Sensitivity and Specificity of Qualitative Visual Field Tests for Screening Visual Hemifield Deficits in Right-Brain-Damaged Stroke Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Formal Visual Field Assessment
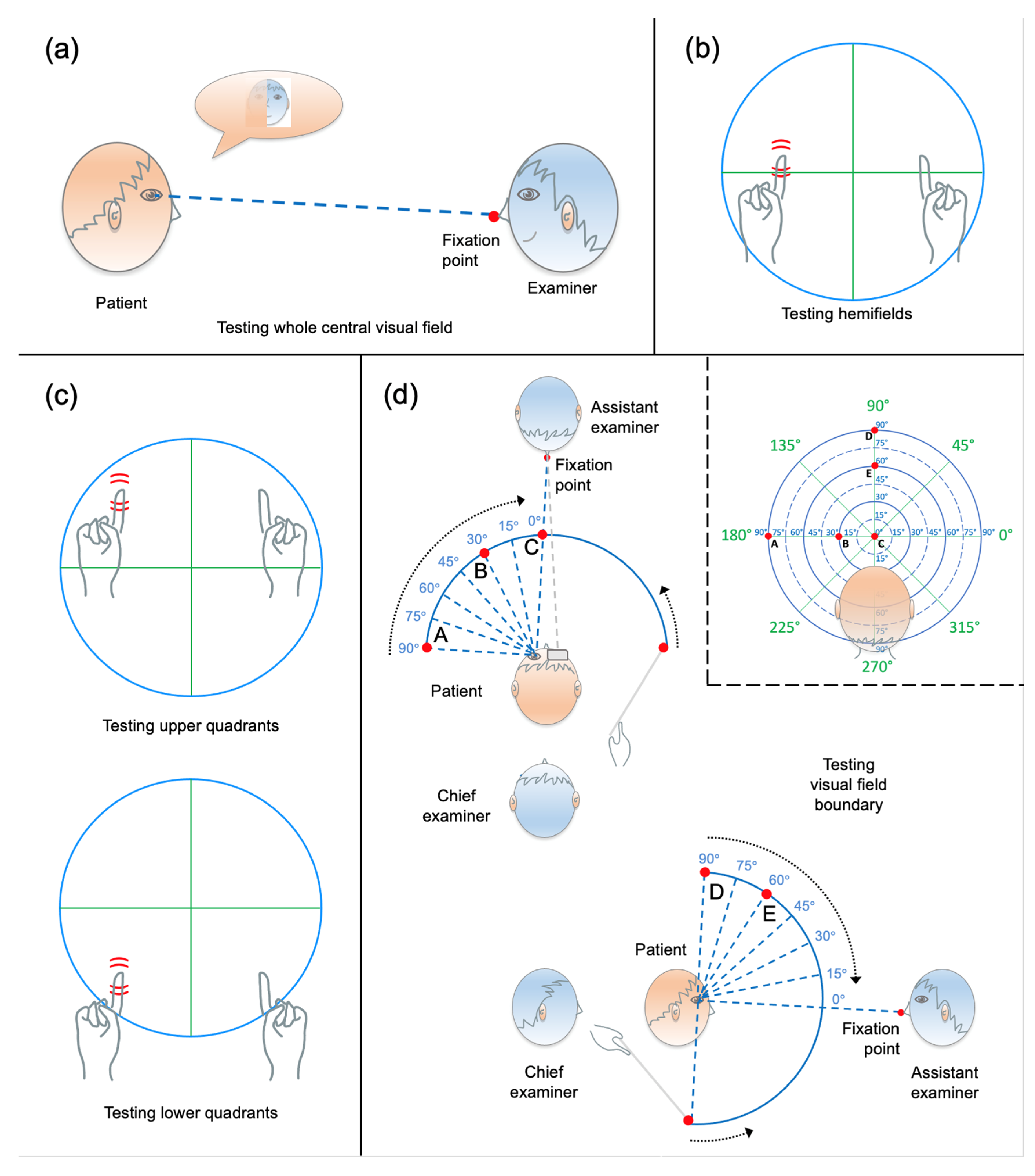
2.3. Qualitative Visual Field Assessment
2.3.1. General Procedure
2.3.2. Face Description Test
2.3.3. Binocular Static Finger Wiggle Test
2.3.4. Monocular Static Finger Wiggle Test
2.3.5. Kinetic Boundary Perimetry
2.4. Data Analysis
3. Results
3.1. Participants
| RBD Stroke Patients (n = 67) | ||||
|---|---|---|---|---|
| (a) Individual Tests | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
| Face description | 13.0 (−0.7–26.8) | 100 (100–100) | 100 (100–100) | 53.5 (41.3–65.7) |
| Binocular static finger wiggle | 60.9 (40.9–80.8) | 100 (100–100) | 100 (100–100) | 71.9 (59.8–84.0) |
| Monocular static finger wiggle | 87.0 (73.2–100) | 100 (100–100) | 100 (100–100) | 88.5 (79.3–97.6) |
| Kinetic boundary perimetry | 91.3 (79.8–100) | 100 (100–100) | 100 (100–100) | 92.0 (84.2–99.8) |
| (b) Combinations of tests | ||||
| 2-test | ||||
| Face description + Binocular static finger wiggle | 60.9 (40.9–80.8) | 100 | 100 | 71.9 (59.8–84.0) |
| Face description + Monocular static finger wiggle | 87.0 (73.2–100) | 100 | 100 | 88.5 (79.3–97.6) |
| Binocular static finger wiggle + Monocular static finger wiggle | 87.0 (73.2–100) | 100 | 100 | 88.5 (79.3–97.6) |
| Face description + Kinetic boundary perimetry | 95.7 (87.3–100) | 100 | 100 | 95.8 (90.0–100) |
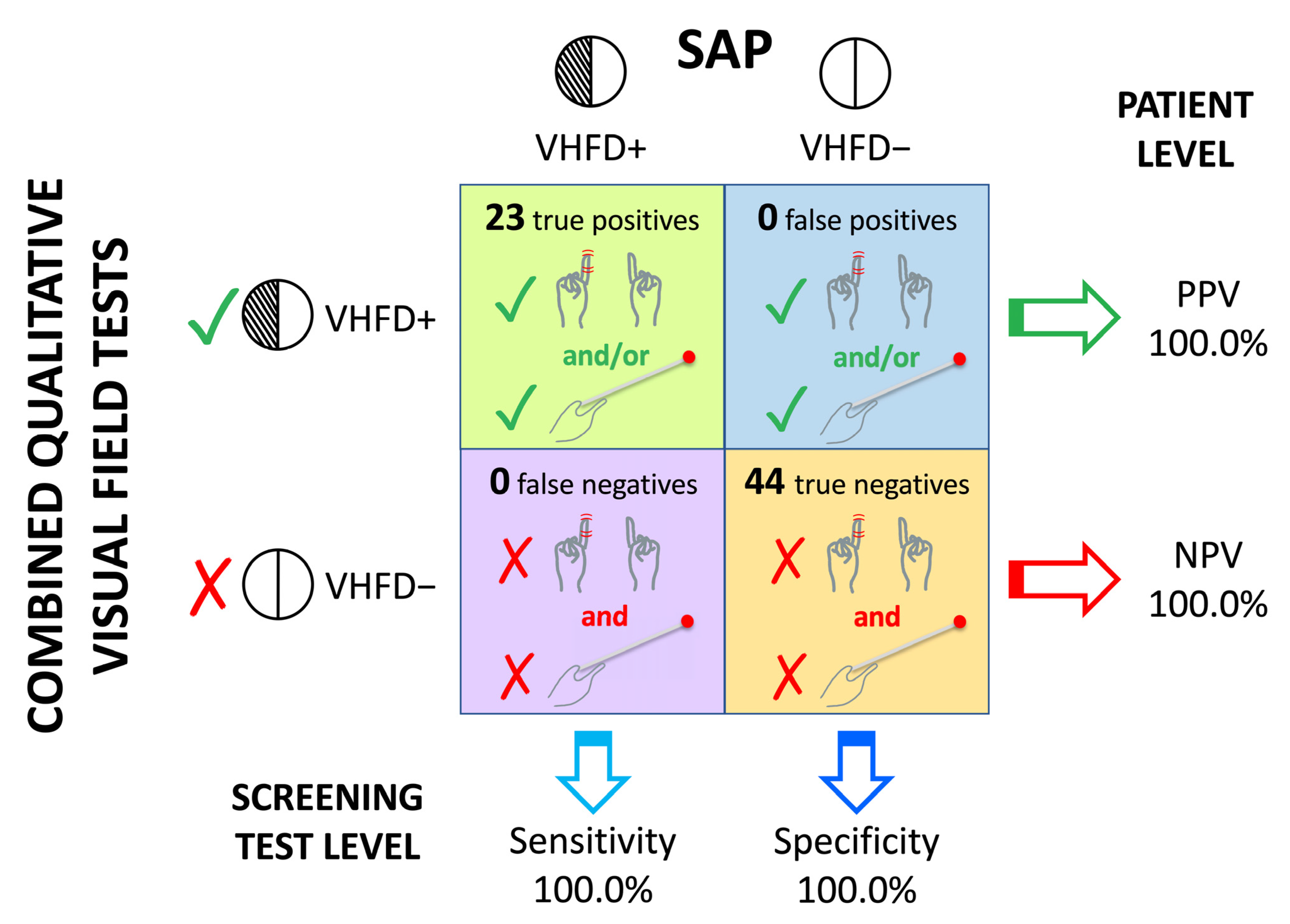
| Binocular static finger wiggle + Kinetic boundary perimetry | 100 (100–100) | 100 | 100 | 100 |
| Monocular static finger wiggle + Kinetic boundary perimetry | 100 (100–100) | 100 | 100 | 100 |
| 3-test | ||||
| Face description + Binocular static finger wiggle + Monocular static finger wiggle | 87.0 (73.2–100) | 100 | 100 | 88.5 (79.3–97.6) |
| Face description + Binocular static finger wiggle + Kinetic boundary perimetry | 100 (100–100) | 100 | 100 | 100 |
| Face description + Monocular static finger wiggle + Kinetic boundary perimetry | 100 (100–100) | 100 | 100 | 100 |
| Binocular static finger wiggle + Monocular static finger wiggle + Kinetic boundary perimetry | 100 (100–100) | 100 | 100 | 100 |
| 4-test | ||||
| Face description + Binocular static finger wiggle + Monocular static finger wiggle + Kinetic boundary perimetry | 100 (100–100) | 100 | 100 | 100 |
3.2. Qualitative Visual Field Assessment
3.2.1. Face Description Test
3.2.2. Binocular Static Finger Wiggle Test
3.2.3. Monocular Static Finger Wiggle Test
3.2.4. Kinetic Boundary Perimetry
3.3. Sensitivity and Specificity of the Qualitative Visual Field Assessment
3.4. Characterisation of the Type of VHFD
4. Discussion
4.1. Changes to Existing Procedures
4.1.1. Kinetic Boundary Perimetry Manoeuvring the Rod from A Rear Position with the Help of An Assistant Examiner
4.1.2. More Trials in the Static Finger Wiggle Tests
4.1.3. Catch Trials
4.1.4. Testing Quadrants Rather Than Whole Hemifields
4.1.5. Using Eye Patches
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cassidy, T.P.; Bruce, D.W.; Gray, C.S. Visual field loss after stroke: Confrontation and perimetry in the assessment of recovery. J. Stroke Cerebrovasc. Dis. 2001, 10, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Jariyakosol, S.; Jaru-Ampornpan, P.; Manassakorn, A.; Itthipanichpong, R.; Hirunwiwatkul, P.; Tantisevi, V.; Somkijrungroj, T.; Rojanapongpun, P. Sensitivity and specificity of new visual field screening software for diagnosing hemianopia. Eye Brain. 2021, 13, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Rowe, F.J.; Wright, D.; Brand, D.; Jackson, C.; Harrison, S.; Maan, T.; Scott, C.; Vogwell, L.; Peel, S.; Akerman, N.; et al. A prospective profile of visual field loss following stroke: Prevalence, type, rehabilitation, and outcome. Biomed. Res. Int. 2013, 2013, 719096. [Google Scholar] [CrossRef]
- Rowe, F.J.; VIS writing Group. Vision in stroke cohort: Profile overview of visual impairment. Brain Behav. 2017, 7, e00771. [Google Scholar] [CrossRef] [PubMed]
- Suchoff, I.B.; Kapoor, N.; Ciuffreda, K.J.; Rutner, D.; Han, E.; Craig, S. The frequency of occurrence, types, and characteristics of visual field defects in acquired brain injury: A retrospective analysis. Optometry 2008, 79, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Cubbidge, R. Visual Fields; Elsevier Health Sciences: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Eperjesi, F.; Bartlett, H.; Dunne, M.C. Ophthalmic Clinical Procedures: A Multimedia Guide; Elsevier Health Sciences: Amsterdam, The Netherlands, 2007; pp. 31–32. [Google Scholar]
- Elliott, D.B.; North, I.; Flanagan, J. Confrontation visual field tests. Ophthalmic Physiol. Opt. 1997, 17 (Suppl. 2), S17–S24. [Google Scholar] [CrossRef]
- Barton, J.J.S.; Benatar, M. Field of Vision: A Manual and Atlas of Perimetry; Humana Press: Totowa, NJ, USA, 2003. [Google Scholar]
- Eperjesi, F. Qualitative Visual Field Analysis Using the Aston Perimetry Tool. Article for Aston University. 2010. Available online: https://docet.info/mod/resource/view.php?id=2457 (accessed on 9 September 2020).
- Hanna, K.L.; Hepworth, L.R.; Rowe, F.J. The treatment methods for post-stroke visual impairment: A systematic review. Brain Behav. 2017, 7, e00682. [Google Scholar] [CrossRef]
- Kerkhoff, G. Neurovisual rehabilitation: Recent developments and future directions. J. Neurol. Neurosurg. Psychiatry 2000, 68, 691–706. [Google Scholar] [CrossRef]
- Liu, K.P.Y.; Hanly, J.; Fahey, P.; Fong, S.S.M.; Bye, R. A Systematic Review and Meta-Analysis of Rehabilitative Interventions for Unilateral Spatial Neglect and Hemianopia Poststroke From 2006 Through 2016. Arch. Phys. Med. Rehabil. 2019, 100, 956–979. [Google Scholar] [CrossRef]
- Grunda, T.; Marsalek, P.; Sykorova, P. Homonymous hemianopia and related visual defects: Restoration of vision after a stroke. Acta Neurobiol. Exp. (Wars) 2013, 73, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Saionz, E.L.; Tadin, D.; Melnick, M.D.; Huxlin, K.R. Functional preservation and enhanced capacity for visual restoration in subacute occipital stroke. Brain 2020, 143, 1857–1872. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.N.; Baloh, F.G. The accuracy of confrontation visual field test in comparison with automated perimetry. J. Natl. Med. Assoc. 1991, 83, 895–898. [Google Scholar] [PubMed]
- Kerr, N.M.; Chew, S.S.; Eady, E.K.; Gamble, G.D.; Danesh-Meyer, H.V. Diagnostic accuracy of confrontation visual field tests. Neurology 2010, 74, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Balcer, L.J.; Volpe, N.J.; Liu, G.T.; Ying, G.S.; Galetta, S.L. Laser pointer visual field screening. J. Neuroophthalmol. 2003, 23, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.J.; Gales, K.; Griffiths, P.G. Effectiveness of testing visual fields by confrontation. Lancet 2001, 358, 1339–1340, Erratum in Lancet 2001, 358, 1820. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.C.; Fernández, V.; Galano, S.; Gómez, R. Confiabilidad de la campimetría manual por confrontación para detectar defectosde campos visuales en patologías neurológicas. Reliability of confrontation testing of visual fields in neurological diseases 2014. Rev. Chil. Neuro-Psiquiat. 2014, 52, 73–80. [Google Scholar]
- Shahinfar, S.; Johnson, L.N.; Madsen, R.W. Confrontation visual field loss as a function of decibel sensitivity loss on automated static perimetry. Implications on the accuracy of confrontation visual field testing. Ophthalmology 1995, 102, 872–877. [Google Scholar] [CrossRef]
- Trobe, J.D.; Acosta, P.C.; Krischer, J.P.; Trick, G.L. Confrontation visual field techniques in the detection of anterior visual pathway lesions. Ann. Neurol. 1981, 10, 28–34. [Google Scholar] [CrossRef]
- Halligan, P.W.; Marshall, J.C.; Wade, D.T. Do visual field deficits exacerbate visuo-spatial neglect? J. Neurol. Neurosurg. Psychiatry 1990, 53, 487–491. [Google Scholar] [CrossRef]
- Buxbaum, L.J.; Ferraro, M.K.; Veramonti, T.; Farne, A.; Whyte, J.; Ladavas, E.; Frassinetti, F.; Coslett, H.B. Hemispatial neglect: Subtypes, neuroanatomy, and disability. Neurology 2004, 62, 749–756. [Google Scholar] [CrossRef]
- Cassidy, T.P.; Bruce, D.W.; Lewis, S.; Gray, C.S. The association of visual field deficits and visuo-spatial neglect in acute right-hemisphere stroke patients. Age Ageing 1999, 28, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Karnath, H.O.; Rennig, J.; Johannsen, L.; Rorden, C. The anatomy underlying acute versus chronic spatial neglect: A longitudinal study. Brain 2011, 134 Pt3, 903–912. [Google Scholar] [CrossRef]
- Nardo, D.; De Luca, M.; Rotondaro, F.; Spanò, B.; Bozzali, M.; Doricchi, F.; Paolucci, S.; Macaluso, E. Left hemispatial neglect and overt orienting in naturalistic conditions: Role of high-level and stimulus-driven signals. Cortex 2019, 113, 329–346. [Google Scholar] [CrossRef]
- Vallar, G.; Perani, D. The anatomy of spatial neglect in humans. Adv. Psychol. 1987, 45, 235–258. [Google Scholar]
- Vuilleumier, P.O.; Rafal, R.D. A systematic study of visual extinction: Between- and within-field deficits of attention in hemispatial neglect. Brain 2000, 123, 1263–1279. [Google Scholar] [CrossRef]
- Azouvi, P.; Samuel, C.; Louis-Dreyfus, A.; Bernati, T.; Bartolomeo, P.; Beis, J.M.; Chokron, S.; Leclercq, M.; Marchal, F.; Martin, Y.; et al. Sensitivity of clinical and behavioural tests of spatial neglect after right hemisphere stroke. J. Neurol. Neurosurg. Psychiatry 2002, 73, 160–166. [Google Scholar] [CrossRef]
- Azouvi, P.; Bartolomeo, P.; Beis, J.M.; Perennou, D.; Pradat-Diehl, P.; Rousseaux, M. A battery of tests for the quantitative assessment of unilateral neglect. Restor. Neurol. Neurosci. 2006, 24, 273–285. [Google Scholar]
- De Luca, M.; Baroncini, M.; Matano, A.; Di Lorenzo, C.; Magnotti, L.; Lucatello, S.; Mulas, M.; Pollarini, V.; Ciurli, M.P.; Nardo, D. Sensitivity and specificity of the Brentano Illusion Test in the detection of visual hemi-field deficits in patients with unilateral spatial neglect. Brain Sci. 2023, 13, 937. [Google Scholar] [CrossRef]
- Bujang, M.A.; Adnan, T.H. Requirements for minimum sample size for sensitivity and specificity analysis. J. Clin. Diagn. Res. 2016, 10, YE01–YE06. [Google Scholar] [CrossRef]
- Maravita, A.; Posteraro, L.; Husain, M.; Vuilleumier, P.; Schwartz, S.; Driver, J. Looking at human eyes affects contralesional stimulus processing after right hemispheric stroke. Neurology 2007, 69, 1619–1621. [Google Scholar] [CrossRef] [PubMed]
- Trevethan, R. Screening, sensitivity, specificity, and so forth: A second, somewhat skeptical, sequel. Mod. Health Sci. 2019, 2, 60–84. [Google Scholar] [CrossRef]
- Trevethan, R. Sensitivity, specificity, and predictive values: Foundations, pliabilities, and pitfalls in research and practice. Front. Public Health 2017, 5, 307. [Google Scholar] [CrossRef] [PubMed]
- Patten, J.P. Neurological Differential Diagnosis, 2nd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1996; pp. 16–38. [Google Scholar]
- Prasad, S.; Cohen, A.B. Diagnostic accuracy of confrontation visual field tests. Neurology 2011, 76, 1192–1193. [Google Scholar] [CrossRef]
- Papanikolaou, A.; Keliris, G.A.; Papageorgiou, T.D.; Shao, Y.; Krapp, E.; Papageorgiou, E.; Stingl, K.; Bruckmann, A.; Schiefer, U.; Logothetis, N.K.; et al. Population receptive field analysis of the primary visual cortex complements perimetry in patients with homonymous visual field defects. Proc. Natl. Acad. Sci. USA 2014, 111, E1656–E1665. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; MacLean, M.W.; Hadid, V.; Lazzouni, L.; Nguyen, D.K.; Tremblay, J.; Dehaes, M.; Lepore, F. Neuronal mechanisms of motion detection underlying blindsight assessed by functional magnetic resonance imaging (fMRI). Neuropsychologia 2019, 128, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.J.; Shuey, N.H.; Wall, M. Rapid confrontation screening for peripheral visual field defects and extinction. Clin. Exp. Optom. 2009, 92, 45–48. [Google Scholar] [CrossRef]
- Chechlaczch, M.; Terry, A.; Demeyere, N.; Douis, H.; Bickerton, W.L.; Rotshtein, P.; Humphreys, G.W. Common and distinct neural mechanisms of visual and tactile extinction: A large scale VBM study in sub-acute stroke. Neuroimage Clin. 2013, 8, 291–302. [Google Scholar] [CrossRef]
- Umarova, R.M.; Saur, D.; Kaller, C.P.; Vry, M.S.; Glauche, V.; Mader, I.; Hennig, J.; Weiller, C. Acute visual neglect and extinction: Distinct functional state of the visuospatial attention system. Brain 2011, 134, 3310–3325. [Google Scholar] [CrossRef]
- Cooper, S.A.; Metcalfe, R.A. Assess and interpret the visual fields at the bedside. Pract. Neurol. 2009, 9, 324–334. [Google Scholar] [CrossRef]
- Hreha, K.; Singsomphone, L.; Kaldenberg, J.; Fielder, J.P.; Watters, K.; Weden, K.; Rizzo, J.R.; Roberts, P.; Wertheimer, J.; Chen, P. Spatial neglect is not a visual field defect: A guide for clinicians. Arch. Phys. Med. Rehabil. 2023, S0003-9993, 00354-4. [Google Scholar] [CrossRef]
- Albert, M.L. A simple test of visual neglect. Neurology 1973, 23, 658–664. [Google Scholar] [CrossRef]
- Bickerton, W.L.; Samson, D.; Williamson, J.; Humphreys, G.W. Separating forms of neglect using the Apples Test: Validation and functional prediction in chronic and acute stroke. Neuropsychology 2011, 25, 567–580. [Google Scholar] [CrossRef]
- Diller, L.; Ben-Yishay, Y.; Gerstamm, L.J. Studies in Cognition and Rehabilitation; New York Medical Center Institute of Rehabilitation Medicine: New York, NY, USA, 1974. [Google Scholar]
- Mancuso, M.; Rosadoni, S.; Capitani, D.; Bickerton, W.L.; Humphreys, G.W.; De Tanti, A.; Zampolini, M.; Galardi, G.; Caputo, M.; De Pellegrin, S.; et al. Italian standardization of the Apples Cancellation Test. Neurol. Sci. 2015, 36, 1233–1240. [Google Scholar] [CrossRef]
- Pizzamiglio, L.; Judica, A.; Razzano, C.; Zoccolotti, P. Toward a comprehensive diagnosis of visual-spatial disorders in unilateral brain damaged patients. Psychol. Assess. 1989, 5, 199.e218. [Google Scholar]
- Spinazzola, L.; Pagliari, C.; Beschin, N. BIT—Behavioural Inattention Test; Italian Adaptation Manual; Giunti O.S: Firenze, Italy, 2010. [Google Scholar]
- Wilson, B.; Cockburn, J.; Halligan, P. Behavioral Inattention Test Manual; Tames Valley Test Company: London, UK, 1987. [Google Scholar]
- Heilman, K.M.; Valenstein, E. Mechanisms underlying hemispatial neglect. Ann. Neurol. 1979, 5, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Guariglia, P.; Matano, A.; Piccardi, L. Bisecting or not bisecting: This is the neglect question. Line bisection performance in the diagnosis of neglect in right brain-damaged patients. PLoS ONE 2014, 9, e99700. [Google Scholar] [CrossRef] [PubMed]
- Massironi, M.; Antonucci, G.; Pizzamiglio, L.; Vitale, M.V.; Zoccolotti, P. The Wundt-Jastrow illusion in the study of spatial hemi-inattention. Neuropsychologia 1988, 26, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Zoccolotti, P.; Antonucci, G.; Judica, A. Psychometric characteristics of two semi-structured scales for the functional evaluation of hemi-inattention in extrapersonal and personal space. Neuropsychol. Rehabil. 1992, 2, 179–191. [Google Scholar] [CrossRef]
- Jehkonen, M.; Ahonen, J.P.; Dastidar, P.; Koivisto, A.M.; Laippala, P.; Vilkki, J. How to detect visual neglect in acute stroke. Lancet 1998, 351, 727–728. [Google Scholar] [CrossRef]
- Rorden, C.; Karnath, H.O. A simple measure of neglect severity. Neuropsychologia 2010, 48, 2758–2763. [Google Scholar] [CrossRef] [PubMed]
- Kooistra, C.A.; Heilman, K.M. Hemispatial visual inattention masquerading as hemianopia. Neurology 1989, 39, 1125–1127. [Google Scholar] [CrossRef] [PubMed]
| n | 67 |
|---|---|
| Sex | 38 M; 29 F |
| Age (years) | 61.5 (14.4) |
| Education (years) | 11.7 (4.7) |
| Time since stroke (days) | 57.4 (48.6) |
| N ischaemic stroke | 46 |
| N haemorrhagic stroke | 21 |
| Patients with a VHFD | 23 |
| Left homonymous hemianopia | 18 |
| Left homonymous inferior quadrantanopia | 4 |
| Left homonymous superior quadrantanopia | 1 |
| With neglect | 21 |
| Without neglect | 2 |
| Patients without aVHFD | 44 |
| With neglect | 23 |
| Without neglect | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, M.; Zeri, F.; Matano, A.; Di Lorenzo, C.; Ciurli, M.P.; Mulas, M.; Pollarini, V.; Paolucci, S.; Nardo, D. Sensitivity and Specificity of Qualitative Visual Field Tests for Screening Visual Hemifield Deficits in Right-Brain-Damaged Stroke Patients. Brain Sci. 2024, 14, 235. https://doi.org/10.3390/brainsci14030235
De Luca M, Zeri F, Matano A, Di Lorenzo C, Ciurli MP, Mulas M, Pollarini V, Paolucci S, Nardo D. Sensitivity and Specificity of Qualitative Visual Field Tests for Screening Visual Hemifield Deficits in Right-Brain-Damaged Stroke Patients. Brain Sciences. 2024; 14(3):235. https://doi.org/10.3390/brainsci14030235
Chicago/Turabian StyleDe Luca, Maria, Fabrizio Zeri, Alessandro Matano, Concetta Di Lorenzo, Maria Paola Ciurli, Martina Mulas, Virginia Pollarini, Stefano Paolucci, and Davide Nardo. 2024. "Sensitivity and Specificity of Qualitative Visual Field Tests for Screening Visual Hemifield Deficits in Right-Brain-Damaged Stroke Patients" Brain Sciences 14, no. 3: 235. https://doi.org/10.3390/brainsci14030235





