|---|
| DMN | 3.19 | 0.001 | 0.626 |
| DAN | 3.2 | 0.001 | 0.628 |
| FMN | 2 | 0.025 | 0.392 |
| LN | 2.28 | 0.013 | 0.447 |
| SMN | 3.08 | 0.002 | 0.604 |
| VAN | 2.67 | 0.005 | 0.524 |
| VN | 3.36 | <0.001 | 0.659 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, H.; Liu, C. Metacontrol Regulates Creative Thinking: An EEG Complexity Analysis Based on Multiscale Entropy. Brain Sci. 2024, 14, 1094. https://doi.org/10.3390/brainsci14111094
Qi H, Liu C. Metacontrol Regulates Creative Thinking: An EEG Complexity Analysis Based on Multiscale Entropy. Brain Sciences. 2024; 14(11):1094. https://doi.org/10.3390/brainsci14111094
Chicago/Turabian StyleQi, Hang, and Chunlei Liu. 2024. "Metacontrol Regulates Creative Thinking: An EEG Complexity Analysis Based on Multiscale Entropy" Brain Sciences 14, no. 11: 1094. https://doi.org/10.3390/brainsci14111094
APA StyleQi, H., & Liu, C. (2024). Metacontrol Regulates Creative Thinking: An EEG Complexity Analysis Based on Multiscale Entropy. Brain Sciences, 14(11), 1094. https://doi.org/10.3390/brainsci14111094






