Spinal Cord Stimulation for Spinal Cord Injury-Related Pain: A Pilot Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Study Population
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Classification of SCI
2.6. Data Collection
2.7. Clinical Assessment and Outcome Measures
2.8. Statistical Analysis
3. Results
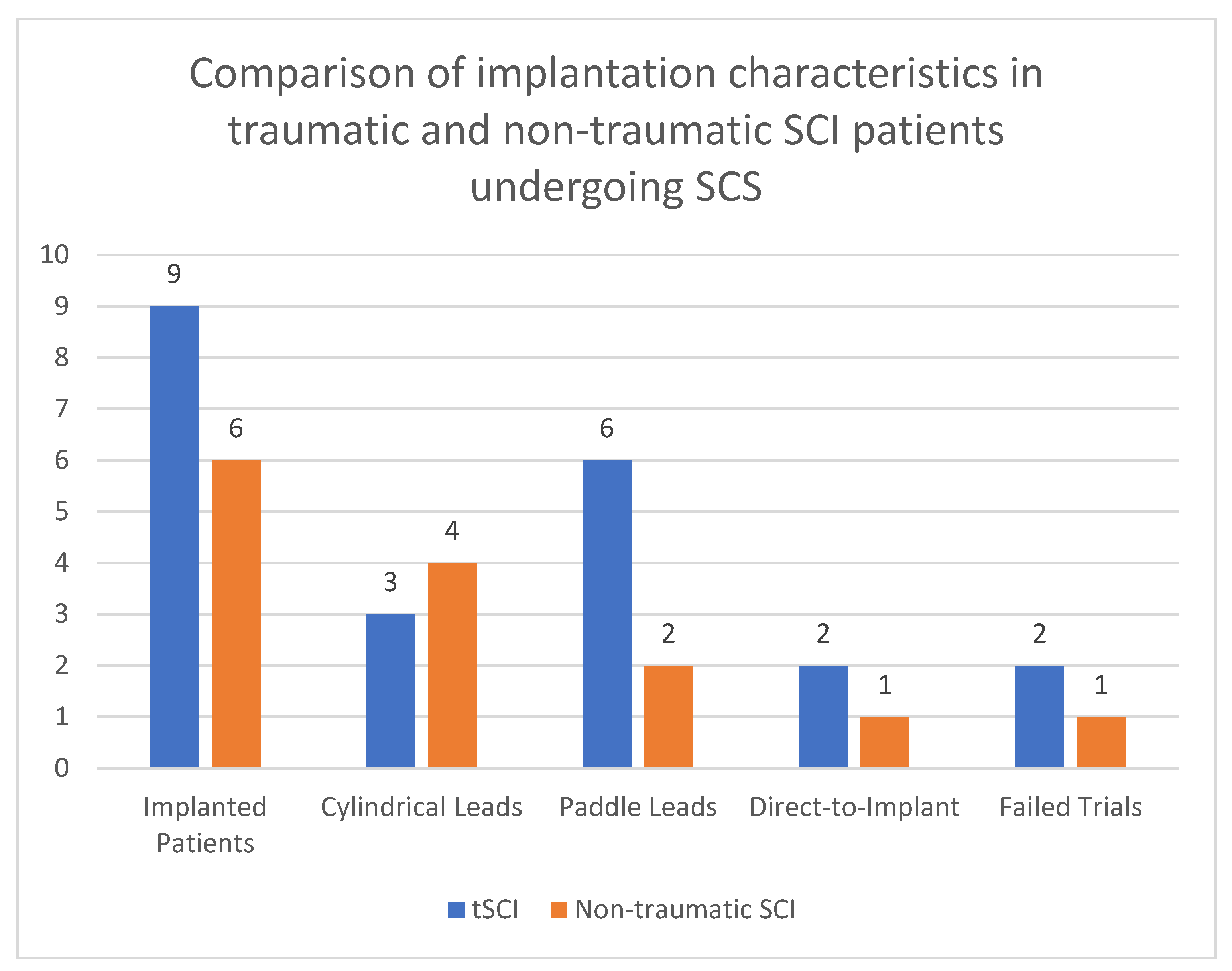
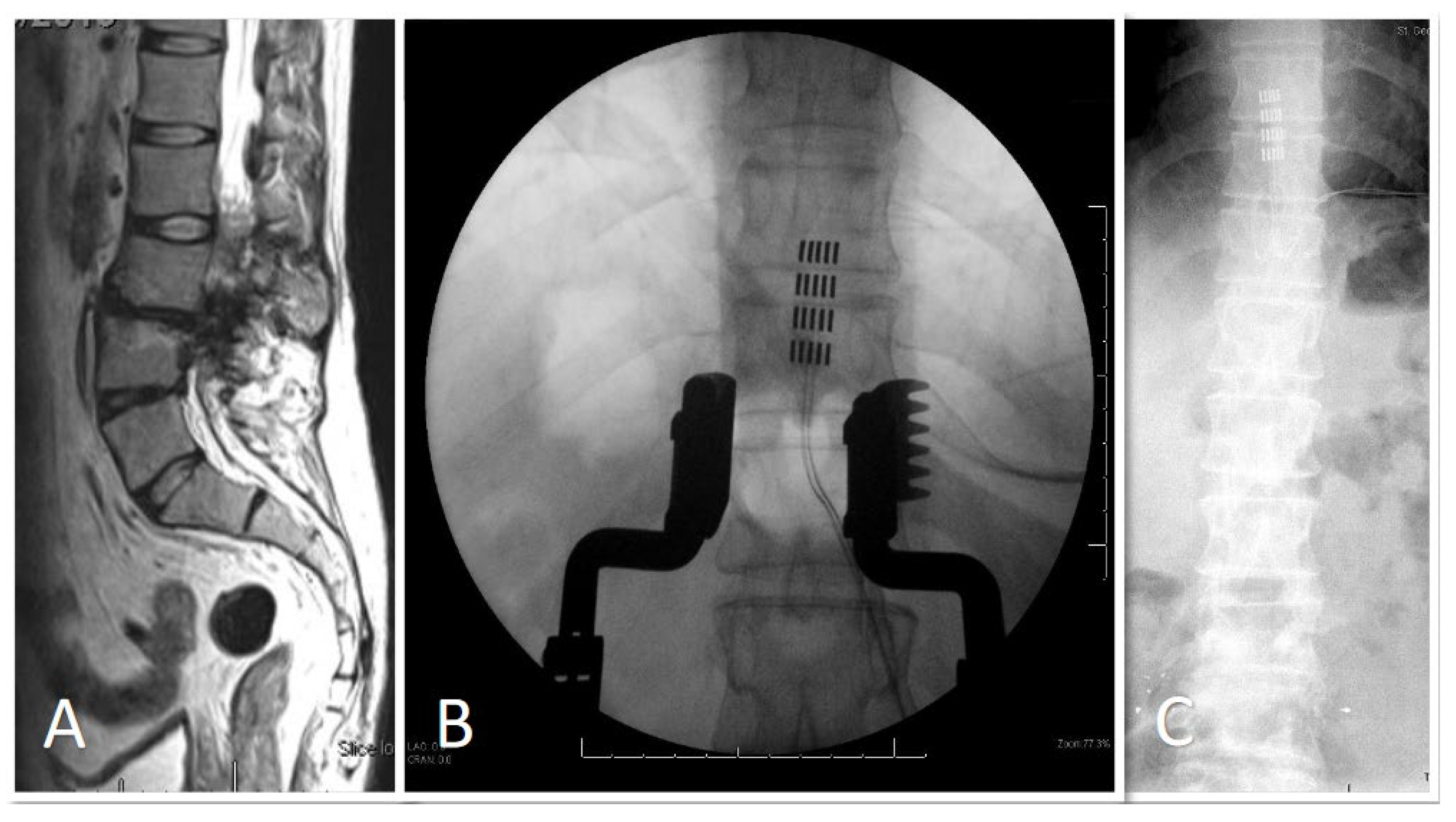
3.1. Demographic and Clinical Data
3.2. Pain Relief Outcomes
3.3. Pain Medication Change Outcome
3.4. Adverse Events
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barkin, R.L.; Lubenow, T.R.; Bruehl, S.; Husfeldt, B.; Ivankovich, O.; Barkin, S.J. Management of Chronic Pain. Part II. Dis. Mon. 1996, 42, 461–507. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.E., 3rd; Kyle, B.N.; Thorp, J.; Wu, Q.; Firnhaber, J. Comparison of Pain, Functioning, Coping, and Psychological Distress in Patients with Chronic Low Back Pain Evaluated for Spinal Cord Stimulator Implant or Behavioral Pain Management. Pain Med. 2015, 16, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; Inglis, J. Pain Tolerance and Narcotic Addiction. Br. J. Soc. Clin. Psychol. 1965, 4, 224–229. [Google Scholar] [CrossRef]
- Fishbain, D.A.; Rosomoff, H.L.; Rosomoff, R.S. Drug Abuse, Dependence, and Addiction in Chronic Pain Patients. Clin. J. Pain 1992, 8, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.L.; White, J.M.; Bochner, F.; Somogyi, A.A.; Semple, T.J.; Rounsefell, B. Hyperalgesia in Opioid-Managed Chronic Pain and Opioid-Dependent Patients. J. Pain 2009, 10, 316–322. [Google Scholar] [CrossRef]
- Margot-Duclot, A.; Tournebise, H.; Ventura, M.; Fattal, C. What Are the Risk Factors of Occurence and Chronicity of Neuropathic Pain in Spinal Cord Injury Patients? Ann. Phys. Rehabil. Med. 2009, 52, 111–123. [Google Scholar] [CrossRef]
- Burke, D.; Fullen, B.M.; Stokes, D.; Lennon, O. Neuropathic Pain Prevalence Following Spinal Cord Injury: A Systematic Review and Meta-Analysis. Eur. J. Pain 2017, 21, 29–44. [Google Scholar] [CrossRef]
- Bryce, T.N.; Biering-Sørensen, F.; Finnerup, N.B.; Cardenas, D.D.; Defrin, R.; Lundeberg, T.; Norrbrink, C.; Richards, J.S.; Siddall, P.; Stripling, T.; et al. International Spinal Cord Injury Pain Classification: Part I. Background and Description. Spinal Cord 2012, 50, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Saulino, M.; Averna, J.F. Evaluation and Management of SCI-Associated Pain. Curr. Pain Headache Rep. 2016, 20, 53. [Google Scholar] [CrossRef]
- Guo, X.; Hu, J.; Feng, S.; Gao, X.; Sun, C.; Ao, Q.; Chen, L.; Chen, L.; Zhang, P.; Dai, Y.; et al. Clinical Neurorestorative Treatment Guidelines for Neurological Dysfunctions of Sequels from Vertebral and Spinal Cord Lesions (CANR 2023 Version). J. Neurorestoratol. 2023, 11, 100070. [Google Scholar] [CrossRef]
- Knotkova, H.; Hamani, C.; Sivanesan, E.; Le Beuffe, M.F.E.; Moon, J.Y.; Cohen, S.P.; Huntoon, M.A. Neuromodulation for Chronic Pain. Lancet 2021, 397, 2111–2124. [Google Scholar] [CrossRef] [PubMed]
- Cameron, T. Safety and Efficacy of Spinal Cord Stimulation for the Treatment of Chronic Pain: A 20-Year Literature Review. J. Neurosurg. 2004, 100, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Spiegelmann, R.; Friedman, W.A. Spinal Cord Stimulation: A Contemporary Series. Neurosurgery 1991, 28, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Deer, T. Stimulation of the Spinal Cord, Peripheral Nerve and Peripheral Nerve Field for the Treatment of Pain. Reg. Anesth. Pain Med. 2010, 35, E8–E10. [Google Scholar] [CrossRef]
- Deer, T.R.; Esposito, M.F.; McRoberts, W.P.; Grider, J.S.; Sayed, D.; Verrills, P.; Lamer, T.J.; Hunter, C.W.; Slavin, K.V.; Shah, J.M.; et al. A Systematic Literature Review of Peripheral Nerve Stimulation Therapies for the Treatment of Pain. Pain Med. 2020, 21, 1590–1603. [Google Scholar] [CrossRef]
- Geurts, J.W.; Joosten, E.A.; van Kleef, M. Current Status and Future Perspectives of Spinal Cord Stimulation in Treatment of Chronic Pain. Pain 2017, 158, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Duan, W.; Sivanesan, E.; Liu, S.; Yang, F.; Chen, Z.; Ford, N.C.; Chen, X.; Guan, Y. Spinal Cord Stimulation for Pain Treatment After Spinal Cord Injury. Neurosci. Bull. 2019, 35, 527. [Google Scholar] [CrossRef]
- Lagauche, D.; Facione, J.; Albert, T.; Fattal, C. The Chronic Neuropathic Pain of Spinal Cord Injury: Which Efficiency of Neuropathics Stimulations? Ann. Phys. Rehabil. Med. 2009, 52, 180–187. [Google Scholar] [CrossRef]
- Thomson, S.; Huygen, F.; Prangnell, S.; De Andrés, J.; Baranidharan, G.; Belaïd, H.; Berry, N.; Billet, B.; Cooil, J.; De Carolis, G.; et al. Appropriate Referral and Selection of Patients with Chronic Pain for Spinal Cord Stimulation: European Consensus Recommendations and E-health Tool. Eur. J. Pain 2020, 24, 1169–1181. [Google Scholar] [CrossRef]
- Logé, D.; De Coster, O.; Washburn, S. Technological Innovation in Spinal Cord Stimulation: Use of a Newly Developed Delivery Device for Introduction of Spinal Cord Stimulation Leads. Neuromodul. Technol. Neural Interface 2012, 15, 392–401. [Google Scholar] [CrossRef]
- Delgado, D.A.; Lambert, B.S.; Boutris, N.; McCulloch, P.C.; Robbins, A.B.; Moreno, M.R.; Harris, J.D. Validation of Digital Visual Analog Scale Pain Scoring with a Traditional Paper-Based Visual Analog Scale in Adults. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2018, 2, e088. [Google Scholar] [CrossRef] [PubMed]
- Dolan, P. Modeling Valuations for EuroQol Health States. Med. Care 1997, 35, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Kapural, L.; Yu, C.; Doust, M.W.; Gliner, B.E.; Vallejo, R.; Sitzman, B.T.; Amirdelfan, K.; Morgan, D.M.; Brown, L.L.; Yearwood, T.L.; et al. Novel 10-KHz High-Frequency Therapy (HF10 Therapy) Is Superior to Traditional Low-Frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: The SENZA-RCT Randomized Controlled Trial. Anesthesiology 2015, 123, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Patterson, D.G.; Baksh, J.; Pope, J.E.; Mehta, P.; Raza, A.; Agnesi, F.; Chakravarthy, K.V. Novel Intermittent Dosing Burst Paradigm in Spinal Cord Stimulation. Neuromodulation 2021, 24, 566–573. [Google Scholar] [CrossRef]
- Hagedorn, J.M.; Falowski, S.M.; Blomme, B.; Capobianco, R.A.; Yue, J.J. Burst Spinal Cord Stimulation Can Attenuate Pain and Its Affective Components in Chronic Pain Patients with High Psychological Distress: Results from the Prospective, International TRIUMPH Study. Spine J. 2022, 22, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Metzger, C.S.; Hammond, M.B.; Paz-Solis, J.F.; Newton, W.J.; Thomson, S.J.; Pei, Y.; Jain, R.; Moffitt, M.; Annecchino, L.; Doan, Q. A Novel Fast-Acting Sub-Perception Spinal Cord Stimulation Therapy Enables Rapid Onset of Analgesia in Patients with Chronic Pain. Expert Rev. Med. Devices 2021, 18, 299–306. [Google Scholar] [CrossRef]
- Sun, C.; Tao, X.; Wan, C.; Zhang, X.; Zhao, M.; Xu, M.; Wang, P.; Liu, Y.; Wang, C.; Xi, Q.; et al. Spinal Cord Stimulation Alleviates Neuropathic Pain by Attenuating Microglial Activation via Reducing Colony-Stimulating Factor 1 Levels in the Spinal Cord in a Rat Model of Chronic Constriction Injury. Anesth. Analg. 2022, 135, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Jongen, J.L.; Smits, H.; Pederzani, T.; Bechakra, M.; Hossaini, M.; Koekkoek, S.K.; Huygen, F.J.; De Zeeuw, C.I.; Holstege, J.C.; Joosten, E.A. Spinal Autofluorescent Flavoprotein Imaging in a Rat Model of Nerve Injury-Induced Pain and the Effect of Spinal Cord Stimulation. PLoS ONE 2014, 9, e109029. [Google Scholar] [CrossRef] [PubMed]
- Brock, J.H.; Elste, A.; Huntley, G.W. Distribution and Injury-Induced Plasticity of Cadherins in Relationship to Identified Synaptic Circuitry in Adult Rat Spinal Cord. J. Neurosci. 2004, 24, 8806–8817. [Google Scholar] [CrossRef]
- Whitt, J.L.; Masri, R.; Pulimood, N.S.; Keller, A. Pathological Activity in Mediodorsal Thalamus of Rats with Spinal Cord Injury Pain. J. Neurosci. 2013, 33, 3915–3926. [Google Scholar] [CrossRef]
- Gwak, Y.S.; Kang, J.; Leem, J.W.; Hulsebosch, C.E. Spinal AMPA Receptor Inhibition Attenuates Mechanical Allodynia and Neuronal Hyperexcitability Following Spinal Cord Injury in Rats. J. Neurosci. Res. 2007, 85, 2352–2359. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.R.; Zhou, H.Y.; Byun, H.S.; Pan, H.L. Nerve Injury Increases GluA2-Lacking AMPA Receptor Prevalence in Spinal Cords: Functional Significance and Signaling Mechanisms. J. Pharmacol. Exp. Ther. 2013, 347, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Malcangio, M.; Ramer, M.S.; Jones, M.G.; McMahon, S.B. Abnormal Substance P Release from the Spinal Cord Following Injury to Primary Sensory Neurons. Eur. J. Neurosci. 2000, 12, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Sun, C.; Fan, B.; Zhao, C.; Zhang, Y.; Duan, H.; Pang, Y.; Shen, W.; Li, B.; Wang, X.; et al. Neurotropin Exerts Neuroprotective Effects after Spinal Cord Injury by Inhibiting Apoptosis and Modulating Cytokines. J. Orthop. Transl. 2021, 26, 74–83. [Google Scholar] [CrossRef]
- Lampert, A.; Hains, B.C.; Waxman, S.G. Upregulation of Persistent and Ramp Sodium Current in Dorsal Horn Neurons after Spinal Cord Injury. Exp. Brain Res. 2006, 174, 660–666. [Google Scholar] [CrossRef]
- Knoblach, S.; Faden, A.; Seo, J.; Hoffman, E. Expression Profiling Months after Experimental Spinal Cord Trauma Reveals a Persistent Dynamic Secondary Injury Response. J. Neurotrauma 2009, 26, A71. [Google Scholar] [CrossRef]
- Castellanos, D.A.; Daniels, L.A.; Morales, M.P.; Hama, A.T.; Sagen, J. Expansion of Formalin-Evoked Fos-Immunoreactivity in Rats with a Spinal Cord Injury. Neurosci. Res. 2007, 58, 386–393. [Google Scholar] [CrossRef]
- Youn, D.H. Peripheral Nerve Injury Alters Excitatory and Inhibitory Synaptic Transmission in Rat Spinal Cord Substantia Gelatinosa. Korean J. Physiol. Pharmacol. 2005, 9, 143–147. [Google Scholar]
- Karri, J.; Li, S.; Chen, Y.T.; Stampas, A.; Li, S. Observations of Autonomic Variability Following Central Neuromodulation for Chronic Neuropathic Pain in Spinal Cord Injury. Neuromodulation 2021, 24, 427–433. [Google Scholar] [CrossRef]
- Hains, B.C.; Saab, C.Y.; Waxman, S.G. Changes in Electrophysiological Properties and Sodium Channel Na v1.3 Expression in Thalamic Neurons after Spinal Cord Injury. Brain 2005, 128, 2359–2371. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, C.S.; Seo, K.J.; Kim, I.Y.; Han, S.; Youn, I.; Yune, T.Y. IL-6/JAK2/STAT3 Axis Mediates Neuropathic Pain by Regulating Astrocyte and Microglia Activation after Spinal Cord Injury. Exp. Neurol. 2023, 370, 114576. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Woolf, C.J. Neuronal Plasticity and Signal Transduction in Nociceptive Neurons: Implications for the Initiation and Maintenance of Pathological Pain. Neurobiol. Dis. 2001, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hains, B.C.; Klein, J.P.; Saab, C.Y.; Craner, M.J.; Black, J.A.; Waxman, S.G. Upregulation of Sodium Channel Nav1.3 and Functional Involvement in Neuronal Hyperexcitability Associated with Central Neuropathic Pain after Spinal Cord Injury. J. Neurosci. 2003, 23, 8881–8892. [Google Scholar] [CrossRef]
- Hains, B.C.; Waxman, S.G. Sodium Channel Expression and the Molecular Pathophysiology of Pain after SCI. Prog. Brain Res. 2007, 161, 195–203. [Google Scholar] [CrossRef]
- Hains, B.C.; Everhart, A.W.; Fullwood, S.D.; Hulsebosch, C.E. Changes in Serotonin, Serotonin Transporter Expression and Serotonin Denervation Supersensitivity: Involvement in Chronic Central Pain after Spinal Hemisection in the Rat. Exp. Neurol. 2002, 175, 347–362. [Google Scholar] [CrossRef]
- Gao, N.; Li, M.; Wang, W.; Liu, Z.; Guo, Y. The Dual Role of TRPV1 in Peripheral Neuropathic Pain: Pain Switches Caused by Its Sensitization or Desensitization. Front. Mol. Neurosci. 2024, 17, 1400118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Waxman, S.G.; Hains, B.C. Extracellular Signal-Regulated Kinase-Regulated Microglia-Neuron Signaling by Prostaglandin E2 Contributes to Pain after Spinal Cord Injury. J. Neurosci. 2007, 27, 2357–2368. [Google Scholar] [CrossRef]
- Hains, B.C.; Waxman, S.G. Activated Microglia Contribute to the Maintenance of Chronic Pain after Spinal Cord Injury. J. Neurosci. 2006, 26, 4308–4317. [Google Scholar] [CrossRef] [PubMed]
- Schomberg, D.; Miranpuri, G.; Duellman, T.; Crowell, A.; Vemuganti, R.; Resnick, D. Spinal Cord Injury Induced Neuropathic Pain: Molecular Targets and Therapeutic Approaches. Metab. Brain Dis. 2015, 30, 645–658. [Google Scholar] [CrossRef]
- Melzack, R.; Wall, P.D. Pain Mechanisms: A New Theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef]
- Larson, S.J.; Sances, A.; Riegel, D.H.; Meyer, G.A.; Dallmann, D.E.; Swiontek, T. Neurophysiological Effects of Dorsal Column Stimulation in Man and Monkey. J. Neurosurg. 1974, 41, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, D. Effect of Dorsal-Column Stimulation on Gelatinosa and Marginal Neurons of Cat Spinal Cord. J. Neurosurg. 1989, 70, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Bantli, H.; Bloedel, J.R.; Thienprasit, P. Supraspinal Interactions Resulting from Experimental Dorsal Column Stimulation. J. Neurosurg. 1975, 42, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Zhai, F.J.; Han, S.P.; Song, T.J.; Huo, R.; Lan, X.Y.; Zhang, R.; Han, J.S. Involvement of Opioid Peptides in the Analgesic Effect of Spinal Cord Stimulation in a Rat Model of Neuropathic Pain. Neurosci. Bull. 2022, 38, 403–416. [Google Scholar] [CrossRef]
- Ultenius, C.; Song, Z.; Lin, P.; Meyerson, B.A.; Linderoth, B. Spinal GABAergic Mechanisms in the Effects of Spinal Cord Stimulation in a Rodent Model of Neuropathic Pain: Is GABA Synthesis Involved? Neuromodulation 2013, 16, 114–120. [Google Scholar] [CrossRef]
- Crosby, N.D.; Weisshaar, C.L.; Smith, J.R.; Zeeman, M.E.; Goodman-Keiser, M.D.; Winkelstein, B.A. Burst and Tonic Spinal Cord Stimulation Differentially Activate Gabaergic Mechanisms to Attenuate Pain in a Rat Model of Cervical Radiculopathy. IEEE Trans. Biomed. Eng. 2015, 62, 1604–1613. [Google Scholar] [CrossRef]
- Meuwissen, K.P.V.; de Vries, L.E.; Gu, J.W.; Zhang, T.C.; Joosten, E.A.J. Burst and Tonic Spinal Cord Stimulation Both Activate Spinal GABAergic Mechanisms to Attenuate Pain in a Rat Model of Chronic Neuropathic Pain. Pain Pract. 2020, 20, 75–87. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Baastrup, C. Spinal Cord Injury Pain: Mechanisms and Management. Curr. Pain Headache Rep. 2012, 16, 207–216. [Google Scholar] [CrossRef]
- Sheldon, B.L.; Olmsted, Z.T.; Sabourin, S.; Heydari, E.; Harland, T.A.; Pilitsis, J.G. Review of the Treatments for Central Neuropathic Pain. Brain Sci. 2022, 12, 1727. [Google Scholar] [CrossRef]
- Tieppo Francio, V.; Leavitt, L.; Alm, J.; Mok, D.; Yoon, B.j.V.; Nazir, N.; Lam, C.M.; Latif, U.; Sowder, T.; Braun, E.; et al. Healthcare Utilization (HCU) Reduction with High-Frequency (10 KHz) Spinal Cord Stimulation (SCS) Therapy. Healthcare 2024, 12, 745. [Google Scholar] [CrossRef]
- Shiao, R.; Lee-Kubli, C.A. Neuropathic Pain After Spinal Cord Injury: Challenges and Research Perspectives. Neurotherapeutics 2018, 15, 635. [Google Scholar] [CrossRef] [PubMed]
- Kirshblum, S.C.; Waring, W.; Biering-Sorensen, F.; Burns, S.P.; Johansen, M.; Schmidt-Read, M.; Donovan, W.; Graves, D.; Jha, A.; Jones, L.; et al. Reference for the 2011 Revision of the International Standards for Neurological Classification of Spinal Cord Injury. J. Spinal Cord Med. 2011, 34, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of Over-Ground Walking after Chronic Motor Complete Spinal Cord Injury. N. Engl. J. Med. 2018, 379, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G.; et al. Effect of Epidural Stimulation of the Lumbosacral Spinal Cord on Voluntary Movement, Standing, and Assisted Stepping after Motor Complete Paraplegia: A Case Study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Malone, I.G.; Nosacka, R.L.; Nash, M.A.; Otto, K.J.; Dale, E.A. Electrical Epidural Stimulation of the Cervical Spinal Cord: Implications for Spinal Respiratory Neuroplasticity after Spinal Cord Injury. J. Neurophysiol. 2021, 126, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Sokal, P.; Palus, D.; Jabłońska, M.; Puk, O.; Kieronska-Siwak, S. Spinal Cord Stimulation for Central Neuropathic Pain After Spinal Cord Injury: A Single-Center Case Series. J. Pain Res. 2024, 17, 2029–2035. [Google Scholar] [CrossRef]
- Kapural, L. Pro Con Debate 19: Neuromodulation for Failed Back Surgery Syndrome Is Theway Forward! Reg. Anesth. Pain Med. 2017, 42 (Suppl. S5), e20–e21. [Google Scholar] [CrossRef]
- Taylor, R.S. Spinal Cord Stimulation in Complex Regional Pain Syndrome and Refractory Neuropathic Back and Leg Pain/Failed Back Surgery Syndrome: Results of a Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2006, 31, S13–S19. [Google Scholar] [CrossRef]
- Russo, M.; Brooker, C.; Poree, L.; Cousins, M.J.; Richard Sullivan, R.; Taylor, N.; Holford, L.; Boesel, T.; Martin, R.; O’Neill, M.; et al. Long-Term Outcomes from the Avalon Study: A Prospective Multicenter Study Evaluating Closed-Loop Scs in the Treatment of Chronic Back and Leg Pain. Neuromodulation 2019, 22, e323–e324. [Google Scholar] [CrossRef]
- De Negri, P.; Paz-Solis, J.F.; Rigoard, P.; Raoul, S.; Kallewaard, J.W.; Gulve, A.; Thomson, S.; Canós-Verdecho, M.A.; Love-Jones, S.; Williams, A.; et al. Real-World Outcomes of Single-Stage Spinal Cord Stimulation in Chronic Pain Patients: A Multicentre, European Case Series. Interv. Pain Med. 2023, 2, 100263. [Google Scholar] [CrossRef]
- Martin, S.C.; Baranidharan, G.; Thomson, S.; Gulve, A.; Manfield, J.H.; Mehta, V.; Love-Jones, S.; Strachan, R.; Bojanić, S.; Eldabe, S.; et al. Spinal Cord Stimulation Improves Quality of Life for Patients with Chronic Pain—Data from the UK and Ireland National Neuromodulation Registry. Neuromodul. Technol. Neural Interface 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- De Jaeger, M.; Goudman, L.; Putman, K.; De Smedt, A.; Rigoard, P.; Geens, W.; Moens, M. The Added Value of High Dose Spinal Cord Stimulation in Patients with Failed Back Surgery Syndrome after Conversion from Standard Spinal Cord Stimulation. J. Clin. Med. 2020, 9, 3126. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, R.; McNaughton, R.; Eldabe, S.; Baranidharan, G.; Bell, J.; Brookes, M.; Duarte, R.V.; Earle, J.; Gulve, A.; Houten, R.; et al. To Trial or Not to Trial Before Spinal Cord Stimulation for Chronic Neuropathic Pain: The Patients’ View From the TRIAL-STIM Randomized Controlled Trial. Neuromodul. Technol. Neural Interface 2021, 24, 459–470. [Google Scholar] [CrossRef] [PubMed]
- North, R.; Desai, M.J.; Vangeneugden, J.; Raftopoulos, C.; Van Havenbergh, T.; Deruytter, M.; Remacle, J.-M.; Shipley, J.; Tan, Y.; Johnson, M.J.; et al. Postoperative Infections Associated with Prolonged Spinal Cord Stimulation Trial Duration (PROMISE RCT). Neuromodul. Technol. Neural Interface 2020, 23, 620–625. [Google Scholar] [CrossRef]
- Chincholkar, M.; Eldabe, S.; Strachan, R.; Brookes, M.; Garner, F.; Chadwick, R.; Gulve, A.; Ness, J. Prospective Analysis of the Trial Period for Spinal Cord Stimulation Treatment for Chronic Pain. Neuromodulation 2011, 14, 523–529. [Google Scholar] [CrossRef]
- Eldabe, S.; Buchser, E.; Duarte, R.V. Complications of Spinal Cord Stimulation and Peripheral Nerve Stimulation Techniques: A Review of the Literature. Pain Med. 2016, 17, 325–336. [Google Scholar] [CrossRef]

| ASIA Classification | Description | Motor Function | Sensory Function |
|---|---|---|---|
| A (Complete) | No motor or sensory function is preserved below the level of injury, including in the sacral segments (S4–S5). | No motor function below the injury level. | No sensory function below the injury level. |
| B (Sensory Incomplete) | Sensory but not motor function is preserved below the level of injury, including the sacral segments (S4–S5). | No motor function below the injury level. | Sensory function preserved below injury level. |
| C (Motor Incomplete) | Motor function is preserved below the level of injury, but more than half of key muscles have strength <3. | Weak motor function (less than grade 3). | Sensory function may or may not be preserved. |
| D (Motor Incomplete) | Motor function is preserved below the level of injury, and at least half of key muscles have strength ≥3. | Strong motor function (grade 3 or higher). | Sensory function may or may not be preserved. |
| E (Normal) | Normal motor and sensory function are preserved below the level of injury. | Normal motor function below injury level. | Normal sensory function below injury level. |
| Age | Sex | Indication | Percutaneous/Paddle | Manufacturer | Program | Anatomical Location of Best Program |
|---|---|---|---|---|---|---|
| 49 | F | Ependymoma | Retrograde paddle T9–10 | Boston (Marlborough, MA, USA) | Microburst | T9 |
| 55 | M | Transverse myelitis | Percutaneous | Boston | FAST | T3 |
| 59 | M | Transverse Myelitis C2–C5 + FBSS L4/L5 | Percutaneous T8–9 | Nevro (Redwood City, CA, USA) | HFX | T8 |
| 53 | M | Cervical myelopathy | Percutaneous C2–6 | Abbott (Green Oaks, IL, USA) | BurstDR | C2 |
| 52 | M | Spinal stroke T3 | Paddle T9 | Abbott | BurstDR | T9–10 |
| Age | Sex | Indication | Percutaneous/Paddle | Manufacturer | ASIA | Program | Anatomical Location of Best Program |
|---|---|---|---|---|---|---|---|
| 55 | M | T8 SCI | Percutaneous | Nevro | D | HFX | |
| 66 | M | T10 to L5 spinal fusion procedure | Paddle T7 to T10 | Nevro | C | HFX | Mid T9 |
| 65 | M | T8 SCI | Paddle over T2 | Abbott | A | Paraesthesia | Mid T2 |
| 40 | M | L1 SCI | Paddle T11 | Abbott | C | BurstDR | T10/T11 |
| 70 | M | L3 SCI | Paddle T10–T11 | Abbott | D | BurstDR | T10/11 |
| 54 | M | C4 SCI | Paddle C4–C7 | Nevro | B | HFX | High C3 |
| 46 | F | T3 SCI | Percutaenous | Nevro | A | HFX |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamri, A.; MacDonald, M.; Al-Mohammad, A.; Ricciardi, L.; Hart, M.G.; Pereira, E.A. Spinal Cord Stimulation for Spinal Cord Injury-Related Pain: A Pilot Study. Brain Sci. 2024, 14, 1173. https://doi.org/10.3390/brainsci14121173
Alamri A, MacDonald M, Al-Mohammad A, Ricciardi L, Hart MG, Pereira EA. Spinal Cord Stimulation for Spinal Cord Injury-Related Pain: A Pilot Study. Brain Sciences. 2024; 14(12):1173. https://doi.org/10.3390/brainsci14121173
Chicago/Turabian StyleAlamri, Alexander, Meredith MacDonald, Alaa Al-Mohammad, Lucia Ricciardi, Michael G. Hart, and Erlick A. Pereira. 2024. "Spinal Cord Stimulation for Spinal Cord Injury-Related Pain: A Pilot Study" Brain Sciences 14, no. 12: 1173. https://doi.org/10.3390/brainsci14121173
APA StyleAlamri, A., MacDonald, M., Al-Mohammad, A., Ricciardi, L., Hart, M. G., & Pereira, E. A. (2024). Spinal Cord Stimulation for Spinal Cord Injury-Related Pain: A Pilot Study. Brain Sciences, 14(12), 1173. https://doi.org/10.3390/brainsci14121173






