The Constrained Disorder Principle May Account for Consciousness
Abstract
:1. Introduction
1.1. The Constrained Disorder Principle (CDP) Accounts for Complex Systems
1.2. CDP Accounts for Consciousness Mandating Internal and External Variability
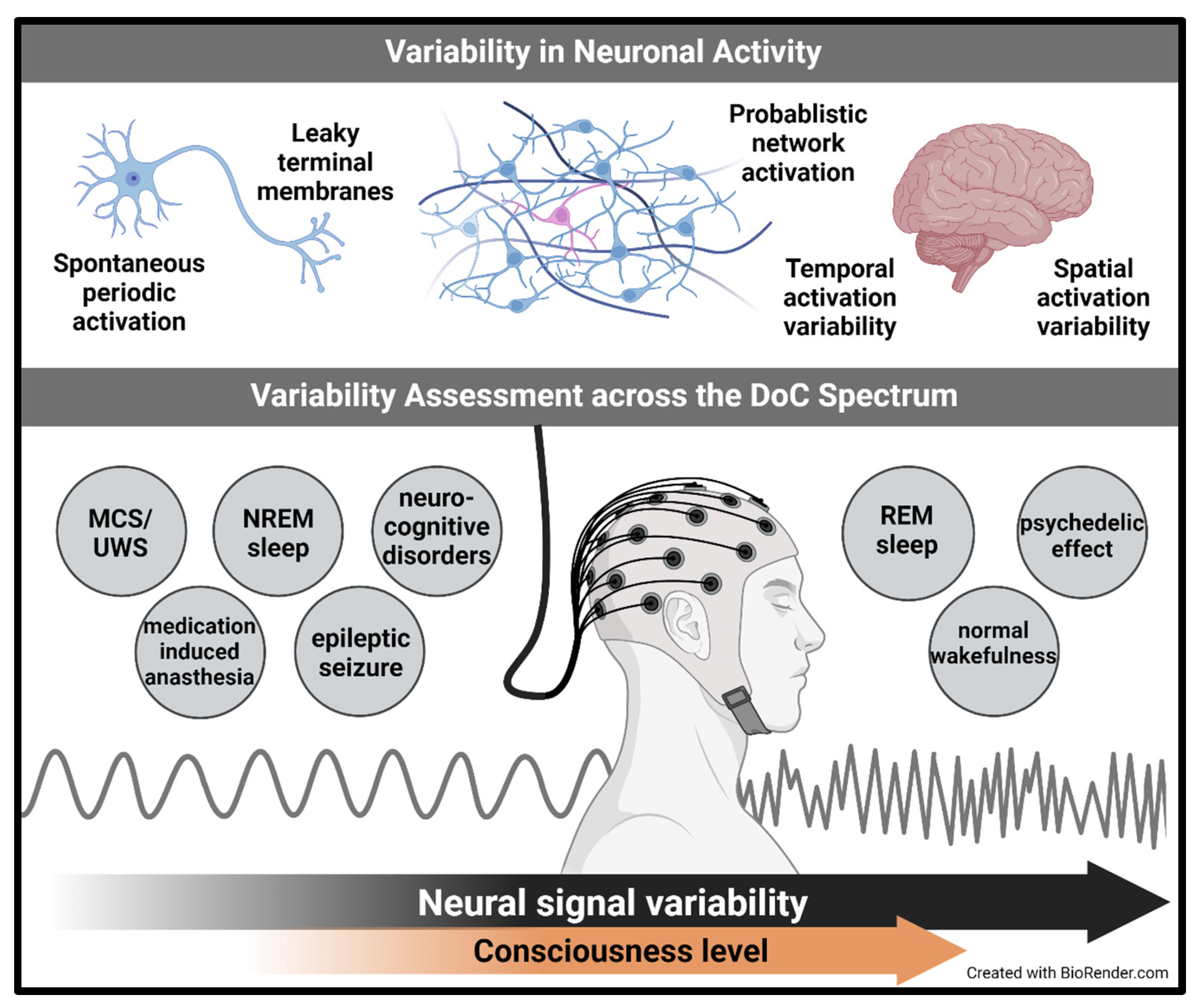
1.3. Methods for Assessing Consciousness Support the Role of Variability in the Process of Consciousness
1.4. CDP Accounts for the Complexity, Entropy, and Uncertainty That Underlie Consciousness
1.5. There Is No Account for the Inherent Variability of Consciousness in Current Theories
1.6. The Variability of Neural Signals in Current Associations of Brain Structures with Consciousness
1.7. CDP Views Consciousness as a Body Adaptation Mechanism Requiring Variability
1.8. The CDP and the Theory of Everything Comprising Consciousness
1.9. CDP-Based Platform to Overcome Drug Tolerance
1.10. Platforms Based on CDP for Improving Consciousness Disorders
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bayne, T.; Hohwy, J.; Owen, A.M. Are there levels of consciousness? Trends Cogn. Sci. 2016, 20, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Peper, A. A general theory of consciousness I: Consciousness and adaptation. Commun. Integr. Biol. 2020, 13, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Dictionary, O.E. Consciousness; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Kotchoubey, B. Human Consciousness: Where Is It From and What Is It for. Front. Psychol. 2018, 9, 567. [Google Scholar] [CrossRef]
- Searle, J.R. How to study consciousness scientifically. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1998, 353, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Tononi, G.; Koch, C. Consciousness: Here, there and everywhere? Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2015, 370, 20140167. [Google Scholar] [CrossRef]
- Signorelli, C.M. Can Computers Become Conscious and Overcome Humans? Front. Robot. AI 2018, 5, 121. [Google Scholar] [CrossRef]
- Ilan, Y. The constrained disorder principle defines living organisms and provides a method for correcting disturbed biological systems. Comput. Struct. Biotechnol. J. 2022, 20, 6087–6096. [Google Scholar] [CrossRef]
- Ilan, Y. Overcoming randomness does not rule out the importance of inherent randomness for functionality. J. Biosci. 2019, 44, 132. [Google Scholar] [CrossRef]
- Ilan, Y. Generating randomness: Making the most out of disordering a false order into a real one. J. Transl. Med. 2019, 17, 49. [Google Scholar] [CrossRef]
- Ilan, Y. Advanced Tailored Randomness: A Novel Approach for Improving the Efficacy of Biological Systems. J. Comput. Biol. 2020, 27, 20–29. [Google Scholar] [CrossRef]
- Ilan, Y. Order Through Disorder: The Characteristic Variability of Systems. Front. Cell Dev. Biol. 2020, 8, 186. [Google Scholar] [CrossRef]
- El-Haj, M.; Kanovitch, D.; Ilan, Y. Personalized inherent randomness of the immune system is manifested by an individualized response to immune triggers and immunomodulatory therapies: A novel platform for designing personalized immunotherapies. Immunol. Res. 2019, 67, 337–347. [Google Scholar] [CrossRef]
- Ilan, Y. Randomness in microtubule dynamics: An error that requires correction or an inherent plasticity required for normal cellular function? Cell Biol. Int. 2019, 43, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Microtubules: From understanding their dynamics to using them as potential therapeutic targets. J. Cell Physiol. 2019, 234, 7923–7937. [Google Scholar] [CrossRef]
- Ilan-Ber, T.; Ilan, Y. The role of microtubules in the immune system and as potential targets for gut-based immunotherapy. Mol. Immunol. 2019, 111, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Forkosh, E.; Kenig, A.; Ilan, Y. Introducing variability in targeting the microtubules: Review of current mechanisms and future directions in colchicine therapy. Pharmacol. Res. Perspect. 2020, 8, e00616. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. beta-Glycosphingolipids as Mediators of Both Inflammation and Immune Tolerance: A Manifestation of Randomness in Biological Systems. Front. Immunol. 2019, 10, 1143. [Google Scholar] [CrossRef] [PubMed]
- Finn, E.H.; Misteli, T. Molecular basis and biological function of variability in spatial genome organization. Science 2019, 365, eaaw9498. [Google Scholar] [CrossRef] [PubMed]
- Chiera, M.; Cerritelli, F.; Casini, A.; Barsotti, N.; Boschiero, D.; Cavigioli, F.; Corti, C.G.; Manzotti, A. Heart Rate Variability in the Perinatal Period: A Critical and Conceptual Review. Front. Neurosci. 2020, 14, 561186. [Google Scholar] [CrossRef]
- Forte, G.; Favieri, F.; Casagrande, M. Heart Rate Variability and Cognitive Function: A Systematic Review. Front. Neurosci. 2019, 13, 710. [Google Scholar] [CrossRef]
- Mitchison, T.; Kirschner, M. Dynamic instability of microtubule growth. Nature 1984, 312, 237–242. [Google Scholar] [CrossRef]
- Kirschner, M.W.; Mitchison, T. Microtubule dynamics. Nature 1986, 324, 621. [Google Scholar] [CrossRef]
- Keshmiri, S. Entropy and the Brain: An Overview. Entropy 2020, 22, 917. [Google Scholar] [CrossRef]
- Nicolini, P.; Ciulla, M.M.; De Asmundis, C.; Magrini, F.; Brugada, P. The prognostic value of heart rate variability in the elderly, changing the perspective: From sympathovagal balance to chaos theory. Pacing Clin. Electrophysiol. 2012, 35, 622–638. [Google Scholar] [CrossRef]
- Varley, T.F.; Craig, M.; Adapa, R.; Finoia, P.; Williams, G.; Allanson, J.; Pickard, J.; Menon, D.K.; Stamatakis, E.A. Fractal dimension of cortical functional connectivity networks & severity of disorders of consciousness. PLoS ONE 2020, 15, e0223812. [Google Scholar] [CrossRef]
- Luppi, A.I.; Craig, M.M.; Coppola, P.; Peattie, A.R.D.; Finoia, P.; Williams, G.B.; Allanson, J.; Pickard, J.D.; Menon, D.K.; Stamatakis, E.A. Preserved fractal character of structural brain networks is associated with covert consciousness after severe brain injury. Neuroimage Clin. 2021, 30, 102682. [Google Scholar] [CrossRef]
- Sarasso, S.; Casali, A.G.; Casarotto, S.; Rosanova, M.; Sinigaglia, C.; Massimini, M. Consciousness and complexity: A consilience of evidence. Neurosci. Conscious. 2021, 7, niab023. [Google Scholar] [CrossRef]
- Boutwell, B.B. On variability & human consciousness. Heliyon 2018, 4, e00905. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, A.R.; Kovacevic, N.; Itier, R.J. Increased brain signal variability accompanies lower behavioral variability in development. PLoS Comput. Biol. 2008, 4, e1000106. [Google Scholar] [CrossRef]
- Zappasodi, F.; Marzetti, L.; Olejarczyk, E.; Tecchio, F.; Pizzella, V. Age-Related Changes in Electroencephalographic Signal Complexity. PLoS ONE 2015, 10, e0141995. [Google Scholar] [CrossRef] [PubMed]
- Turkheimer, E. Three Laws of Behavior Genetics and What They Mean. Curr. Dir. Psychol. Sci. 2000, 9, 160–164. [Google Scholar] [CrossRef]
- Toga, A.W.; Thompson, P.M. Genetics of brain structure and intelligence. Annu. Rev. Neurosci. 2005, 28, 1–23. [Google Scholar] [CrossRef]
- Graziano, M.S.; Kastner, S. Human consciousness and its relationship to social neuroscience: A novel hypothesis. Cogn. Neurosci. 2011, 2, 98–113. [Google Scholar] [CrossRef]
- Toga, A.; Thompson, P. Mapping Brain Maturation. Trends Neurosci. 2006, 29, 148–159. [Google Scholar] [CrossRef]
- Luppi, A.I.; Cain, J.; Spindler, L.R.B.; Górska, U.J.; Toker, D.; Hudson, A.E.; Brown, E.N.; Diringer, M.N.; Stevens, R.D.; Massimini, M.; et al. Mechanisms Underlying Disorders of Consciousness: Bridging Gaps to Move Toward an Integrated Translational Science. Neurocritical Care 2021, 35, 37–54. [Google Scholar] [CrossRef]
- Kumar, S.; Boone, K.; Tuszyński, J.; Barclay, P.; Simon, C. Possible existence of optical communication channels in the brain. Sci. Rep. 2016, 6, 36508. [Google Scholar] [CrossRef]
- He, B.J. Scale-free brain activity: Past, present, and future. Trends Cogn. Sci. 2014, 18, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Salinas, E.; Sejnowski, T.J. Correlated neuronal activity and the flow of neural information. Nat. Rev. Neurosci. 2001, 2, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Roach, J.P.; Pidde, A.; Katz, E.; Wu, J.; Ognjanovski, N.; Aton, S.J.; Zochowski, M.R. Resonance with subthreshold oscillatory drive organizes activity and optimizes learning in neural networks. Proc. Natl. Acad. Sci. USA 2018, 115, E3017–E3025. [Google Scholar] [CrossRef]
- Ghosh, S.; Mondal, A.; Ji, P.; Mishra, A.; Dana, S.K.; Antonopoulos, C.G.; Hens, C. Emergence of Mixed Mode Oscillations in Random Networks of Diverse Excitable Neurons: The Role of Neighbors and Electrical Coupling. Front. Comput. Neurosci. 2020, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Mashour, G.A.; Roelfsema, P.; Changeux, J.-P.; Dehaene, S. Conscious processing and the global neuronal workspace hypothesis. Neuron 2020, 105, 776–798. [Google Scholar] [CrossRef]
- Setareh, H.; Deger, M.; Gerstner, W. Excitable neuronal assemblies with adaptation as a building block of brain circuits for velocity-controlled signal propagation. PLoS Comput. Biol. 2018, 14, e1006216. [Google Scholar] [CrossRef]
- Tallon-Baudry, C. On the neural mechanisms subserving consciousness and attention. Front. Psychol. 2011, 2, 397. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.; Tsuchiya, N. Growing evidence for separate neural mechanisms for attention and consciousness. Atten. Percept. Psychophys. 2021, 83, 558–576. [Google Scholar] [CrossRef]
- Takano, H.; McCartney, M.; Ortinski, P.I.; Yue, C.; Putt, M.E.; Coulter, D.A. Deterministic and stochastic neuronal contributions to distinct synchronous CA3 network bursts. J. Neurosci. 2012, 32, 4743–4754. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, P.R.; Vargas-Caballero, M.; Erdélyi, F.; Szabó, G.; Paulsen, O.; Robinson, H.P. Stochastic and deterministic dynamics of intrinsically irregular firing in cortical inhibitory interneurons. eLife 2016, 5, e16475. [Google Scholar] [CrossRef]
- Braun, H.A. Stochasticity Versus Determinacy in Neurobiology: From Ion Channels to the Question of the “Free Will”. Front. Syst. Neurosci. 2021, 15, 629436. [Google Scholar] [CrossRef] [PubMed]
- Mele, A.; Vohs, K.; Baumeister, R. Free Will and Consciousness: An Introduction and Overview of Perspectives. In Free Will and Consciousness: How Might They Work? Mele, A., Baumeister, R., Vohs, K., Eds.; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Lavazza, A. Free Will and Neuroscience: From Explaining Freedom Away to New Ways of Operationalizing and Measuring It. Front. Hum. Neurosci. 2016, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Ebert, J.P.; Wegner, D.M. Mistaking randomness for free will. Conscious. Cogn. 2011, 20, 965–971. [Google Scholar] [CrossRef]
- Beckett, S. Lessness; Calder and Boyars: London, UK, 1970. [Google Scholar]
- Coetzee, J.M. Samuel Beckett’s “Lessness”: An Exercise in Decomposition. Comput. Humanit. 1973, 7, 195–198. [Google Scholar] [CrossRef]
- Haahr, E.D.a.M. Lessness: Randomness, Consciousness, and Meaning. Available online: https://www.random.org/lessness/paper (accessed on 5 January 2023).
- Schwáb, Z. Mind the gap: The impact of Wolfgang Iser’s reader—Response criticism on biblical studies—A critical assessment. Lit. Theol. 2003, 17, 170–181. [Google Scholar] [CrossRef]
- Harman, G. The intrinsic quality of experience. Philos. Perspect. 1990, 4, 31–52. [Google Scholar] [CrossRef]
- Norretranders, T. The User Illusion: Cutting Consciousness Down to Size; Penguin Books: London, UK, 1999. [Google Scholar]
- Northoff, G.; Zilio, F. From Shorter to Longer Timescales: Converging Integrated Information Theory (IIT) with the Temporo-Spatial Theory of Consciousness (TTC). Entropy 2022, 24, 270. [Google Scholar] [CrossRef]
- Garrett, D.D.; Samanez-Larkin, G.R.; MacDonald, S.W.; Lindenberger, U.; McIntosh, A.R.; Grady, C.L. Moment-to-moment brain signal variability: A next frontier in human brain mapping? Neurosci. Biobehav. Rev. 2013, 37, 610–624. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Wu, J.; Liu, X.; Xu, J.; Zhang, J.; Qin, P.; Dai, R.; Yang, Z.; Mao, Y.; et al. Disrupted neural variability during propofol-induced sedation and unconsciousness. Hum. Brain Mapp. 2018, 39, 4533–4544. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Y.; Han, S.; Xu, L.; Chen, X.; Geng, X.; Bie, L.; He, J. The temporal dynamics of Large-Scale brain network changes in disorders of consciousness: A Microstate-Based study. CNS Neurosci. Ther. 2023, 29, 296–305. [Google Scholar] [CrossRef]
- Fagerholm, E.; Scott, G.; Shew, W.; Song, C.; Leech, R.; Knöpfel, T.; Sharp, D. Cortical Entropy, Mutual Information and Scale-Free Dynamics in Waking Mice. Cereb. Cortex 2016, 26, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Magnani, F.G.; Barbadoro, F.; Cacciatore, M.; Leonardi, M. The importance of instrumental assessment in disorders of consciousness: A comparison between American, European, and UK International recommendations. Crit. Care 2022, 26, 245. [Google Scholar] [CrossRef]
- Casaletto, K.B.; Heaton, R.K. Neuropsychological Assessment: Past and Future. J. Int. Neuropsychol. Soc. 2017, 23, 778–790. [Google Scholar] [CrossRef]
- Young, M.J.; Bodien, Y.G.; Giacino, J.T.; Fins, J.J.; Truog, R.D.; Hochberg, L.R.; Edlow, B.L. The neuroethics of disorders of consciousness: A brief history of evolving ideas. Brain 2021, 144, 3291–3310. [Google Scholar] [CrossRef]
- Goldfine, A.M.; Schiff, N.D. Consciousness: Its neurobiology and the major classes of impairment. Neurol. Clin. 2011, 29, 723–737. [Google Scholar] [CrossRef]
- Blume, C.; Del Giudice, R.; Wislowska, M.; Lechinger, J.; Schabus, M. Across the consciousness continuum-from unresponsive wakefulness to sleep. Front. Hum. Neurosci. 2015, 9, 105. [Google Scholar] [CrossRef]
- Boncompte, G.; Cosmelli, D. Neural Correlates of Conscious Motion Perception. Front. Hum. Neurosci. 2018, 12, 355. [Google Scholar] [CrossRef]
- Eklund, R.; Wiens, S. Visual awareness negativity is an early neural correlate of awareness: A preregistered study with two Gabor sizes. Cogn. Affect. Behav. Neurosci. 2018, 18, 176–188. [Google Scholar] [CrossRef]
- Sinitsyn, D.O.; Poydasheva, A.G.; Bakulin, I.S.; Legostaeva, L.A.; Iazeva, E.G.; Sergeev, D.V.; Sergeeva, A.N.; Kremneva, E.I.; Morozova, S.N.; Lagoda, D.Y.; et al. Detecting the Potential for Consciousness in Unresponsive Patients Using the Perturbational Complexity Index. Brain Sci. 2020, 10, 917. [Google Scholar] [CrossRef]
- Casali, A.G.; Gosseries, O.; Rosanova, M.; Boly, M.; Sarasso, S.; Casali, K.R.; Casarotto, S.; Bruno, M.A.; Laureys, S.; Tononi, G.; et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci. Transl. Med. 2013, 5, 198ra105. [Google Scholar] [CrossRef] [PubMed]
- Snider, S.B.; Hsu, J.; Darby, R.R.; Cooke, D.; Fischer, D.; Cohen, A.L.; Grafman, J.H.; Fox, M.D. Cortical lesions causing loss of consciousness are anticorrelated with the dorsal brainstem. Hum. Brain Mapp. 2020, 41, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Roquet, D.; Foucher, J.R.; Froehlig, P.; Renard, F.; Pottecher, J.; Besancenot, H.; Schneider, F.; Schenck, M.; Kremer, S. Resting-state networks distinguish locked-in from vegetative state patients. NeuroImage. Clin. 2016, 12, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, A.; Sevenius Nilsen, A.; Markhus, R.; Kusztor, A.; HasanzadehMoghadam, F.; Kauppi, N.; Thürer, B.; Storm, J.F.; Juel, B.E. EEG Lempel-Ziv complexity varies with sleep stage, but does not seem to track dream experience. Front. Hum. Neurosci. 2023, 16, 987714. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Massimini, M.; Boly, M.; Tononi, G. Neural correlates of consciousness: Progress and problems. Nat. Rev. Neurosci. 2016, 17, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Arai, N.; Nakanishi, T.; Nakajima, S.; Li, X.; Wada, M.; Daskalakis, Z.J.; Goodman, M.S.; Blumberger, D.M.; Mimura, M.; Noda, Y. Insights of neurophysiology on unconscious state using combined transcranial magnetic stimulation and electroencephalography: A systematic review. Neurosci. Biobehav. Rev. 2021, 131, 293–312. [Google Scholar] [CrossRef]
- Casarotto, S.; Comanducci, A.; Rosanova, M.; Sarasso, S.; Fecchio, M.; Napolitani, M.; Pigorini, A.; Casali, A.G.; Trimarchi, P.D.; Boly, M.; et al. Stratification of unresponsive patients by an independently validated index of brain complexity. Ann. Neurol. 2016, 80, 718–729. [Google Scholar] [CrossRef]
- Morgan, C.J.; Noronha, L.A.; Muetzelfeldt, M.; Feilding, A.; Curran, H.V. Harms and benefits associated with psychoactive drugs: Findings of an international survey of active drug users. J. Psychopharmacol. 2013, 27, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Berlucchi, G.; Marzi, C.A. Neuropsychology of Consciousness: Some History and a Few New Trends. Front. Psychol. 2019, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, H. Impaired consciousness in epilepsy. Lancet. Neurol. 2012, 11, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Boly, M.; Seth, A.K.; Wilke, M.; Ingmundson, P.; Baars, B.; Laureys, S.; Edelman, D.B.; Tsuchiya, N. Consciousness in humans and non-human animals: Recent advances and future directions. Front. Psychol. 2013, 4, 625. [Google Scholar] [CrossRef]
- Feinberg, T.E.; Mallatt, J. The evolutionary and genetic origins of consciousness in the Cambrian Period over 500 million years ago. Front. Psychol. 2013, 4, 667. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, C.; Marino, M.; Carozzo, S.; Russo, M.; Ursino, M.; Ruggiero, V.; Ragno, C.; Proto, S.; Tonin, P. Fractal Dimension Feature as a Signature of Severity in Disorders of Consciousness: An EEG Study. Int. J. Neural Syst. 2022, 32, 2250031. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, A.; Juel, B.; Thürer, B.; Storm, J. Proposed EEG measures of consciousness: A systematic, comparative review. PsyArXiv 2020. [Google Scholar] [CrossRef]
- Thul, A.; Lechinger, J.; Donis, J.; Michitsch, G.; Pichler, G.; Kochs, E.F.; Jordan, D.; Ilg, R.; Schabus, M. EEG entropy measures indicate decrease of cortical information processing in Disorders of Consciousness. Clin. Neurophysiol. 2016, 127, 1419–1427. [Google Scholar] [CrossRef]
- Visani, E.; Luria, G.; Sattin, D.; Rossi Sebastiano, D.; Ferraro, S.; Panzica, F.; Leonardi, M.; Franceschetti, S. Entropy Metrics Correlating with Higher Residual Functioning in Patients with Chronic Disorders of Consciousness. Brain Sci. 2022, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, Z.; Xia, X.; Bai, Y.; Liang, Z.; He, J.; Li, X. Application of Fast Perturbational Complexity Index to the Diagnosis and Prognosis for Disorders of Consciousness. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Gorska, U.; Rupp, A.; Celikel, T.; Englitz, B. Assessing the state of consciousness for individual patients using complex, statistical stimuli. Neuroimage Clin. 2021, 29, 102471. [Google Scholar] [CrossRef] [PubMed]
- Sitt, J.D.; King, J.R.; El Karoui, I.; Rohaut, B.; Faugeras, F.; Gramfort, A.; Cohen, L.; Sigman, M.; Dehaene, S.; Naccache, L. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain 2014, 137, 2258–2270. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Miras, J.; Soler, F.; Iglesias-Parro, S.; Ibáñez-Molina, A.J.; Casali, A.G.; Laureys, S.; Massimini, M.; Esteban, F.J.; Navas, J.; Langa, J.A. Fractal dimension analysis of states of consciousness and unconsciousness using transcranial magnetic stimulation. Comput. Methods Programs Biomed. 2019, 175, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Schartner, M.M.; Pigorini, A.; Gibbs, S.A.; Arnulfo, G.; Sarasso, S.; Barnett, L.; Nobili, L.; Massimini, M.; Seth, A.K.; Barrett, A.B. Global and local complexity of intracranial EEG decreases during NREM sleep. Neurosci. Conscious. 2017, 2017, niw022. [Google Scholar] [CrossRef]
- Mateos, D.M.; Guevara Erra, R.; Wennberg, R.; Perez Velazquez, J.L. Measures of entropy and complexity in altered states of consciousness. Cogn. Neurodyn 2018, 12, 73–84. [Google Scholar] [CrossRef]
- Gonzalez, J.; Mateos, D.; Cavelli, M.; Mondino, A.; Pascovich, C.; Torterolo, P.; Rubido, N. Low frequency oscillations drive EEG’s complexity changes during wakefulness and sleep. Neuroscience 2022, 494, 1–11. [Google Scholar] [CrossRef]
- Schartner, M.; Seth, A.; Noirhomme, Q.; Boly, M.; Bruno, M.A.; Laureys, S.; Barrett, A. Complexity of Multi-Dimensional Spontaneous EEG Decreases during Propofol Induced General Anaesthesia. PLoS ONE 2015, 10, e0133532. [Google Scholar] [CrossRef]
- Artoni, F.; Maillard, J.; Britz, J.; Seeber, M.; Lysakowski, C.; Bréchet, L.; Tramèr, M.R.; Michel, C.M. EEG microstate dynamics indicate a U-shaped path to propofol-induced loss of consciousness. Neuroimage 2022, 256, 119156. [Google Scholar] [CrossRef]
- Li, D.; Fabus, M.S.; Sleigh, J.W. Brain Complexities and Anesthesia: Their Meaning and Measurement. Anesthesiology 2022, 137, 290–302. [Google Scholar] [CrossRef]
- Fuentes, N.; Garcia, A.; Guevara, R.; Orofino, R.; Mateos, D.M. Complexity of Brain Dynamics as a Correlate of Consciousness in Anaesthetized Monkeys. Neuroinformatics 2022, 20, 1041–1054. [Google Scholar] [CrossRef]
- Yang, A.C.; Wang, S.J.; Lai, K.L.; Tsai, C.F.; Yang, C.H.; Hwang, J.P.; Lo, M.T.; Huang, N.E.; Peng, C.K.; Fuh, J.L. Cognitive and neuropsychiatric correlates of EEG dynamic complexity in patients with Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 47, 52–61. [Google Scholar] [CrossRef]
- Maturana-Candelas, A.; Gomez, C.; Poza, J.; Pinto, N.; Hornero, R. EEG Characterization of the Alzheimer’s Disease Continuum by Means of Multiscale Entropies. Entropy 2019, 21, 544. [Google Scholar] [CrossRef]
- Schartner, M.M.; Carhart-Harris, R.L.; Barrett, A.B.; Seth, A.K.; Muthukumaraswamy, S.D. Increased spontaneous MEG signal diversity for psychoactive doses of ketamine, LSD and psilocybin. Sci. Rep. 2017, 7, 46421. [Google Scholar] [CrossRef]
- Alnes, S.L.; Lucia, M.; Rossetti, A.O.; Tzovara, A. Complementary roles of neural synchrony and complexity for indexing consciousness and chances of surviving in acute coma. Neuroimage 2021, 245, 118638. [Google Scholar] [CrossRef]
- Liu, Z.; Si, L.; Xu, W.; Zhang, K.; Wang, Q.; Chen, B.; Wang, G. Characteristics of EEG Microstate Sequences During Propofol-Induced Alterations of Brain Consciousness States. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Varley, T.F.; Carhart-Harris, R.; Roseman, L.; Menon, D.K.; Stamatakis, E.A. Serotonergic psychedelics LSD & psilocybin increase the fractal dimension of cortical brain activity in spatial and temporal domains. Neuroimage 2020, 220, 117049. [Google Scholar] [CrossRef] [PubMed]
- Riganello, F.; Larroque, S.K.; Bahri, M.A.; Heine, L.; Martial, C.; Carrière, M.; Charland-Verville, V.; Aubinet, C.; Vanhaudenhuyse, A.; Chatelle, C.; et al. A Heartbeat Away From Consciousness: Heart Rate Variability Entropy Can Discriminate Disorders of Consciousness and Is Correlated With Resting-State fMRI Brain Connectivity of the Central Autonomic Network. Front. Neurol. 2018, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.D.; Goldberger, A.L. Heart rate fragmentation: Using cardiac pacemaker dynamics to probe the pace of biological aging. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H1341–H1344. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L. Non-linear dynamics for clinicians: Chaos theory, fractals, and complexity at the bedside. Lancet 1996, 347, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.; Jen, H.I.; Lin, Y.H.; Hung, C.S.; Jou, W.J.; Huang, P.W.; Shieh, J.S.; Ho, Y.L.; Lai, D.M.; Wu, A.Y.; et al. Complexity of heart rate variability predicts outcome in intensive care unit admitted patients with acute stroke. J. Neurol. Neurosurg. Psychiatry 2015, 86, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Tobaldini, E.; Toschi-Dias, E.; Trimarchi, P.D.; Brena, N.; Comanducci, A.; Casarotto, S.; Montano, N.; Devalle, G. Cardiac autonomic responses to nociceptive stimuli in patients with chronic disorders of consciousness. Clin. Neurophysiol. 2018, 129, 1083–1089. [Google Scholar] [CrossRef]
- Sattin, D.; Duran, D.; Visintini, S.; Schiaffi, E.; Panzica, F.; Carozzi, C.; Rossi Sebastiano, D.; Visani, E.; Tobaldini, E.; Carandina, A.; et al. Analyzing the Loss and the Recovery of Consciousness: Functional Connectivity Patterns and Changes in Heart Rate Variability During Propofol-Induced Anesthesia. Front. Syst. Neurosci. 2021, 15, 652080. [Google Scholar] [CrossRef] [PubMed]
- Sara, M.; Sebastiano, F.; Sacco, S.; Pistoia, F.; Onorati, P.; Albertini, G.; Carolei, A. Heart rate non linear dynamics in patients with persistent vegetative state: A preliminary report. Brain Inj. 2008, 22, 33–37. [Google Scholar] [CrossRef]
- Riganello, F.; Dolce, G.; Sannita, W.G. Heart rate variability and the central autonomic network in the severe disorder of consciousness. J. Rehabil. Med. 2012, 44, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Cortese, M.D.; Riganello, F.; Arcuri, F.; Pugliese, M.E.; Lucca, L.F.; Dolce, G.; Sannita, W.G. Coma recovery scale-r: Variability in the disorder of consciousness. BMC Neurol. 2015, 15, 186. [Google Scholar] [CrossRef]
- Candelieri, A.; Cortese, M.D.; Dolce, G.; Riganello, F.; Sannita, W.G. Visual pursuit: Within-day variability in the severe disorder of consciousness. J. Neurotrauma 2011, 28, 2013–2017. [Google Scholar] [CrossRef]
- Kaskinoro, K.; Maksimow, A.; Langsjo, J.; Aantaa, R.; Jaaskelainen, S.; Kaisti, K.; Sarkela, M.; Scheinin, H. Wide inter-individual variability of bispectral index and spectral entropy at loss of consciousness during increasing concentrations of dexmedetomidine, propofol, and sevoflurane. Br. J. Anaesth. 2011, 107, 573–580. [Google Scholar] [CrossRef]
- Rolls, E.T.; Cheng, W.; Feng, J. Brain dynamics: The temporal variability of connectivity, and differences in schizophrenia and ADHD. Transl. Psychiatry 2021, 11, 70. [Google Scholar] [CrossRef]
- Liu, B.; Macellaio, M.V.; Osborne, L.C. Efficient sensory cortical coding optimizes pursuit eye movements. Nat. Commun. 2016, 7, 12759. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Wu, J.; Qin, P.; Wu, X.; Wang, Z.; Dai, R.; Li, Y.; Liang, W.; Mao, Y.; et al. Decoupled temporal variability and signal synchronization of spontaneous brain activity in loss of consciousness: An fMRI study in anesthesia. Neuroimage 2016, 124, 693–703. [Google Scholar] [CrossRef]
- Wannez, S.; Heine, L.; Thonnard, M.; Gosseries, O.; Laureys, S. The repetition of behavioral assessments in diagnosis of disorders of consciousness. Ann. Neurol. 2017, 81, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, C.; Kandeepan, S.; Aiello, M.; Ribeiro de Paula, D.; Marchitelli, R.; Fiorenza, S.; Orsini, M.; Trojano, L.; Masotta, O.; St Lawrence, K.; et al. Multimodal Neuroimaging Approach to Variability of Functional Connectivity in Disorders of Consciousness: A PET/MRI Pilot Study. Front. Neurol. 2018, 9, 861. [Google Scholar] [CrossRef] [PubMed]
- Comanducci, A.; Boly, M.; Claassen, J.; De Lucia, M.; Gibson, R.M.; Juan, E.; Laureys, S.; Naccache, L.; Owen, A.M.; Rosanova, M.; et al. Clinical and advanced neurophysiology in the prognostic and diagnostic evaluation of disorders of consciousness: Review of an IFCN-endorsed expert group. Clin. Neurophysiol. 2020, 131, 2736–2765. [Google Scholar] [CrossRef] [PubMed]
- Blum, L.; Blum, M. A theory of consciousness from a theoretical computer science perspective: Insights from the Conscious Turing Machine. Proc. Natl. Acad. Sci. USA 2022, 119, e2115934119. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.A.; Mridha, M.F.; Das, S.C.; Kabir, M.M.; Islam, M.R.; Watanobe, Y. A Comprehensive Survey on the Detection, Classification, and Challenges of Neurological Disorders. Biology 2022, 11, 469. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, R.; Martínez, F.; Zamora, A. Improving the Statistical Qualities of Pseudo Random Number Generators. Symmetry 2022, 14, 269. [Google Scholar] [CrossRef]
- Radin, D.I.; Nelson, R.D. Evidence for consciousness-related anomalies in random physical systems. Found. Phys. 1989, 19, 1499–1514. [Google Scholar] [CrossRef]
- Ilan, Y. Microtubules as a potential platform for energy transfer in biological systems: A target for implementing individualized, dynamic variability patterns to improve organ function. Mol. Cell Biochem. 2022, 478, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Leech, R.; Hellyer, P.J.; Shanahan, M.; Feilding, A.; Tagliazucchi, E.; Chialvo, D.R.; Nutt, D. The entropic brain: A theory of conscious states informed by neuroimaging research with psychedelic drugs. Front. Hum. Neurosci. 2014, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L. The entropic brain-Revisited. Neuropharmacology 2018, 142, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, L.; Gollo, L.L.; Zalesky, A.; Breakspear, M. Criticality in the brain: A synthesis of neurobiology, models and cognition. Prog. Neurobiol. 2017, 158, 132–152. [Google Scholar] [CrossRef] [PubMed]
- Tononi, G.; Edelman, G.M. Consciousness and complexity. Science 1998, 282, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Sarasso, S.; Rosanova, M.; Casali, A.G.; Casarotto, S.; Fecchio, M.; Boly, M.; Gosseries, O.; Tononi, G.; Laureys, S.; Massimini, M. Quantifying cortical EEG responses to TMS in (un) consciousness. Clin. EEG Neurosci. 2014, 45, 40–49. [Google Scholar] [CrossRef]
- Viol, A.; Palhano-Fontes, F.; Onias, H.; de Araujo, D.B.; Viswanathan, G. Shannon entropy of brain functional complex networks under the influence of the psychedelic Ayahuasca. Sci. Rep. 2017, 7, 7388. [Google Scholar] [CrossRef] [PubMed]
- Zmeskal, O.; Dzik, P.; Vesely, M. Entropy of fractal systems. Comput. Math. Appl. 2013, 66, 135–146. [Google Scholar] [CrossRef]
- Ke, D.-G. Unifying complexity and information. Sci. Rep. 2013, 3, 1585. [Google Scholar] [CrossRef]
- Wang, J.; Noh, G.-J.; Choi, B.-M.; Ku, S.-W.; Joo, P.; Jung, W.-S.; Kim, S.; Lee, H. Suppressed neural complexity during ketamine-and propofol-induced unconsciousness. Neurosci. Lett. 2017, 653, 320–325. [Google Scholar] [CrossRef]
- Beggs, J.M.; Timme, N. Being critical of criticality in the brain. Front. Physiol. 2012, 3, 163. [Google Scholar] [CrossRef]
- Shew, W.L.; Yang, H.; Petermann, T.; Roy, R.; Plenz, D. Neuronal avalanches imply maximum dynamic range in cortical networks at criticality. J. Neurosci. 2009, 29, 15595–15600. [Google Scholar] [CrossRef]
- Shew, W.L.; Yang, H.; Yu, S.; Roy, R.; Plenz, D. Information capacity and transmission are maximized in balanced cortical networks with neuronal avalanches. J. Neurosci. 2011, 31, 55–63. [Google Scholar] [CrossRef]
- Mustafa, N.; Ahearn, T.S.; Waiter, G.D.; Murray, A.D.; Whalley, L.J.; Staff, R.T. Brain structural complexity and life course cognitive change. Neuroimage 2012, 61, 694–701. [Google Scholar] [CrossRef]
- Walter, N.; Hinterberger, T. Determining states of consciousness in the electroencephalogram based on spectral, complexity, and criticality features. Neurosci. Conscious. 2022, 2022, niac008. [Google Scholar] [CrossRef]
- Yu, S.; Chen, X.; Yang, K.; Wang, J.; Zhao, K.; Dong, W.; Yan, W.; Su, G.; Zhao, S. Correlation between left ventricular fractal dimension and impaired strain assessed by cardiac MRI feature tracking in patients with left ventricular noncompaction and normal left ventricular ejection fraction. Eur. Radiol. 2022, 32, 2594–2603. [Google Scholar] [CrossRef]
- Foss, J.M.; Apkarian, A.V.; Chialvo, D.R. Dynamics of pain: Fractal dimension of temporal variability of spontaneous pain differentiates between pain States. J. Neurophysiol. 2006, 95, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Galletly, D.; Larsen, P. Ventilatory frequency variability in spontaneously breathing anaesthetized subjects. Br. J. Anaesth. 1999, 83, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Y.; Wang, X.-W.; Chen, D.-Y.; Smith, C.M.; Jin, X.-M. Quantum transport in fractal networks. Nat. Photonics 2021, 15, 703–710. [Google Scholar] [CrossRef]
- Hillen, M.A.; Gutheil, C.M.; Strout, T.D.; Smets, E.M.A.; Han, P.K.J. Tolerance of uncertainty: Conceptual analysis, integrative model, and implications for healthcare. Soc. Sci. Med. 2017, 180, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.C.; Carleton, R.N.; Diefenbach, M.; Han, P.K.J. The Relationship Between Uncertainty and Affect. Front. Psychol. 2019, 10, 2504. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Meng, F.; Wang, C.; Haponenko, H.; Li, A. Motor expertise affects the unconscious processing of geometric forms. PeerJ 2020, 8, e9520. [Google Scholar] [CrossRef]
- Hirsh, J.B.; Mar, R.A.; Peterson, J.B. Psychological entropy: A framework for understanding uncertainty-related anxiety. Psychol. Rev. 2012, 119, 304–320. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, F.; Deng, X.; Jiang, W. A New Total Uncertainty Measure from A Perspective of Maximum Entropy Requirement. Entropy 2021, 23, 1061. [Google Scholar] [CrossRef]
- Kozyreva, A.; Hertwig, R. The interpretation of uncertainty in ecological rationality. Synthese 2021, 198, 1517–1547. [Google Scholar] [CrossRef]
- Ferry, D.K.; Nedjalkov, M.; Weinbub, J.; Ballicchia, M.; Welland, I.; Selberherr, S. Complex Systems in Phase Space. Entropy 2020, 22, 1103. [Google Scholar] [CrossRef] [PubMed]
- Schlitter, J. The Second Law of Thermodynamics as a Force Law. Entropy 2018, 20, 234. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, J.; Wu, J.; Mashour, G.A.; Hudetz, A.G. Temporal circuit of macroscale dynamic brain activity supports human consciousness. Sci. Adv. 2020, 6, eaaz0087. [Google Scholar] [CrossRef] [PubMed]
- Syzranov, S.V.; Gorshkov, A.V.; Galitski, V.M. Interaction-induced transition in the quantum chaotic dynamics of a disordered metal. Ann. Phys. 2019, 405, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.E. Vacuum Radiation, Entropy, and Molecular Chaos. Found. Phys. 2007, 37, 1727–1737. [Google Scholar] [CrossRef]
- Burnes, J. The Action of Consciousness and the Uncertainty Principle. J. Non. Locality 2012, 1. [Google Scholar]
- Huang, K. Introduction to Statistical Physics; Chapman and Hall/CRC: Boca Raton, FL, USA, 2009. [Google Scholar]
- Xie, C. Searching for unity in diversity of animal magnetoreception: From biology to quantum mechanics and back. Innovation 2022, 3, 100229. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chen, X.; Dong, J.; Yang, J.F.; Xiao, H.; Ye, Y.; Li, L.H.; Zhan, C.G.; Yang, W.C.; Yang, G.F. Rational Redesign of Enzyme via the Combination of Quantum Mechanics/Molecular Mechanics, Molecular Dynamics, and Structural Biology Study. J. Am. Chem. Soc. 2021, 143, 15674–15687. [Google Scholar] [CrossRef]
- Yurenko, Y.P.; Novotny, J.; Marek, R. Weak Supramolecular Interactions Governing Parallel and Antiparallel DNA Quadruplexes: Insights from Large-Scale Quantum Mechanics Analysis of Experimentally Derived Models. Chemistry 2017, 23, 5573–5584. [Google Scholar] [CrossRef] [PubMed]
- Sponer, J.; Sponer, J.E.; Mladek, A.; Jurecka, P.; Banas, P.; Otyepka, M. Nature and magnitude of aromatic base stacking in DNA and RNA: Quantum chemistry, molecular mechanics, and experiment. Biopolymers 2013, 99, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Poltev, V.; Anisimov, V.M.; Dominguez, V.; Gonzalez, E.; Deriabina, A.; Garcia, D.; Rivas, F.; Polteva, N.A. Biologically important conformational features of DNA as interpreted by quantum mechanics and molecular mechanics computations of its simple fragments. J. Mol. Model. 2018, 24, 46. [Google Scholar] [CrossRef] [PubMed]
- Annila, A. On the Character of Consciousness. Front. Syst. Neurosci. 2016, 10, 27. [Google Scholar] [CrossRef]
- Pepperell, R. Consciousness as a Physical Process Caused by the Organization of Energy in the Brain. Front. Psychol. 2018, 9, 2091. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.D.; Prakash, C. Objects of consciousness. Front. Psychol. 2014, 5, 577. [Google Scholar] [CrossRef] [PubMed]
- Burns, J. The social brain hypothesis of schizophrenia. World Psychiatry 2006, 5, 77–81. [Google Scholar] [PubMed]
- Kress, G.J.; Mennerick, S. Action potential initiation and propagation: Upstream influences on neurotransmission. Neuroscience 2009, 158, 211–222. [Google Scholar] [CrossRef]
- Cook, N.D. The neuron-level phenomena underlying cognition and consciousness: Synaptic activity and the action potential. Neuroscience 2008, 153, 556–570. [Google Scholar] [CrossRef]
- Lamme, V.A.F. Challenges for theories of consciousness: Seeing or knowing, the missing ingredient and how to deal with panpsychism. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2018, 373, 20170344. [Google Scholar] [CrossRef]
- Seth, A.K.; Dienes, Z.; Cleeremans, A.; Overgaard, M.; Pessoa, L. Measuring consciousness: Relating behavioural and neurophysiological approaches. Trends Cogn. Sci. 2008, 12, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.K.; Bayne, T. Theories of consciousness. Nat. Rev. Neurosci. 2022, 23, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Graziano, M.S.A. A conceptual framework for consciousness. Proc. Natl. Acad. Sci. USA 2022, 119, e2116933119. [Google Scholar] [CrossRef] [PubMed]
- Velmans, M. Making Sense of Causal Interactions Between Consciousness and Brain. J. Conscious. Stud. 2002, 9, 1. [Google Scholar]
- Freris, L. Mind and matter. Commun. Integr. Biol. 2013, 6, e26658. [Google Scholar] [CrossRef] [PubMed]
- Perlovsky, L.I. Physics of the Mind. Front. Syst. Neurosci. 2016, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Pitts, J.B. Conservation Laws and the Philosophy of Mind: Opening the Black Box, Finding a Mirror. Philosophia 2020, 48, 673–707. [Google Scholar] [CrossRef]
- Jylkkä, J.; Railo, H. Consciousness as a concrete physical phenomenon. Conscious. Cogn. 2019, 74, 102779. [Google Scholar] [CrossRef]
- Schaffer, J. Monism: The Priority of the Whole. Philos. Rev. 2010, 119, 31–76. [Google Scholar] [CrossRef]
- Goertzel, B. Quantum Theory and Consciousness. J. Mind Behav. 1992, 13, 29–36. [Google Scholar]
- Tegmark, M. Consciousness as a state of matter. Chaos Solitons Fractals 2015, 76, 238–270. [Google Scholar] [CrossRef]
- Suojanen, M. Conscious Experience and Quantum Consciousness Theory: Theories, Causation, and Identity. E-LOGOS 2019, 26, 14–34. [Google Scholar] [CrossRef]
- McKilliam, A. What is a global state of consciousness? Philos. Mind Sci. 2020, 1. [Google Scholar] [CrossRef]
- Park, H.-D.; Tallon-Baudry, C. The neural subjective frame: From bodily signals to perceptual consciousness. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130208. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J. Higher-order theories of consciousness and what-it-is-like-ness. Philos. Stud. 2018, 175, 2743–2761. [Google Scholar] [CrossRef]
- Brown, R.; Lau, H.; LeDoux, J.E. Understanding the Higher-Order Approach to Consciousness. Trends Cogn. Sci. 2019, 23, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, B.; Chang, M.Y.; Lau, H.; Cheung, S.-H. Inflation versus filling-in: Why we feel we see more than we actually do in peripheral vision. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170345. [Google Scholar] [CrossRef]
- Lau, H.C.; Passingham, R.E. Relative blindsight in normal observers and the neural correlate of visual consciousness. Proc. Natl. Acad. Sci. USA 2006, 103, 18763–18768. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.; Rosenthal, D. Empirical support for higher-order theories of conscious awareness. Trends Cogn. Sci. 2011, 15, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.M.; Ryu, J.; Golfinos, J.G.; Blackmon, K.E. Domain-specific impairment in metacognitive accuracy following anterior prefrontal lesions. Brain 2014, 137, 2811–2822. [Google Scholar] [CrossRef]
- Fox, K.C.; Shi, L.; Baek, S.; Raccah, O.; Foster, B.L.; Saha, S.; Margulies, D.S.; Kucyi, A.; Parvizi, J. Intrinsic network architecture predicts the effects elicited by intracranial electrical stimulation of the human brain. Nat. Hum. Behav. 2020, 4, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Demertzi, A.; Tagliazucchi, E.; Dehaene, S.; Deco, G.; Barttfeld, P.; Raimondo, F.; Martial, C.; Fernández-Espejo, D.; Rohaut, B.; Voss, H. Human consciousness is supported by dynamic complex patterns of brain signal coordination. Sci. Adv. 2019, 5, eaat7603. [Google Scholar] [CrossRef] [PubMed]
- Dehaene, S.; Naccache, L. Towards a cognitive neuroscience of consciousness: Basic evidence and a workspace framework. Cognition 2001, 79, 1–37. [Google Scholar] [CrossRef]
- Dehaene, S.; Changeux, J.-P. Experimental and theoretical approaches to conscious processing. Neuron 2011, 70, 200–227. [Google Scholar] [CrossRef]
- Dehaene, S.; Sergent, C.; Changeux, J.-P. A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proc. Natl. Acad. Sci. USA 2003, 100, 8520–8525. [Google Scholar] [CrossRef]
- Koch, C. What Is Consciousness? Nature 2018, 557, S8–S12. [Google Scholar] [CrossRef]
- Tononi, G.; Boly, M.; Massimini, M.; Koch, C. Integrated information theory: From consciousness to its physical substrate. Nat. Rev. Neurosci. 2016, 17, 450–461. [Google Scholar] [CrossRef]
- Oizumi, M.; Albantakis, L.; Tononi, G. From the phenomenology to the mechanisms of consciousness: Integrated information theory 3.0. PLoS Comput. Biol. 2014, 10, e1003588. [Google Scholar] [CrossRef]
- Song, C.; Haun, A.M.; Tononi, G. Plasticity in the Structure of Visual Space. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Block, N. Consciousness, accessibility, and the mesh between psychology and neuroscience. Behav. Brain Sci. 2007, 30, 481–499; discussion 499–548. [Google Scholar] [CrossRef]
- Tononi, G. An information integration theory of consciousness. BMC Neurosci. 2004, 5, 42. [Google Scholar] [CrossRef]
- Tononi, G. Consciousness as integrated information: A provisional manifesto. Biol. Bull. 2008, 215, 216–242. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.S.; Augustine, G.J. The claustrum and consciousness: An update. Int. J. Clin. Health Psychol. 2023, 23, 100405. [Google Scholar] [CrossRef] [PubMed]
- Tsytsarev, V. Methodological aspects of studying the mechanisms of consciousness. Behav. Brain Res. 2022, 419, 113684. [Google Scholar] [CrossRef]
- Nikolenko, V.N.; Rizaeva, N.A.; Beeraka, N.M.; Oganesyan, M.V.; Kudryashova, V.A.; Dubovets, A.A.; Borminskaya, I.D.; Bulygin, K.V.; Sinelnikov, M.Y.; Aliev, G. The mystery of claustral neural circuits and recent updates on its role in neurodegenerative pathology. Behav. Brain Funct. 2021, 17, 8. [Google Scholar] [CrossRef]
- Northoff, G.; Huang, Z. How do the brain’s time and space mediate consciousness and its different dimensions? Temporo-spatial theory of consciousness (TTC). Neurosci. Biobehav. Rev. 2017, 80, 630–645. [Google Scholar] [CrossRef] [PubMed]
- Klar, P.; Çatal, Y.; Langner, R.; Huang, Z.; Northoff, G. Scale-free dynamics in the core-periphery topography and task alignment decline from conscious to unconscious states. Commun. Biol. 2023, 6, 499. [Google Scholar] [CrossRef]
- Hohwy, J.; Seth, A. Predictive processing as a systematic basis for identifying the neural correlates of consciousness. Philos. Mind Sci. 2020, 1. [Google Scholar] [CrossRef]
- Clark, A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav. Brain Sci. 2013, 36, 181–204. [Google Scholar] [CrossRef]
- Friston, K. The free-energy principle: A unified brain theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. Am I Self-Conscious? (Or Does Self-Organization Entail Self-Consciousness?). Front. Psychol. 2018, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.P.N.; Ballard, D.H. Predictive coding in the visual cortex: A functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 1999, 2, 79–87. [Google Scholar] [CrossRef]
- Parr, T.; Friston, K.J. Generalised free energy and active inference. Biol. Cybern. 2019, 113, 495–513. [Google Scholar] [CrossRef]
- Seth, A.K. A predictive processing theory of sensorimotor contingencies: Explaining the puzzle of perceptual presence and its absence in synesthesia. Cogn. Neurosci. 2014, 5, 97–118. [Google Scholar] [CrossRef]
- Parr, T.; Corcoran, A.W.; Friston, K.J.; Hohwy, J. Perceptual awareness and active inference. Neurosci. Conscious. 2019, 2019, niz012. [Google Scholar] [CrossRef]
- Friston, K.; FitzGerald, T.; Rigoli, F.; Schwartenbeck, P.; Pezzulo, G. Active Inference: A Process Theory. Neural Comput. 2017, 29, 1–49. [Google Scholar] [CrossRef]
- Parr, T.; Friston, K.J. Working memory, attention, and salience in active inference. Sci. Rep. 2017, 7, 14678. [Google Scholar] [CrossRef]
- Lamme, V.A. Towards a true neural stance on consciousness. Trends Cogn. Sci. 2006, 10, 494–501. [Google Scholar] [CrossRef]
- Boehler, C.; Schoenfeld, M.; Heinze, H.-J.; Hopf, J.-M. Rapid recurrent processing gates awareness in primary visual cortex. Proc. Natl. Acad. Sci. USA 2008, 105, 8742–8747. [Google Scholar] [CrossRef] [PubMed]
- Velmans, M. A reflexive science of consciousness. Ciba Found. Symp. 1993, 174, 81–91; discussion 91–89. [Google Scholar] [CrossRef] [PubMed]
- Peebles, G. Reflexive theories of consciousness and unconscious perception. Philos. Psychol. 2018, 31, 25–43. [Google Scholar] [CrossRef]
- Mehta, N.; Mashour, G. General and specific consciousness: A first-order representationalist approach. Front. Psychol. 2013, 4, 407. [Google Scholar] [CrossRef] [PubMed]
- Tye, M. 253 Representationalist Theories of Consciousness. In The Oxford Handbook of Philosophy of Mind; Beckermann, A., McLaughlin, B.P., Walter, S., Eds.; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Yeshurun, Y.; Swanson, S.; Simony, E.; Chen, J.; Lazaridi, C.; Honey, C.J.; Hasson, U. Same Story, Different Story: The neural representation of interpretive frameworks. Psychol. Sci. 2017, 28, 307–319. [Google Scholar] [CrossRef]
- Dennett, D.C. Consciousness Explained; Penguin Books UK: London, UK, 1993. [Google Scholar]
- Del Pin, S.H.; Skóra, Z.; Sandberg, K.; Overgaard, M.; Wierzchoń, M. Comparing theories of consciousness: Why it matters and how to do it. Neurosci. Conscious. 2021, 2021, niab019. [Google Scholar] [CrossRef]
- Baars, B.J. Global workspace theory of consciousness: Toward a cognitive neuroscience of human experience. Prog. Brain Res. 2005, 150, 45–53. [Google Scholar]
- Polák, M.; Marvan, T. Neural Correlates of Consciousness Meet the Theory of Identity. Front. Psychol. 2018, 9, 1269. [Google Scholar] [CrossRef]
- Kent, L.; Wittmann, M. Time consciousness: The missing link in theories of consciousness. Neurosci. Conscious. 2021, 2021, niab011. [Google Scholar] [CrossRef]
- Metzinger, T. Neural Correlates of Consciousness: Empirical and Conceptual Questions; MIT Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Långsjö, J.W.; Alkire, M.T.; Kaskinoro, K.; Hayama, H.; Maksimow, A.; Kaisti, K.K.; Aalto, S.; Aantaa, R.; Jääskeläinen, S.K.; Revonsuo, A.; et al. Returning from oblivion: Imaging the neural core of consciousness. J. Neurosci. 2012, 32, 4935–4943. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, J.; Hoel, E. Falsification and consciousness. Neurosci. Conscious. 2021, 2021, niab001. [Google Scholar] [CrossRef]
- Olcese, U.; Oude Lohuis, M.N.; Pennartz, C.M.A. Sensory Processing Across Conscious and Nonconscious Brain States: From Single Neurons to Distributed Networks for Inferential Representation. Front. Syst. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- Walsh, E.; Oakley, D.A. Editing reality in the brain. Neurosci. Conscious. 2022, 2022, niac009. [Google Scholar] [CrossRef]
- Tononi, G.; Koch, C. The neural correlates of consciousness: An update. Ann. N. Y. Acad. Sci. 2008, 1124, 239–261. [Google Scholar] [CrossRef]
- Li, M.; Han, Y.; Aburn, M.J.; Breakspear, M.; Poldrack, R.A.; Shine, J.M.; Lizier, J.T. Transitions in information processing dynamics at the whole-brain network level are driven by alterations in neural gain. PLoS Comput. Biol. 2019, 15, e1006957. [Google Scholar] [CrossRef]
- Torres, J.J.; Marro, J. Brain Performance versus Phase Transitions. Sci. Rep. 2015, 5, 12216. [Google Scholar] [CrossRef]
- Ridder, D.; Vanneste, S.; Freeman, W. The Bayesian brain: Phantom percepts resolve sensory uncertainty. Neurosci. Biobehav. Rev. 2012, 44, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Kveraga, K.; Ghuman, A.S.; Bar, M. Top-down predictions in the cognitive brain. Brain Cogn. 2007, 65, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Calabro, R.S.; Cacciola, A.; Bramanti, P.; Milardi, D. Neural correlates of consciousness: What we know and what we have to learn! Neurol. Sci. 2015, 36, 505–513. [Google Scholar] [CrossRef]
- Afrasiabi, M.; Redinbaugh, M.J.; Phillips, J.M.; Kambi, N.A.; Mohanta, S.; Raz, A.; Haun, A.M.; Saalmann, Y.B. Consciousness depends on integration between parietal cortex, striatum, and thalamus. Cell Syst. 2021, 12, 363–373 e311. [Google Scholar] [CrossRef]
- Askenasy, J.; Lehmann, J. Consciousness, brain, neuroplasticity. Front. Psychol. 2013, 4, 412. [Google Scholar] [CrossRef]
- Budson, A.E.; Richman, K.A.; Kensinger, E.A. Consciousness as a Memory System. Cogn. Behav. Neurol. 2022, 35, 263–297. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, V.; Dwarakanath, A.; Safavi, S.; Werner, J.; Besserve, M.; Panagiotaropoulos, T.I.; Logothetis, N.K. Decoding internally generated transitions of conscious contents in the prefrontal cortex without subjective reports. Nat. Commun. 2022, 13, 1535. [Google Scholar] [CrossRef]
- Kelso, J.A. Multistability and metastability: Understanding dynamic coordination in the brain. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2012, 367, 906–918. [Google Scholar] [CrossRef]
- Alderson, T.H.; Bokde, A.L.W.; Kelso, J.A.S.; Maguire, L.; Coyle, D. Metastable neural dynamics underlies cognitive performance across multiple behavioural paradigms. Hum. Brain Mapp. 2020, 41, 3212–3234. [Google Scholar] [CrossRef] [PubMed]
- Peper, A. Intermittent adaptation: A mathematical model of drug tolerance, dependence and addiction. In Computational Neuroscience of Drug Addiction; Springer: Berlin/Heidelberg, Germany, 2012; pp. 19–56. [Google Scholar]
- Peper, A. Intermittent adaptation. Pharmacopsychiatry 2009, 42, S129–S143. [Google Scholar] [CrossRef] [PubMed]
- Prinz, J.J. Is emotion a form of perception? Can. J. Philos. Suppl. Vol. 2006, 32, 136–160. [Google Scholar] [CrossRef]
- Peper, A. A theory of drug tolerance and dependence II: The mathematical model. J. Theor. Biol. 2004, 229, 491–500. [Google Scholar] [CrossRef]
- Peper, A.; Grimbergen, C.; Kraal, J.; Engelbart, J. An approach to the modeling of the tolerance mechanism in the drug effect. I: The drug effect as a disturbance of regulations. J. Theor. Biol. 1987, 127, 413–426. [Google Scholar] [CrossRef]
- Peper, A.; Grimbergen, C.A.; Kraal, J.W.; Engelbart, J.H. An approach to the modeling of the tolerance mechanism in the drug effect. II: On the implications of compensatory regulation. J. Theor. Biol. 1988, 132, 29–41. [Google Scholar] [CrossRef]
- Cleeremans, A.; Jiménez, L. Implicit learning and consciousness: A graded, dynamic perspective. In Implicit Learning and Consciousness; French, R.M., Cleeremans, A., Eds.; Psychology Press: Hove, UK, 2002; pp. 1–40. [Google Scholar]
- Peper, A. A theory of drug tolerance and dependence I: A conceptual analysis. J. Theor. Biol. 2004, 229, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Earl, B. The biological function of consciousness. Front. Psychol. 2014, 5, 697. [Google Scholar] [CrossRef]
- Ilan, Y. Enhancing the plasticity, proper function and efficient use of energy of the Sun, genes and microtubules using variability. Clin. Transl. Discov. 2022, 2, e103. [Google Scholar] [CrossRef]
- Cranford, S. Wabi Sabi Science and Embracing Emperfection. Matter 2021, 4, 3367–3368. [Google Scholar] [CrossRef]
- Demekas, D.; Parr, T.; Friston, K.J. An Investigation of the Free Energy Principle for Emotion Recognition. Front. Comput. Neurosci. 2020, 14, 30. [Google Scholar] [CrossRef]
- Badcock, P.B.; Friston, K.J.; Ramstead, M.J.D.; Ploeger, A.; Hohwy, J. The hierarchically mechanistic mind: An evolutionary systems theory of the human brain, cognition, and behavior. Cogn. Affect. Behav. Neurosci. 2019, 19, 1319–1351. [Google Scholar] [CrossRef] [PubMed]
- t Hooft, G.; Susskind, L.; Witten, E.; Fukugita, M.; Randall, L.; Smolin, L.; Stachel, J.; Rovelli, C.; Ellis, G.; Weinberg, S.; et al. A theory of everything? Nature 2005, 433, 257–259. [Google Scholar] [CrossRef]
- Aerts, D. Relativity Theory Refounded. Found. Sci. 2018, 23, 511–547. [Google Scholar] [CrossRef]
- Frank, A. Uncertain for a century: Quantum mechanics and the dilemma of interpretation. Ann. N. Y. Acad. Sci. 2015, 1361, 69–73. [Google Scholar] [CrossRef]
- Kauffman, S.A. Quantum Gravity If Non-Locality Is Fundamental. Entropy 2022, 24, 554. [Google Scholar] [CrossRef]
- Khrennikov, A. The Present Situation in Quantum Theory and its Merging with General Relativity. Found. Phys. 2017, 47, 1077–1099. [Google Scholar] [CrossRef]
- Ashtekar, A.; Bianchi, E. A short review of loop quantum gravity. Rep. Prog. Phys. Phys. Soc. 2021, 84, 042001. [Google Scholar] [CrossRef] [PubMed]
- Kallosh, R. M-theory, black holes and cosmology. Proc. Math. Phys. Eng. Sci. 2021, 477, 20200786. [Google Scholar] [CrossRef] [PubMed]
- Rovelli, C. Loop Quantum Gravity. Living Rev. Relativ. 2008, 11, 5. [Google Scholar] [CrossRef]
- Schurger, A.; Graziano, M. Consciousness explained or described? Neurosci. Conscious. 2022, 2022, niac001. [Google Scholar] [CrossRef]
- Hunt, T.; Schooler, J.W. The Easy Part of the Hard Problem: A Resonance Theory of Consciousness. Front. Hum. Neurosci. 2019, 13, 378. [Google Scholar] [CrossRef]
- Halligan, P.W.; Oakley, D.A. Giving Up on Consciousness as the Ghost in the Machine. Front. Psychol. 2021, 12, 571460. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, D.J. The puzzle of conscious experience. Sci. Am. 1995, 273, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Vimal, R.L.P. Towards a Theory of Everything Part III—Introduction of Consciousness in Loop Quantum Gravity and String Theory and Unification of Experiences with Fundamental Forces. Neuroquantology 2010, 8, 571–599. [Google Scholar] [CrossRef]
- Attariani, H.; Momeni, K.; Adkins, K. Defect Engineering: A Path toward Exceeding Perfection. ACS Omega 2017, 2, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mi, X.; Li, X.; Wang, B. Defect Engineering in Metal—Organic Frameworks as Futuristic Options for Purification of Pollutants in an Aqueous Environment. Front. Chem. 2021, 9, 673738. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Overcoming Compensatory Mechanisms toward Chronic Drug Administration to Ensure Long-Term, Sustainable Beneficial Effects. Mol. Ther. Methods Clin. Dev. 2020, 18, 335–344. [Google Scholar] [CrossRef]
- Ilan, Y. Second-Generation Digital Health Platforms: Placing the Patient at the Center and Focusing on Clinical Outcomes. Front. Digit. Health 2020, 2, 569178. [Google Scholar] [CrossRef]
- Ilan, Y. Improving Global Healthcare and Reducing Costs Using Second-Generation Artificial Intelligence-Based Digital Pills: A Market Disruptor. Int. J. Env. Res. Public Health 2021, 18, 811. [Google Scholar] [CrossRef]
- Ilan, Y. Next-Generation Personalized Medicine: Implementation of Variability Patterns for Overcoming Drug Resistance in Chronic Diseases. J. Pers. Med. 2022, 12, 1303. [Google Scholar] [CrossRef]
- Pomatto, L.C.D.; Davies, K.J.A. The role of declining adaptive homeostasis in ageing. J. Physiol. 2017, 595, 7275–7309. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Weksler-Zangen, S.; Ilan, Y. Role of the Immune System and the Circadian Rhythm in the Pathogenesis of Chronic Pancreatitis: Establishing a Personalized Signature for Improving the Effect of Immunotherapies for Chronic Pancreatitis. Pancreas 2020, 49, 1024–1032. [Google Scholar] [CrossRef]
- Ishay, Y.; Kolben, Y.; Kessler, A.; Ilan, Y. Role of circadian rhythm and autonomic nervous system in liver function: A hypothetical basis for improving the management of hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G400–G412. [Google Scholar] [CrossRef]
- Kolben, Y.; Weksler-Zangen, S.; Ilan, Y. Adropin as a potential mediator of the metabolic system-autonomic nervous system-chronobiology axis: Implementing a personalized signature-based platform for chronotherapy. Obes. Rev. 2021, 22, e13108. [Google Scholar] [CrossRef]
- Kenig, A.; Kolben, Y.; Asleh, R.; Amir, O.; Ilan, Y. Improving Diuretic Response in Heart Failure by Implementing a Patient-Tailored Variability and Chronotherapy-Guided Algorithm. Front. Cardiovasc. Med. 2021, 8, 695547. [Google Scholar] [CrossRef]
- Azmanov, H.; Ross, E.L.; Ilan, Y. Establishment of an Individualized Chronotherapy, Autonomic Nervous System, and Variability-Based Dynamic Platform for Overcoming the Loss of Response to Analgesics. Pain. Physician 2021, 24, 243–252. [Google Scholar]
- Potruch, A.; Khoury, S.T.; Ilan, Y. The role of chronobiology in drug-resistance epilepsy: The potential use of a variability and chronotherapy-based individualized platform for improving the response to anti-seizure drugs. Seizure 2020, 80, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Isahy, Y.; Ilan, Y. Improving the long-term response to antidepressants by establishing an individualized platform based on variability and chronotherapy. Int. J. Clin. Pharmacol. Ther. 2021, 59, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Khoury, T.; Ilan, Y. Introducing Patterns of Variability for Overcoming Compensatory Adaptation of the Immune System to Immunomodulatory Agents: A Novel Method for Improving Clinical Response to Anti-TNF Therapies. Front. Immunol. 2019, 10, 2726. [Google Scholar] [CrossRef]
- Khoury, T.; Ilan, Y. Platform introducing individually tailored variability in nerve stimulations and dietary regimen to prevent weight regain following weight loss in patients with obesity. Obes. Res. Clin. Pract. 2021, 15, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Kenig, A.; Ilan, Y. A Personalized Signature and Chronotherapy-Based Platform for Improving the Efficacy of Sepsis Treatment. Front. Physiol. 2019, 10, 1542. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Why targeting the microbiome is not so successful: Can randomness overcome the adaptation that occurs following gut manipulation? Clin. Exp. Gastroenterol. 2019, 12, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Gelman, R.; Bayatra, A.; Kessler, A.; Schwartz, A.; Ilan, Y. Targeting SARS-CoV-2 receptors as a means for reducing infectivity and improving antiviral and immune response: An algorithm-based method for overcoming resistance to antiviral agents. Emerg. Microbes Infect. 2020, 9, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Ishay, Y.; Potruch, A.; Schwartz, A.; Berg, M.; Jamil, K.; Agus, S.; Ilan, Y. A digital health platform for assisting the diagnosis and monitoring of COVID-19 progression: An adjuvant approach for augmenting the antiviral response and mitigating the immune-mediated target organ damage. Biomed. Pharmacother. 2021, 143, 112228. [Google Scholar] [CrossRef]
- Ilan, Y.; Spigelman, Z. Establishing patient-tailored variability-based paradigms for anti-cancer therapy: Using the inherent trajectories which underlie cancer for overcoming drug resistance. Cancer Treat. Res. Commun. 2020, 25, 100240. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, N.; Azmanov, H.; Kesler, A.; Ilan, Y. Establishing a second-generation artificial intelligence-based system for improving diagnosis, treatment, and monitoring of patients with rare diseases. Eur. J. Hum. Genet. 2021, 29, 1485–1490. [Google Scholar] [CrossRef]
- Ilan, Y. Digital Medical Cannabis as Market Differentiator: Second-Generation Artificial Intelligence Systems to Improve Response. Front. Med. 2021, 8, 788777. [Google Scholar] [CrossRef] [PubMed]
- Gelman, R.; Berg, M.; Ilan, Y. A Subject-Tailored Variability-Based Platform for Overcoming the Plateau Effect in Sports Training: A Narrative Review. Int. J. Env. Res. Public Health 2022, 19, 1722. [Google Scholar] [CrossRef] [PubMed]
- Azmanov, H.; Bayatra, A.; Ilan, Y. Digital Analgesic Comprising a Second-Generation Digital Health System: Increasing Effectiveness by Optimizing the Dosing and Minimizing Side Effects. J. Pain. Res. 2022, 15, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, N.; Elkhateeb, N.; Sigawi, T.; Rinsky-Halivni, L.; Ilan, Y. Improving the effectiveness of anti-aging modalities by using the constrained disorder principle-based management algorithms. Front. Aging 2022, 3, 1044038. [Google Scholar] [CrossRef]
- Kolben, Y.; Azmanov, H.; Gelman, R.; Dror, D.; Ilan, Y. Using chronobiology-based second-generation artificial intelligence digital system for overcoming antimicrobial drug resistance in chronic infections. Ann. Med. 2023, 55, 311–318. [Google Scholar] [CrossRef]
- Adar, O.; Hollander, A.; Ilan, Y. The Constrained Disorder Principle Accounts for the Variability That Characterizes Breathing: A Method for Treating Chronic Respiratory Diseases and Improving Mechanical Ventilation. Adv. Respir. Med. 2023, 91, 350–367. [Google Scholar] [CrossRef]
- Adar, T.; Mizrahi, M.; Lichtenstein, Y.; Shabat, Y.; Sakhnini, R.; Zolotarov, L.; Shehadeh, N.; Ilan, Y. Increased hepatic Akt phosphorylation alleviated glucose intolerance and improved liver function in leptin-deficient mice. Clin. Exp. Hepatol. 2023, 9, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Bayatra, A.; Nasserat, R.; Ilan, Y. Overcoming Low Adherence to Chronic Medications by Improving their Effectiveness Using a Personalized Second-generation Digital System. Curr. Pharm. Biotechnol. 2024, 25, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gelman, R.; Hurvitz, N.; Nesserat, R.; Kolben, Y.; Nachman, D.; Jamil, K.; Agus, S.; Asleh, R.; Amir, O.; Berg, M.; et al. A second-generation artificial intelligence-based therapeutic regimen improves diuretic resistance in heart failure: Results of a feasibility open-labeled clinical trial. Biomed. Pharmacother. 2023, 161, 114334. [Google Scholar] [CrossRef]
- Hurvitz, N.; Ilan, Y. The Constrained-Disorder Principle Assists in Overcoming Significant Challenges in Digital Health: Moving from "Nice to Have" to Mandatory Systems. Clin. Pract. 2023, 13, 994–1014. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Department of Medicine 2040: Implementing a Constrained Disorder Principle-Based Second-Generation Artificial Intelligence System for Improved Patient Outcomes in the Department of Internal Medicine. Inquiry 2023, 60, 469580231221285. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Constrained disorder principle-based variability is fundamental for biological processes: Beyond biological relativity and physiological regulatory networks. Prog. Biophys. Mol. Biol. 2023, 180–181, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Making use of noise in biological systems. Prog. Biophys. Mol. Biol. 2023, 178, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sigawi, T.; Ilan, Y. Using Constrained-Disorder Principle-Based Systems to Improve the Performance of Digital Twins in Biological Systems. Biomimetics 2023, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Edlow, B.L.; Sanz, L.R.D.; Polizzotto, L.; Pouratian, N.; Rolston, J.D.; Snider, S.B.; Thibaut, A.; Stevens, R.D.; Gosseries, O.; Curing Coma, C.; et al. Therapies to Restore Consciousness in Patients with Severe Brain Injuries: A Gap Analysis and Future Directions. Neurocrit Care 2021, 35, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.; Carhart-Harris, R.L. Psychedelics as a treatment for disorders of consciousness. Neurosci. Conscious. 2019, 2019, niz003. [Google Scholar] [CrossRef]
- Guo, Y.; Bai, Y.; Xia, X.; Li, J.; Wang, X.; Dai, Y.; Dang, Y.; He, J.; Liu, C.; Zhang, H. Effects of Long-Lasting High-Definition Transcranial Direct Current Stimulation in Chronic Disorders of Consciousness: A Pilot Study. Front. Neurosci. 2019, 13, 412. [Google Scholar] [CrossRef]
- Vanhoecke, J.; Hariz, M. Deep brain stimulation for disorders of consciousness: Systematic review of cases and ethics. Brain Stimul. 2017, 10, 1013–1023. [Google Scholar] [CrossRef]
- Schiff, N.D.; Giacino, J.T.; Kalmar, K.; Victor, J.D.; Baker, K.; Gerber, M.; Fritz, B.; Eisenberg, B.; Biondi, T.; O’Connor, J.; et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature 2007, 448, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Rezaei Haddad, A.; Lythe, V.; Green, A.L. Deep Brain Stimulation for Recovery of Consciousness in Minimally Conscious Patients After Traumatic Brain Injury: A Systematic Review. Neuromodulation 2019, 22, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Osinska, A.; Rynkiewicz, A.; Binder, M.; Komendzinski, T.; Borowicz, A.; Leszczynski, A. Non-invasive Vagus Nerve Stimulation in Treatment of Disorders of Consciousness—Longitudinal Case Study. Front. Neurosci. 2022, 16, 834507. [Google Scholar] [CrossRef]
- Mancuso, M.; Abbruzzese, L.; Canova, S.; Landi, G.; Rossi, S.; Santarnecchi, E. Transcranial Random Noise Stimulation Does Not Improve Behavioral and Neurophysiological Measures in Patients with Subacute Vegetative-Unresponsive Wakefulness State (VS-UWS). Front. Hum. Neurosci. 2017, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Potok, W.; van der Groen, O.; Bachinger, M.; Edwards, D.; Wenderoth, N. Transcranial Random Noise Stimulation Modulates Neural Processing of Sensory and Motor Circuits, from Potential Cellular Mechanisms to Behavior: A Scoping Review. eNeuro 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Murillo, M.A.; Trevino, M.; Manjarrez, E. Random noise stimulation in the treatment of patients with neurological disorders. Neural Regen. Res. 2022, 17, 2557–2562. [Google Scholar] [CrossRef]
- Sanfilippo, K.R.M.; Spiro, N.; Molina-Solana, M.; Lamont, A. Do the shuffle: Exploring reasons for music listening through shuffled play. PLoS ONE 2020, 15, e0228457. [Google Scholar] [CrossRef]
- Naik, B.I.; Lynch, C., 3rd; Durbin, C.G., Jr. Variability in Mechanical Ventilation: What’s All the Noise About? Respir. Care 2015, 60, 1203–1210. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigawi, T.; Hamtzany, O.; Shakargy, J.D.; Ilan, Y. The Constrained Disorder Principle May Account for Consciousness. Brain Sci. 2024, 14, 209. https://doi.org/10.3390/brainsci14030209
Sigawi T, Hamtzany O, Shakargy JD, Ilan Y. The Constrained Disorder Principle May Account for Consciousness. Brain Sciences. 2024; 14(3):209. https://doi.org/10.3390/brainsci14030209
Chicago/Turabian StyleSigawi, Tal, Omer Hamtzany, Josef Daniel Shakargy, and Yaron Ilan. 2024. "The Constrained Disorder Principle May Account for Consciousness" Brain Sciences 14, no. 3: 209. https://doi.org/10.3390/brainsci14030209
APA StyleSigawi, T., Hamtzany, O., Shakargy, J. D., & Ilan, Y. (2024). The Constrained Disorder Principle May Account for Consciousness. Brain Sciences, 14(3), 209. https://doi.org/10.3390/brainsci14030209





