Neurorehabilitation including Virtual-Reality-Based Balance Therapy: Factors Associated with Training Response
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Device and Therapy Program
2.3. Collected VR Exergame Parameters
2.4. Clinical Outcome Measurements
2.4.1. Berg Balance Scale
2.4.2. Trunk Impairment Scale
2.4.3. Dynamic Gait Index
2.4.4. Timed Up and Go Test
2.4.5. Functional Ambulation Categories
2.4.6. Intrinsic Motivation Inventory
2.5. Data Analysis
3. Results
3.1. Overview
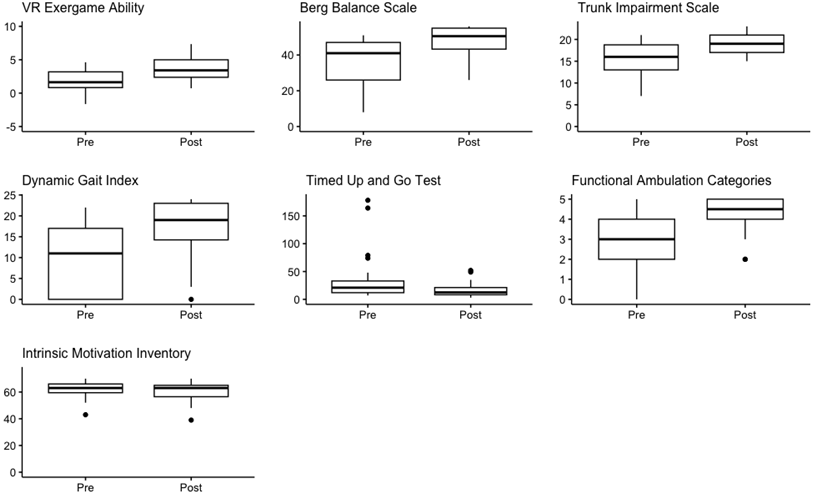
3.2. Change over Time
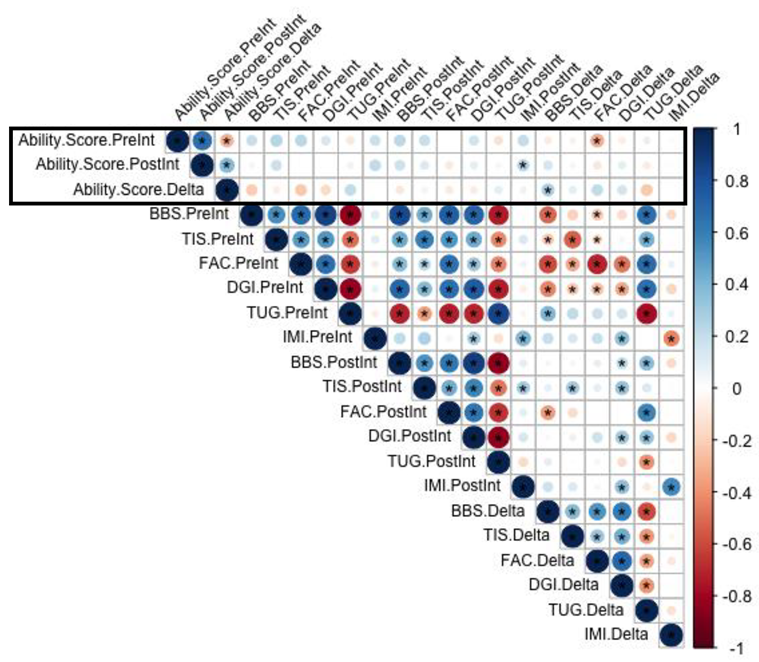
3.3. Correlation between Clinical Outcome Measurements and Exergame Ability Score
3.4. Comparison of Responders to Nonresponders
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Change over Time of Clinical Measurements in Persons with Stroke
 | ||||
| Preintervention Median (IQR) | Postintervention Median (IQR) | p-Value | Effect Size | |
| VR Exergame ability | 1.64 (0.83–3.19) | 3.41 (2.38–5.00) | <0.001 | 0.52 |
| Berg Balance Scale [range 0–56] | 41 (26–47) | 51 (43–55) | <0001 | 0.61 |
| Trunk Impairment Scale [range 0–23] | 16 (13–19) | 19 (17–21) | <0.001 | 0.58 |
| Dynamic Gait Index [range 0–24] | 11 (0–17) | 19 (14–23) | <0.001 | 0.61 |
| Timed Up and Go Test | 16 (11–28) | 13 (10–24) | <0.001 | 0.68 |
| Functional Ambulation Categories [range 0–5] | 3 (2–4) | 5 (4–5) | <0.001 | 0.60 |
| Intrinsic Motivation Inventory [range 0–70] | 63 (60–66) | 63 (57–65) | 0.54 | 0.08 |
Appendix B. Change over Time of Clinical Measurements in Persons with Multiple Sclerosis
 | ||||
| Preintervention Median (IQR) | Postintervention Median (IQR) | p-Value | Effect Size | |
| VR Exergame ability | 2.34 (1.24–3.02) | 3.63 (2.31–4.68) | <0.001 | 0.49 |
| Berg Balance Scale [range 0–56] | 44 (34–47) | 47 (41–51) | <0.001 | 0.57 |
| Trunk Impairment Scale [range 0–23] | 17 (15–18) | 18 (16–20) | <0.001 | 0.42 |
| Dynamic Gait Index [range 0–24] | 13 (8–17) | 16 (12–19) | <0.001 | 0.43 |
| Timed Up and Go Test | 16 (11–28) | 13 (10–24) | <0.001 | 0.50 |
| Functional Ambulation Categories [range 0–5] | 4 (4–5) | 5 (4–5) | <0.001 | 0.36 |
| Intrinsic Motivation Inventory [range 0–70] | 60 (54–64) | 60 (56–65) | 0.28 | 0.10 |
Appendix C. Spearman Rho Correlations between Clinical Measurements and Exergame Ability Score
References
- Quinn, G.; Comber, L.; McGuigan, C.; Hannigan, A.; Galvin, R.; Coote, S. Risk Factors for Falling for People with Multiple Sclerosis Identified in a Prospective Cohort Study. Clin. Rehabil. 2021, 35, 765–774. [Google Scholar] [CrossRef]
- Khan, F.; Chevidikunnan, M.F. Prevalence of Balance Impairment and Factors Associated with Balance among Patients with Stroke. A Cross Sectional Retrospective Case Control Study. Healthcare 2021, 9, 320. [Google Scholar] [CrossRef]
- Sosnoff, J.J.; Socie, M.J.; Boes, M.K.; Sandroff, B.M.; Pula, J.H.; Suh, Y.; Weikert, M.; Balantrapu, S.; Morrison, S.; Motl, R.W. Mobility, Balance and Falls in Persons with Multiple Sclerosis. PLoS ONE 2011, 6, e28021. [Google Scholar] [CrossRef]
- Oh, Y.; Yang, S. Defining Exergames & Exergaming. In Proceedings of the Meaningful Play, East Lansing, MI, USA, 21–23 October 2010; pp. 21–23. [Google Scholar]
- Lohse, K.R.; Hilderman, C.G.E.; Cheung, K.L.; Tatla, S.; Van der Loos, H.F.M. Virtual Reality Therapy for Adults Post-Stroke: A Systematic Review and Meta-Analysis Exploring Virtual Environments and Commercial Games in Therapy. PLoS ONE 2014, 9, e93318. [Google Scholar] [CrossRef] [PubMed]
- Casuso-Holgado, M.J.; Martín-Valero, R.; Carazo, A.F.; Medrano-Sánchez, E.M.; Cortés-Vega, M.D.; Montero-Bancalero, F.J. Effectiveness of Virtual Reality Training for Balance and Gait Rehabilitation in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2018, 32, 1220–1234. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Pérez, I.; Osuna-Pérez, M.C.; Montoro-Cárdenas, D.; Lomas-Vega, R.; Obrero-Gaitán, E.; Nieto-Escamez, F.A. Virtual Reality-Based Therapy Improves Balance and Reduces Fear of Falling in Patients with Multiple Sclerosis. A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Neuroeng. Rehabil. 2023, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual Reality for Stroke Rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef]
- Corbetta, D.; Imeri, F.; Gatti, R. Rehabilitation That Incorporates Virtual Reality Is More Effective than Standard Rehabilitation for Improving Walking Speed, Balance and Mobility after Stroke: A Systematic Review. J. Physiother. 2015, 61, 117–124. [Google Scholar] [CrossRef]
- Wiskerke, E.; Kool, J.; Hilfiker, R.; Sattelmayer, K.M.; Verheyden, G. Determining the Optimal Virtual Reality Exergame Approach for Balance Therapy in Persons With Neurological Disorders Using a Rasch Analysis: Longitudinal Observational Study. JMIR Serious Games 2022, 10, e30366. [Google Scholar] [CrossRef]
- Smith, M.-C.; Barber, P.A.; Stinear, C.M. The TWIST Algorithm Predicts Time to Walking Independently After Stroke. Neurorehabilit. Neural Repair 2017, 31, 955–964. [Google Scholar] [CrossRef]
- Veerbeek, J.M.; Van Wegen, E.E.H.; Harmeling Van Der Wel, B.C.; Kwakkel, G. Is Accurate Prediction of Gait in Nonambulatory Stroke Patients Possible within 72 Hours Poststroke? The EPOS Study. Neurorehabilit. Neural Repair 2011, 25, 268–274. [Google Scholar] [CrossRef]
- Coscia, M.; Wessel, M.J.; Chaudary, U.; Millán, J.d.R.; Micera, S.; Guggisberg, A.; Vuadens, P.; Donoghue, J.; Birbaumer, N.; Hummel, F.C. Neurotechnology-Aided Interventions for upper Limb Motor Rehabilitation in Severe Chronic Stroke. Brain 2019, 142, 2182–2197. [Google Scholar] [CrossRef] [PubMed]
- Langdon, D.W.; Thompson, A.J. Multiple Sclerosis: A Preliminary Study of Selected Variables Affecting Rehabilitation Outcome. Mult. Scler. J. 1999, 5, 94–100. [Google Scholar] [CrossRef]
- Stinear, C.M.; Smith, M.-C.; Byblow, W.D. Prediction Tools for Stroke Rehabilitation. Stroke 2019, 50, 3314–3322. [Google Scholar] [CrossRef]
- Boyd, L.A.; Hayward, K.S.; Ward, N.S.; Stinear, C.M.; Rosso, C.; Fisher, R.J.; Carter, A.R.; Leff, A.P.; Copland, D.A.; Carey, L.M.; et al. Biomarkers of Stroke Recovery: Consensus-Based Core Recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int. J. Stroke 2017, 12, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of Precision Cancer Medicine: Evolution of the Treatment Paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef] [PubMed]
- Prat-Luri, A.; Moreno-Navarro, P.; García, J.A.; Barbado, D.; Vera-Garcia, F.J.; Elvira, J.L.L. Do Initial Trunk Impairment, Age, Intervention Onset, and Training Volume Modulate the Effectiveness of Additional Trunk Exercise Programs after Stroke? A Systematic Review with Meta-Analyses. Int. J. Environ. Res. Public Health 2020, 17, 8714. [Google Scholar] [CrossRef]
- Schwenk, M.; Sabbagh, M.; Lin, I.; Morgan, P.; Grewal, G.S.; Mohler, J.; Coon, D.W.; Najafi, B. Sensor-Based Balance Training with Motion Feedback in People with Mild Cognitive Impairment. J. Rehabil. Res. Dev. 2016, 53, 945–958. [Google Scholar] [CrossRef]
- Werner, C.; Rosner, R.; Wiloth, S.; Lemke, N.C.; Bauer, J.M.; Hauer, K. Time Course of Changes in Motor-Cognitive Exergame Performances during Task-Specific Training in Patients with Dementia: Identification and Predictors of Early Training Response. J. Neuroeng. Rehabil. 2018, 15, 100. [Google Scholar] [CrossRef]
- Maclean, N.; Pound, P.; Wolfe, C.; Rudd, A. The Concept of Patient Motivation. Stroke 2002, 33, 444–448. [Google Scholar] [CrossRef]
- World Medical Association World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
- Bond, T.G.; Fox, C.M. Applying the Rasch Model; Fundamental Measurements in the Human Sciences, 3rd ed.; Routledge: New York, NY, USA, 2015; ISBN 9781315814698. [Google Scholar]
- La Porta, F.; Caselli, S.; Susassi, S.; Cavallini, P.; Tennant, A.; Franceschini, M. Is the Berg Balance Scale an Internally Valid and Reliable Measure of Balance Across Different Etiologies in Neurorehabilitation? A Revisited Rasch Analysis Study. Arch. Phys. Med. Rehabil. 2012, 93, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Blum, L.; Korner-Bitensky, N. Usefulness of the Berg Balance Scale in Stroke Rehabilitation: A Systematic Review. Phys. Ther. 2008, 88, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Verheyden, G.; Nieuwboer, A.; Mertin, J.; Preger, R.; Kiekens, C.; De Weerdt, W. The Trunk Impairment Scale: A New Tool to Measure Motor Impairment of the Trunk after Stroke. Clin. Rehabil. 2004, 18, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Verheyden, G.; Nuyens, G.; Nieuwboer, A.; Van Asch, P.; Ketelaer, P.; De Weerdt, W. Reliability and Validity of Trunk Assessment for People With Multiple Sclerosis. Phys. Ther. 2006, 86, 66–76. [Google Scholar] [CrossRef]
- Torchio, A.; Corrini, C.; Anastasi, D.; Parelli, R.; Meotti, M.; Spedicato, A.; Groppo, E.; D’arma, A.; Grosso, C.; Montesano, A.; et al. Identification of Modified Dynamic Gait Index Cutoff Scores for Assessing Fall Risk in People with Parkinson Disease, Stroke and Multiple Sclerosis. Gait Posture 2022, 91, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir, J.; Cattaneo, D. Reliability and Validity of the Dynamic Gait Index in Persons With Chronic Stroke. Arch. Phys. Med. Rehabil. 2007, 88, 1410–1415. [Google Scholar] [CrossRef]
- McConvey, J.; Bennett, S.E. Reliability of the Dynamic Gait Index in Individuals with Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2005, 86, 130–133. [Google Scholar] [CrossRef]
- Lexell, J.; Flansbjer, U.-B.; Holmbäck, A.M.; Downham, D.; Patten, C. Reliability of gait performance tests in men and women with hemiparesis after stroke. J. Rehabil. Med. 2005, 37, 75–82. [Google Scholar] [CrossRef]
- Sebastião, E.; Sandroff, B.M.; Learmonth, Y.C.; Motl, R.W. Validity of the Timed Up and Go Test as a Measure of Functional Mobility in Persons With Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2016, 97, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Wagner, K.; Rutte, K.; Meiβner, D.; Pohl, M. Predictive Validity and Responsiveness of the Functional Ambulation Category in Hemiparetic Patients After Stroke. Arch. Phys. Med. Rehabil. 2007, 88, 1314–1319. [Google Scholar] [CrossRef]
- Holden, M.K.; Gill, K.M.; Magliozzi, M.R. Gait Assessment for Neurologically Impaired Patients. Phys. Ther. 1986, 66, 1530–1539. [Google Scholar] [CrossRef]
- Ryan, R.M.; Mims, V.; Koestner, R. Relation of Reward Contingency and Interpersonal Context to Intrinsic Motivation: A Review and Test Using Cognitive Evaluation Theory. J. Pers. Soc. Psychol. 1983, 45, 736–750. [Google Scholar] [CrossRef]
- Lloréns, R.; Noé, E.; Colomer, C.; Alcañiz, M. Effectiveness, Usability, and Cost-Benefit of a Virtual Reality–Based Telerehabilitation Program for Balance Recovery After Stroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2015, 96, 418–425.e2. [Google Scholar] [CrossRef]
- Bergmann, J.; Krewer, C.; Bauer, P.; Koenig, A.; Riener, R.; Müller, F. Virtual Reality to Augment Robot-Assisted Gait Training in Non-Ambulatory Patients with a Subacute Stroke: A Pilot Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2018, 54, 397–407. [Google Scholar] [CrossRef] [PubMed]
- McAuley, E.; Duncan, T.; Tammen, V.V. Psychometric Properties of the Intrinsic Motivation Inventory in a Competitive Sport Setting: A Confirmatory Factor Analysis. Res. Q. Exerc. Sport 1989, 60, 48–58. [Google Scholar] [CrossRef] [PubMed]
- de Vries, A.W.; van Dieën, J.H.; van den Abeele, V.; Verschueren, S.M.P. Understanding Motivations and Player Experiences of Older Adults in Virtual Reality Training. Games Health J. 2018, 7, 369–376. [Google Scholar] [CrossRef]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Portney, L.G.; Watkins, M. Foundations of Clinical Research: Application to Practice, 3rd ed.; Pearson/Prentice Hall: Hoboken, NJ, USA, 2009. [Google Scholar]
- Baert, I.; Smedal, T.; Kalron, A.; Rasova, K.; Heric-Mansrud, A.; Ehling, R.; Minguez, I.E.; Nedeljkovic, U.; Tacchino, A.; Hellinckx, P.; et al. Responsiveness and Meaningful Improvement of Mobility Measures Following MS Rehabilitation. Neurology 2018, 91, E1880–E1892. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-J.; Lee, J.-H.; Shin, W.-S. Minimal Clinically Important Difference of Berg Balance Scale Scores in People with Acute Stroke. Phys. Ther. Rehabil. Sci. 2018, 7, 102–108. [Google Scholar] [CrossRef]
- Widmer, M.; Held, J.P.O.; Wittmann, F.; Valladares, B.; Lambercy, O.; Sturzenegger, C.; Palla, A.; Lutz, K.; Luft, A.R. Reward During Arm Training Improves Impairment and Activity After Stroke: A Randomized Controlled Trial. Neurorehabilit. Neural Repair 2022, 36, 140–150. [Google Scholar] [CrossRef]
- Oesch, P.; Kool, J.; Fernandez-Luque, L.; Brox, E.; Evertsen, G.; Civit, A.; Hilfiker, R.; Bachmann, S. Exergames versus Self-Regulated Exercises with Instruction Leaflets to Improve Adherence during Geriatric Rehabilitation: A Randomized Controlled trial. BMC Geriatr. 2017, 17, 77. [Google Scholar] [CrossRef]
- Lyons, E.J. Cultivating Engagement and Enjoyment in Exergames Using Feedback, Challenge, and Rewards. Games Health J. 2018, 4, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Kwakkel, G.; Wagenaar, R.C.; Twisk, J.W.R.; Lankhorst, G.J.; Koetsier, J.C. Intensity of Leg and Arm Training after Primary Middle-Cerebral-Artery Stroke: A Randomised Trial. Lancet 1999, 354, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Farlie, M.K.; Robins, L.; Keating, J.L.; Molloy, E.; Haines, T.P. Intensity of Challenge to the Balance System Is Not Reported in the Prescription of Balance Exercises in Randomised Trials: A Systematic Review. J. Physiother. 2013, 59, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Gervasoni, E.; Jonsdottir, J.; Montesano, A.; Cattaneo, D. Minimal Clinically Important Difference of Berg Balance Scale in People With Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2017, 98, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Miyata, K.; Kobayashi, S.; Takeda, R.; Iwamoto, H. The Minimal Clinically Important Difference in Berg Balance Scale Scores among Patients with Early Subacute Stroke: A Multicenter, Retrospective, Observational Study. Top. Stroke Rehabil. 2022, 29, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, T.J. Detecting Change in Patients with Stroke Using the Berg Balance Scale. Aust. J. Physiother. 2001, 47, 29–38. [Google Scholar] [CrossRef]
- Snapinn, S.M.; Jiang, Q. Responder Analyses and the Assessment of a Clinically Relevant Treatment Effect. Trials 2007, 8, 31. [Google Scholar] [CrossRef]


| Characteristic | All (n = 81) | Stroke (n = 30) | MS (n = 51) |
|---|---|---|---|
| Age, (years) median (IQR) | 57 (51–66) | 65 (56–77) | 55 (46–60) |
| Gender, n (%) | |||
| Male | 35 (43) | 22 (73) | 13 (25) |
| Female | 46 (57) | 8 (27) | 38 (75) |
| Stroke, n (%) | 30 (37) | ||
| Ischemic | 27 (90) | n.a. | |
| Haemorrhagic | 3 (10) | n.a. | |
| Multiple sclerosis, n (%) | 51 (63) | ||
| Primary-progressive MS | n.a. | 13 (25) | |
| Secondary-progressive MS | n.a. | 19 (37) | |
| Relapse-remitting MS | n.a. | 19 (37) | |
| Hemiparetic or weaker body side, n (%) | |||
| Left | 38 (47) | 12 (40) | 26 (51) |
| Right | 40 (49) | 16 (53) | 24 (47) |
| Bilateral | 3 (4) | 2 (7) | 1 (2) |
| Time post diagnosis, median (IQR) | |||
| Time post stroke (days) | 14 (11–21) | n.a. | |
| Time since MS diagnosis (years) | n.a. | 16 (10–21) | |
| Montreal Cognitive Assessment score, median (IQR) [range 0–30] | 24 (22–26) | 23 (20–25) | 25 (23–27) |
| Exergame ability score, median (IQR) | 2.18 (0.83–3.07) | 1.64 (0.83–3.19) | 2.34 (1.24–3.02) |
| Berg Balance Scale score, median (IQR) [range 0–56] | 42 (29–47) | 41 (26–47) | 44 (34–47) |
| Trunk Impairment Scale score, median (IQR) [range 0–23] | 17 (14–18) | 16 (13–19) | 17 (15–18) |
| Dynamic Gait Index score, median (IQR) [range 0–24] | 13 (8–17) | 11 (0–17) | 13 (8–17) |
| Timed Up and Go test time (seconds), median (IQR) | 18 (11–31) | 21 (12–33) | 16 (11–28) |
| With walking aid, n (%) | 38 (47) | 19 (63) | 35 (69) |
| Without walking aid, n (%) | 43 (53) | 11 (37) | 16 (31) |
| Functional Ambulation Category, n (%) | |||
| 0–2 | 13 (16) | 13 (43) | 0 (0) |
| 3–5 | 68 (84) | 17 (57) | 51 (100) |
| Intrinsic Motivation Inventory, median (IQR) [range 0–70] | 61 (54–64) | 63 (60–66) | 60 (54–64) |
 | ||||
|---|---|---|---|---|
| Preintervention Median (IQR) | Postintervention Median (IQR) | p-Value | Effect Size | |
| VR Exergame ability | 2.18 (0.83–3.07) | 3.53 (2.32–4.83) | <0.001 | 0.50 |
| Berg Balance Scale [range 0–56] | 42 (29–47) | 48 (41–53) | <0.001 | 0.59 |
| Trunk Impairment Scale[range 0–23] | 17 (14–18) | 19 (17–20) | <0.001 | 0.50 |
| Dynamic Gait Index [range 0–24] | 13 (8–17) | 16 (13–21) | <0.001 | 0.52 |
| Timed Up and Go Test | 18 (11–31) | 13 (10–23) | <0.001 | 0.55 |
| Functional Ambulation Categories [range 0–5] | 4 (3–5) | 5 (4–5) | <0.001 | 0.45 |
| Intrinsic Motivation Inventory [range 0–70] | 61 (54–64) | 61 (56–65) | 0.54 | 0.05 |
| Variable | Nonresponder (n = 32) | Responder (n = 49) | p-Value |
|---|---|---|---|
| Demographic variables | |||
| Age | 62 (54–73) | 56 (51–61) | 0.13 |
| Gender | 18 female;14 male | 28 female; 21 male | 0.94 ** |
| Diagnosis | 18 stroke; 14 MS | 12 stroke; 37 MS | 0.00 * |
| Weaker bodyside | 2 bilateral; 12 left; 16 right | 1 bilateral; 26 left; 24 right | 0.18 ** |
| Cognitive function (MoCA) | 24 (21–26) | 25 (22–27) | 0.34 |
| Clinical measures at baseline | |||
| Berg Balance Scale score t0 [range 0–56] | 45 (37–50) | 39 (27–46) | 0.02 |
| Trunk Impairment Scale score t0 [range 0–23] | 17 (15–19) | 16 (13–18) | 0.13 |
| Dynamic Gait Index score t0 [range 0–24] | 16 (11–18) | 11 (6–15) | 0.03 |
| Timed Up and Go test time t0 | 14 (11–26) | 21 (13–33) | 0.17 |
| Functional Ambulation Category t0 [range 0–5] | 4 (3–5) | 4 (3–5) | 0.54 |
| Intrinsic motivation t0 [range 0–70] | 61 (54–64) | 61 (54–64) | 0.84 |
| Development of motivation over time | |||
| Intrinsic motivation change [range 0–70] | −2 (−7–3) | 1 (−1–5) | 0.03 |
| Exergame parameters | |||
| Exergame ability score baseline, logits | 2.02 (0.735–3.015) | 2.23 (1.26–3.190) | 0.50 |
| Total duration, minutes | 96 (84–120) | 84 (84–98) | 0.13 |
| Subjective average difficulty [range 0–5] | 1.64 (1.46–1.93) | 1.65 (1.45–1.98) | 0.99 |
| Exercises with objective high difficulty, n | 15 (10–21) | 11 (8–17) | 0.03 |
| Exercises with subjective high difficulty, n | 26 (20–36) | 29 (19–35) | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiskerke, E.; Kool, J.; Hilfiker, R.; Sattelmayer, M.; Verheyden, G. Neurorehabilitation including Virtual-Reality-Based Balance Therapy: Factors Associated with Training Response. Brain Sci. 2024, 14, 263. https://doi.org/10.3390/brainsci14030263
Wiskerke E, Kool J, Hilfiker R, Sattelmayer M, Verheyden G. Neurorehabilitation including Virtual-Reality-Based Balance Therapy: Factors Associated with Training Response. Brain Sciences. 2024; 14(3):263. https://doi.org/10.3390/brainsci14030263
Chicago/Turabian StyleWiskerke, Evelyne, Jan Kool, Roger Hilfiker, Martin Sattelmayer, and Geert Verheyden. 2024. "Neurorehabilitation including Virtual-Reality-Based Balance Therapy: Factors Associated with Training Response" Brain Sciences 14, no. 3: 263. https://doi.org/10.3390/brainsci14030263
APA StyleWiskerke, E., Kool, J., Hilfiker, R., Sattelmayer, M., & Verheyden, G. (2024). Neurorehabilitation including Virtual-Reality-Based Balance Therapy: Factors Associated with Training Response. Brain Sciences, 14(3), 263. https://doi.org/10.3390/brainsci14030263






