Terahertz Radiation Modulates Neuronal Morphology and Dynamics Properties
Abstract
:1. Introduction
2. The Mechanisms of Terahertz Radiation Modulation on Neurons
2.1. Thermal Effect
2.2. Non-Thermal Effect
3. Methods for Terahertz Radiation Regulation of Neurons
3.1. Radiation System for Terahertz Regulation of Neurons
3.2. Radiation Protocol for Terahertz Radiation Regulation of Neurons
4. The Impact of Terahertz Radiation on Neuronal Morphology and Dynamic Properties
4.1. The Influence of Terahertz Radiation on the Growth and Development of Neurons
4.2. The Impact of Terahertz Radiation on Neuronal Membrane Permeability and Integrity
4.3. The Impact of Terahertz Radiation on Neuronal Dynamical Properties
4.4. The Impact of Terahertz Radiation on Animal Behavior
5. The Current Challenges and Future Perspectives
5.1. Safety of Terahertz Regulation of the Nervous System
5.2. Development of Terahertz Radiation System for Neurobiological Effects Research
5.3. The Mechanism of Terahertz Modulation of the Nervous System
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Li, S.; Le, W. Advances of Terahertz Technology in Neuroscience: Current Status and a Future Perspective. iScience 2021, 24, 103548. [Google Scholar] [CrossRef]
- Cherkasova, O.P.; Serdyukov, D.S.; Nemova, E.F.; Ratushnyak, A.S.; Kucheryavenko, A.S.; Dolganova, I.N.; Xu, G.; Skorobogatiy, M.; Reshetov, I.V.; Timashev, P.S.; et al. Cellular Effects of Terahertz Waves. J. Biomed. Opt. 2021, 26, 090902. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zuo, H.; Li, Y. Effects of Radiofrequency Electromagnetic Radiation on Neurotransmitters in the Brain. Front. Public Health 2021, 9, 691880. [Google Scholar] [CrossRef] [PubMed]
- Cherkasova, O.P.; Serdyukov, D.S.; Ratushnyak, A.S.; Nemova, E.F.; Kozlov, E.N.; Shidlovskii, Y.V.; Zaytsev, K.I.; Tuchin, V.V. Effects of Terahertz Radiation on Living Cells: A Review. Opt. Spectrosc. 2020, 128, 855–866. [Google Scholar] [CrossRef]
- Chen, X.; Lindley-Hatcher, H.; Stantchev, R.I.; Wang, J.; Li, K.; Serrano, A.; Taylor, Z.; Castro-Camus, E.; Pickwell-MacPherson, E. Terahertz (THz) Biophotonics Technology: Instrumentation, Techniques, and Biomedical Applications. Chem. Phys. Rev. 2022, 3, 011311. [Google Scholar] [CrossRef]
- Bo, W.; Che, R.; Kong, L.; Zhang, M.; Zhang, X. Research Progress of Biological Effects of Cell Membrane under Infrared and Terahertz Irradiation. Acta Phys. Sin. 2021, 70, 248707. [Google Scholar] [CrossRef]
- Peng, X.; Zhou, H. Biological Effects of Terahertz Waves. Acta Phys. Sin. 2021, 70, 240701. [Google Scholar] [CrossRef]
- Bakels, S.; Gaigeot, M.; Rijs, A. Gas-Phase Infrared Spectroscopy of Neutral Peptides: Insights from the Far-IR and THz Domain. Chem. Rev. 2020, 120, 3233–3260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, R.; Li, B.; Liu, D.; Xu, D. Progress of Terahertz Radiation and Its Biological Effects. Prog. Biochem. Biophys. 2021, 48, 1471–1482. [Google Scholar] [CrossRef]
- Tang, M.; Xia, L.; Wei, D.; Yan, S.; Zhang, M.; Yang, Z.; Wang, H.; Du, C.; Cui, H. Rapid and Label-Free Metamaterial-Based Biosensor for Fatty Acid Detection with Terahertz Time-Domain Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117736. [Google Scholar] [CrossRef] [PubMed]
- Afsah, L.; Hajeb, P.; Ara, P.; Ehsani, R. A Comprehensive Review on Food Applications of Terahertz Spectroscopy and Imaging. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1563–1621. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Mao, P.; Peng, P.; Yan, S.; Zang, Z.; Yao, C. Terahertz Spectra of Proteinuria and Non-Proteinuria. Front. Bioeng. Biotechnol. 2023, 11, 1119694. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Cai, J.; Zhang, W.; Hu, J.; Deng, Y.; Miao, J.; Tan, Z.; Li, H.; Cao, J.; Wu, X. Deep Learning Enhanced Terahertz Imaging of Silkworm Eggs Development. iScience 2021, 24, 103316. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, X.; Wu, J.; Fan, K.; Chen, B.; Zhang, C.; Hu, W.; Cao, X.; Jin, B.; Lu, Y.; et al. Dual-Color Terahertz Spatial Light Modulator for Single-Pixel Imaging. Light Sci. Appl. 2022, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shin, S.; Roh, Y.; Oh, S.; Lee, S.; Song, H.; Ryu, Y.; Kim, Y.; Seo, M. Label-Free Brain Tissue Imaging Using Large-Area Terahertz Metamaterials. Biosens. Bioelectron. 2020, 170, 112663. [Google Scholar] [CrossRef] [PubMed]
- Mancini, T.; Mosetti, R.; Marcelli, A.; Petrarca, M.; Lupi, S.; D’Arco, A. Terahertz Spectroscopic Analysis in Protein Dynamics: Current Status. Radiation 2022, 2, 100–123. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, L.; Peng, R. Research progress in the effects of terahertz waves on biomacromolecules. Mil. Med. Res. 2021, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Wilmink, G.; Grundt, J. Invited Review Article: Current State of Research on Biological Effects of Terahertz Radiation. J. Infrared Millim. Terahertz Waves 2011, 32, 1074–1122. [Google Scholar] [CrossRef]
- Echchgadda, I.; Grundt, J.; Cerna, C.; Roth, C.; Payne, J.; Ibey, B.; Wilmink, G. Terahertz Radiation: A Non-contact Tool for the Selective Stimulation of Biological Responses in Human Cells. IEEE Trans. Terahertz Sci. Technol. 2016, 6, 54–68. [Google Scholar] [CrossRef]
- Niessen, K.; Xu, M.; George, D.; Chen, M.; Ferré-D’Amaré, A.; Snell, E.; Cody, V.; Pace, J.; Schmidt, M.; Markelz, A. Protein and RNA dynamical fingerprinting. Nat. Commun. 2019, 10, 1026. [Google Scholar] [CrossRef]
- Varvdekar, B.; Prabhakant, A.; Krishnan, M. Response of Terahertz Protein Vibrations to Ligand Binding: Calmodulin-Peptide Complexes as a Case Study. J. Chem. Inf. Model. 2022, 62, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chang, C.; Zhu, Z.; Sun, L.; Fan, C. Terahertz Wave Enhances Permeability of the Voltage-Gated Calcium Channel. J. Am. Chem. Soc. 2021, 143, 4311–4318. [Google Scholar] [CrossRef] [PubMed]
- Castro-Camus, E.; Johnston, M. Conformational changes of photoactive yellow protein monitored by terahertz spectroscopy. Chem. Phys. Lett. 2008, 455, 289–292. [Google Scholar] [CrossRef]
- Adams, E.; Pezzotti, S.; Ahlers, J.; Rüttermann, M.; Levin, M.; Goldenzweig, A.; Peleg, Y.; Fleishman, J.; Sagi, I.; Havenith, M. Local Mutations Can Serve as a Game Changer for Global Protein Solvent Interaction. JACS Au 2021, 1, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Walther, M.; Uhd Jepsen, P. Far-infrared vibrational modes of DNA components studied by terahertz time-domain spectroscopy. Phys. Med. Biol. 2002, 47, 3807–3814. [Google Scholar] [CrossRef] [PubMed]
- Cherkasova, O.; Fedorov, V.; Nemova, E.; Pogodin, A. Influence of terahertz laser radiation on the spectral characteristics and functional properties of albumin. Opt. Spectrosc. 2009, 107, 534–537. [Google Scholar] [CrossRef]
- Lundholm, I.; Rodilla, H.; Wahlgren, W.; Duelli, A.; Bourenkov, G.; Vukusic, J.; Friedman, R.; Stake, J.; Schneider, T.; Katona, G. Terahertz radiation induces non-thermal structural changes associated with Fröhlich condensation in a protein crystal. Struct. Dyn. 2015, 2, 054702. [Google Scholar] [CrossRef]
- Bo, W.; Guo, L.; Wang, K.; Ma, J.; Tang, J.; Wu, Z.; Zeng, B.; Gong, Y. Modulation of Voltage-Gated Calcium Influx by Electromagnetic Irradiation with Terahertz Gaussian Pulse. IEEE Access 2020, 8, 133673–133680. [Google Scholar] [CrossRef]
- Zhu, Z.; Chang, C.; Shu, Y.; Song, B. Transition to a Superpermeation Phase of Confined Water Induced by a Terahertz Electromagnetic Wave. J. Phys. Chem. Lett. 2020, 11, 256–262. [Google Scholar] [CrossRef]
- Dimitrov, A. Resting membrane state as an interplay of electrogenic transporters with various pumps. Pflug. Arch.-Eur. J. Physiol. 2023, 475, 1113–1128. [Google Scholar] [CrossRef]
- Wu, H.; Meng, Z.; Wang, J.; Yao, G.; Yang, L.; Zeng, Z.; She, K.; Zhao, S.; Wang, G.; Zhang, Y.; et al. Aptamer functionalized cell membrane for brain and nerve cell sensing with high sensitivity and stability. Biosens. Bioelectron. 2023, 227, 115149. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Lu, J. Terahertz Spectroscopy of the Interfacial Water in Phospholipid Membranes. Laser Optoelectron. Prog. 2017, 54, 043001. [Google Scholar] [CrossRef]
- Etherington, S.; Moorhouse, A.; Moorhouse, T.; Towstoless, M.; Hayes, A.; Hayes, D.; Hayes, L.; Tangalakis, K.; Force, T. Unpacking and validating the “cell membrane” core concept of physiology by an Australian team. Adv. Physiol. Educ. 2023, 47, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, S.; MacRae, M.; Dancel-Manning, K.; Bhabha, G.; Bhabha, D. Lipid Transport Across Bacterial Membranes. Annu. Rev. Cell Dev. Biol. 2022, 38, 125–153. [Google Scholar] [CrossRef] [PubMed]
- Bo, W.; Xu, J.; Tang, J.; Yang, Y.; Ma, J.; Wang, Z.; Gong, Y. Numerical simulation on voltage-activated calcium flux of neuroblastoma cells in response to 2.5THz electric pulses. In Proceedings of the 2017 42nd International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), Cancun, Mexico, 27 August–1 September 2017; pp. 1–2. [Google Scholar] [CrossRef]
- Bo, W.; Che, R.; Guo, L.; Wang, Y.; Guo, L.; Gao, X.; Sun, K.; Wang, S.; Gong, Y. Numerical Simulation on Permeability Change in Cell Membrane by Terahertz Irradiation-Induced Hydrophilic Pores. In Proceedings of the 2021 46th International Conference on Infrared, Millimeter and Terahertz Waves (IRMMW-THz), Chengdu, China, 29 August–3 September 2021; pp. 1–2. [Google Scholar] [CrossRef]
- Liu, X.; Qiao, Z.; Chai, Y.; Zhu, Z.; Wu, K.; Ji, W.; Li, D.; Xiao, Y.; Mao, L.; Chang, C.; et al. Nonthermal and reversible control of neuronal signaling and behavior by midinfrared stimulation. Proc. Natl. Acad. Sci. USA 2021, 118, e2015685118. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, S.; Rana, J.N.; Choi, E.H.; Han, I. Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects. Int. J. Mol. Sci. 2022, 23, 9288. [Google Scholar] [CrossRef]
- Fan, S.; Ma, Y.; Shu, G.; Qian, Z. The interaction of terahertz with water molecules: Mechanism, applications, and new trends. J. Shenzhen Univ. (Sci. Eng.) 2019, 36, 200–206. [Google Scholar] [CrossRef]
- Ladanyi, B.; Skaf, M. Computer Simulation of Hydrogen-Bonding Liquids. Annu. Rev. Phys. Chem. 1993, 44, 335–368. [Google Scholar] [CrossRef]
- Russo, D.; Hura, G.; Head-Gordon, T. Hydration dynamics near a model protein surface. Biophys. J. 2004, 86, 1852–1862. [Google Scholar] [CrossRef]
- Yada, H.; Nagai, M.; Tanaka, K. Origin of the fast relaxation component of water and heavy water revealed by terahertz time-domain attenuated total reflection spectroscopy. Chem. Phys. Lett. 2008, 464, 166–170. [Google Scholar] [CrossRef]
- Ol’shevskaia, I.S.; Kozlov, A.; Petrov, A.; Zapara, T.; Ratushniak, A. Influence of Terahertz (Submillimeter) Laser Radiation on Neurons in vitro. Zhurnal Vyss. Nervn. Deyatelnosti Im. I P Pavlov. 2009, 59, 353–359. [Google Scholar]
- Chen, C.; Ma, Q.; Tao, J.; Lu, Y.; Lin, M.; Gao, P.; Deng, P.; He, M.; Pi, H.; Zhang, L.; et al. Effects of terahertz exposure on skin injury in mouse model. J. Third Mil. Med. Univ. 2020, 42, 2282–2289. [Google Scholar] [CrossRef]
- Xia, Q. Photothermal Effect of Near-Infrared Laser on the Electrical Activity of Cortical Neurons in Rats. Ph.D. Thesis, Chongqing University, Chongqing, China, 2019; pp. 10–42. [Google Scholar] [CrossRef]
- Alexandrov, B.; Rasmussen, K.; Bishop, A.; Usheva, A.; Alexandrov, L.; Chong, S.; Dagon, Y.; Booshehri, L.; Mielke, C.; Phipps, M.; et al. Non-thermal effects of terahertz radiation on gene expression in mouse stem cells. Biomed. Opt. Express 2011, 2, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- Kirichuck, V.F.; Ivanov, A.N.; Antipova, O.N.; Krenickiy, A.P.; Mayborodin, A.V.; Tupikin, V.D. Sex-specific differences in changes of disturbed functional activity of platelets in albino rats under the effect of terahertz electromagnetic radiation at nitric oxide frequencies. Bull. Exp. Biol. Med. 2008, 145, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, H. The extraordinary dielectric properties of biological materials and the action of enzymes. Proc. Natl. Acad. Sci. USA 1975, 72, 4211–4215. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Luo, Y. Terahertz Technology and Its Biological Applications; People’s Medical Publishing House: Beijing, China, 2017; pp. 150–200. [Google Scholar]
- Alexandrov, B.; Gelev, V.; Bishop, A.; Usheva, A.; Rasmussen, K. DNA Breathing Dynamics in the Presence of a Terahertz Field. Phys. Lett. A 2010, 374, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.; Paik, J.; Choi, M.; Yang, H.; Son, J. Detection and manipulation of methylation in blood cancer DNA using terahertz radiation. Sci. Rep. 2019, 9, 6413. [Google Scholar] [CrossRef]
- Cheon, H.; Yang, H.J.; Choi, M.; Son, J.H. Effective demethylation of melanoma cells using terahertz radiation. Biomed. Opt. Express 2019, 10, 4931–4941. [Google Scholar] [CrossRef]
- Ye, Q.; Yang, C. Recent progress in THz sources based on photonics methods. Chin. Opt. 2012, 5, 1–11. [Google Scholar] [CrossRef]
- Vicario, C.; Jazbinsek, M.; Ovchinnikov, A.; Chefonov, O.; Ashitkov, S.; Agranat, M.; Hauri, C. High efficiency THz generation in DSTMS, DAST and OH1 pumped by Cr: Forsterite laser. Opt. Express 2015, 23, 4573–4580. [Google Scholar] [CrossRef]
- Kim, K.; Taylor, A.; Glownia, J.; Rodriguez, G. Coherent control of terahertz supercontinuum generation in ultrafast laser–gas interactions. Nat. Photonics 2008, 2, 605–609. [Google Scholar] [CrossRef]
- Ma, S.; Gong, S.; Zhang, W.; Lu, C.; Li, X.; Li, Y. Neuronal growth and development promoted by low-intensity roadband terahertz radiation. Acta Phys. Sin. 2022, 71, 208701. [Google Scholar] [CrossRef]
- Tsurkan, M.; Smolyanskaya, O.; Bespalov, V.; Penniyainen, V.; Kipenko, A.; Lopatina, E.; Krylov, B. Changing Growth of Neurites of Sensory Ganglion by Terahertz Radiation. Proc. SPIE 2012, 8261, 82610. [Google Scholar]
- Sulatsky, M.; Duka, M.; Smolyanskaya, O. Stimulation of neurite growth under broadband pulsed THz radiation. Phys. Wave Phenom. 2014, 22, 197–201. [Google Scholar] [CrossRef]
- Tan, P.; Huang, J.; Liu, K.; Xiong, Y.; Fan, M. Terahertz radiation sources based on free electron lasers and their applications. Sci. China-Inf. Sci. 2011, 55, 1–15. [Google Scholar] [CrossRef]
- Votintsev, A.; Borisov, A.; Makashev, D.; Stoyanova, M.; Kistenev, Y. Widely tunable compact terahertz gas lasers. Science 2019, 366, 856–860. [Google Scholar] [CrossRef]
- Köhler, R.; Tredicucci, A.; Beltram, F.; Beere, H.; Linfield, E.; Davies, A.; Ritchie, D.; Iotti, R.; Rossi, F. Terahertz semiconductor-heterostructure laser. Nature 2002, 417, 156–159. [Google Scholar] [CrossRef]
- Xi, H.; Wang, P.; Bao, C.; Liu, Y. The Research on Backward Wave Oscillator with Wide Tunable Bandwidth and High Power. Infocommun. Radio Technol. 2022, 5, 101–107. [Google Scholar] [CrossRef]
- Castellano, F.; Li, L.; Linfield, E.; Davies, A.; Vitiello, M. Frequency and amplitude modulation of ultra-compact terahertz quantum cascade lasers using an integrated avalanche diode oscillator. Sci. Rep. 2016, 6, 23053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, M.; Zhao, J.; Chen, X.; Liu, L.; Lu, X.; Tian, T.; Chen, M.; Wang, P. Effect of 0.1 THz Radiation on Excitability of Hippocampal Neurons in Sprague Dawley Rats. Chin. J. Lasers 2020, 47, 295–301. [Google Scholar] [CrossRef]
- Qi, M.; Liu, R.; Li, B.; Wang, S.; Fan, R.; Zhao, X.; Xu, D. Behavioral Effect of Terahertz Waves in C57BL/6 Mice. Biosensors 2022, 12, 79. [Google Scholar] [CrossRef]
- Tan, S.; Tan, P.; Luo, L.; Chi, Y.; Yang, Z.; Zhao, X.; Zhao, L.; Dong, J.; Zhang, J.; Yao, B.; et al. Exposure Effects of Terahertz Waves on Primary Neurons and Neuron-like Cells Under Nonthermal Conditions. Biomed. Environ. Sci. 2019, 32, 739–754. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, M.; Liu, Y.; Liu, H.; Ren, K.; Xue, Q.; Zhang, H.; Zhi, N.; Wang, W.; Wu, S. Terahertz exposure enhances neuronal synaptic transmission and oligodendrocyte differentiation in vitro. iScience 2021, 24, 103485. [Google Scholar] [CrossRef]
- Deghoyan, A.; Heqimyan, A.; Nikoghosyan, A.; Dadasyan, E.; Ayrapetyan, S. Cell bathing medium as a target for non thermal effect of millimeter waves. Electromagn. Biol. Med. 2012, 31, 132–142. [Google Scholar] [CrossRef]
- Akiyama, H.; Kamiguchi, H. Second Messenger Networks for Accurate Growth Cone Guidance. Dev. Neurobiol. 2013, 75, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Goodhill, G.; Faville, R.; Sutherland, D.; Bicknell, B.; Thompson, A.; Pujic, Z.; Sun, B.; Kita, E.; Scott, E. The Dynamics of Growth Cone Morphology. BMC Biol. 2015, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Titushkin, I.; Rao, V.; Pickard, W.; Moros, E.; Shafirstein, G.; Cho, M. Altered Calcium Dynamics Mediates P19-Derived Neuron-Like Cell Responses to Millimeter-Wave Radiation. Radiat. Res. 2009, 172, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Samsonov, A.; Popov, S. The Effect of a 94 GHz Electromagnetic Field on Neuronal Microtubules. Bioelectromagnetics 2012, 34, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yi, R.; Liu, S.; Chi, Y.; Tan, S.; Dong, J.; Wang, H.; Zhang, J.; Wang, H.; Xu, X.; et al. Biological Responses to Terahertz Radiation with Different Power Density in Primary Hippocampal Neurons. PLoS ONE 2023, 18, e0267064. [Google Scholar] [CrossRef] [PubMed]
- Wilmink, G.; Rivest, B.; Roth, C.; Ibey, B.; Payne, J.; Cundin, L.; Grundt, J.; Peralta, X.; Mixon, D.; Roach, W. In vitro investigation of the biological effects associated with human dermal fibroblasts exposed to 2.52 THz radiation. Laser Surg. Med. 2011, 43, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Pennes, H. Analysis of Tissue and Arterial Blood Temperatures in the Resting Human Forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef]
- Ma, S.; Li, Z.; Gong, S.; Lu, C.; Li, X.; Li, Y. The laws and effects of terahertz wave interactions with neurons. Front. Bioeng. Biotechnol. 2023, 11, 1147684. [Google Scholar] [CrossRef]
- Duka, M.; Dvoretskaya, L.; Babelkin, N.; Khodzitskii, M.; Chivilikhin, S.; Smolyanskaya, O. Numerical and Experimental Studies of Mechanisms Underlying the Effect of Pulsed Broadband Terahertz Radiation on Nerve Cells. Quantum Electron. 2014, 44, 707–712. [Google Scholar] [CrossRef]
- Guo, L.; Wang, S.; Yang, L.; Wang, K.; Ma, J.; Zhou, J.; Gong, Y. Weak resonance effects of THz wave transimission in nerve cell. Acta Phys. Sin. 2021, 70, 340301. [Google Scholar] [CrossRef]
- Liu, W.; Lu, Y.; She, R.; Wei, G.; Jiao, G.; Lv, J.; Li, G. Thermal Analysis of Cornea Heated with Terahertz Radiation. Appl. Sci. 2019, 9, 917. [Google Scholar] [CrossRef]
- Generalov, V.; Safatov, A.; Kruchinina, M.; Gromov, A.; Buryak, G.; Generalov, K.; Kruchinin, V. Dielectric properties of the human red blood cell Izmer. Meas. Tech. 2020, 63, 580–586. [Google Scholar] [CrossRef]
- Winkle, C.C.; Gupton, S.L. Membrane Trafficking in Neuronal Development: Ins and Outs of Neural Connectivity. Int. Rev. Cell Mol. Biol. 2016, 322, 247–280. [Google Scholar] [CrossRef]
- Ramachandran, K.V.; Fu, J.B.; Schaffer, T.; Na, C.H.; Delannoy, M.; Margolis, S.A. Activity-Dependent Degradation of the Nascentome by the Neuronal Membrane Proteasome. Mol. Cell 2018, 71, 169–177.e6. [Google Scholar] [CrossRef] [PubMed]
- Lemire, S.; Jeromin, A.; Boisselier, É. Membrane Binding of Neuronal Calcium Sensor-1 (NCS1). Colloids Surf. B Biointerfaces 2016, 139, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Karasmanis, E.P.; Phan, C.-T.; Angelis, D.; Kesisova, I.A.; Hoogenraad, C.C.; McKenney, R.J.; Spiliotis, E.T. Polarity of Neuronal Membrane Traffic Requires Sorting of Kinesin Motor Cargo during Entry into Dendrites by a Microtubule-Associated Septin. Dev. Cell 2018, 46, 204–218.e7. [Google Scholar] [CrossRef] [PubMed]
- Zapara, T.A.; Treskova, S.I.; Ratushniak, A.S. Effect of Antioxidants on the Interaction of Terahertz (Submillimeter) Laser Radiation and Neuronal Membrane. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2015, 9, 869–871. [Google Scholar] [CrossRef]
- Paulsen, B.; Velasco, S.; Kedaigle, A.; Pigoni, M.; Quadrato, G.; Deo, A.; Adiconis, X.; Uzquiano, A.; Sartore, R.; Yang, S.; et al. Autism Genes Converge on Asynchronous Development of Shared Neuron Classes. Nature 2022, 602, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Bo, W.; Wang, K.; Wang, S.; Gong, Y. Theoretical Investigation on the Effect of Terahertz Wave on Ca2+ Transport in the Calcium Channel. iScience 2021, 25, 103561. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Titushkin, I.; Varner, J.; Cho, M. Millimeter Wave-Induced Modulation of Calcium Dynamics in an Engineered Skin Co-culture Model: Role of Secreted ATP on Calcium Spiking. J. Radiat. Res. 2012, 53, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Li, Z.; Gong, S.; Lu, C.; Li, X.; Li, Y. High Frequency Electromagnetic Radiation Stimulates Neuronal Growth and Hippocampal Synaptic Transmission. Brain Sci. 2023, 13, 686. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhong, Y.; Li, R.; Chang, C. Neuromodulation of Chemical Synaptic Transmission Driven by THz. Research 2022, 2022, 0010. [Google Scholar] [CrossRef]
- Kirichuk, V.; Antipova, O.; Krylova, Y. Effect of Continuous Irradiation with Terahertz Electromagnetic Waves of the NO Frequency Range on Behavioral Reactions of Male Albino Rats under Stress Conditions. Bull. Exp. Biol. Med. 2014, 157, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Bondar, N.; Kovalenko, I.; Avgustinovich, D.; Khamoyan, A.; Kudryavtseva, N. Behavioral Effect of Terahertz Waves in Male Mice. Bull. Exp. Biol. Med. 2008, 145, 401–405. [Google Scholar] [CrossRef]
- Kirichuk, V.; Efimova, N.; Evgeny, A. Effect of High Power Terahertz Irradiation on Platelet Aggregation and Behavioral Reactions of Albino Rats. Bull. Exp. Biol. Med. 2009, 148, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Kirichuk, V.; Ivanov, A. Regulatory Effects of Terahertz Waves. Russ. Open Med. J. 2013, 2, 0402. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Pei, G.; Wang, Z.; Chen, N. Neurotrophic basis to the pathogenesis of depression and phytotherapy. Front. Pharmacol. 2022, 14, 1182666. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Ma, R.; Wen, J.; Zhai, Y.; Wang, Y.; Wang, F.; Liu, D.; Zhao, X.; Sun, B.; Jia, P.; et al. Pathogenesis, Animal Models, and Drug Discovery of Alzheimer’s Disease. J. Alzheimers Dis. 2023, 94, 1265–1301. [Google Scholar] [CrossRef]
- Stübling, E.; Rehn, A.; Siebrecht, T.; Bauckhage, Y.; Öhrström, L.; Eppenberger, P.; Balzer, J.; Rühli, F.; Koch, M. Application of a robotic THz imaging system for sub-surface analysis of ancient human remains. Sci. Rep. 2019, 9, 3390. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Guan, L.; Liu, L.; Liu, R.; Cai, N.; Wang, S. Design and energy research of terahertz optical systems for cell stimulation. Microw. Opt. Technol. Lett. 2022, 65, 981–987. [Google Scholar] [CrossRef]

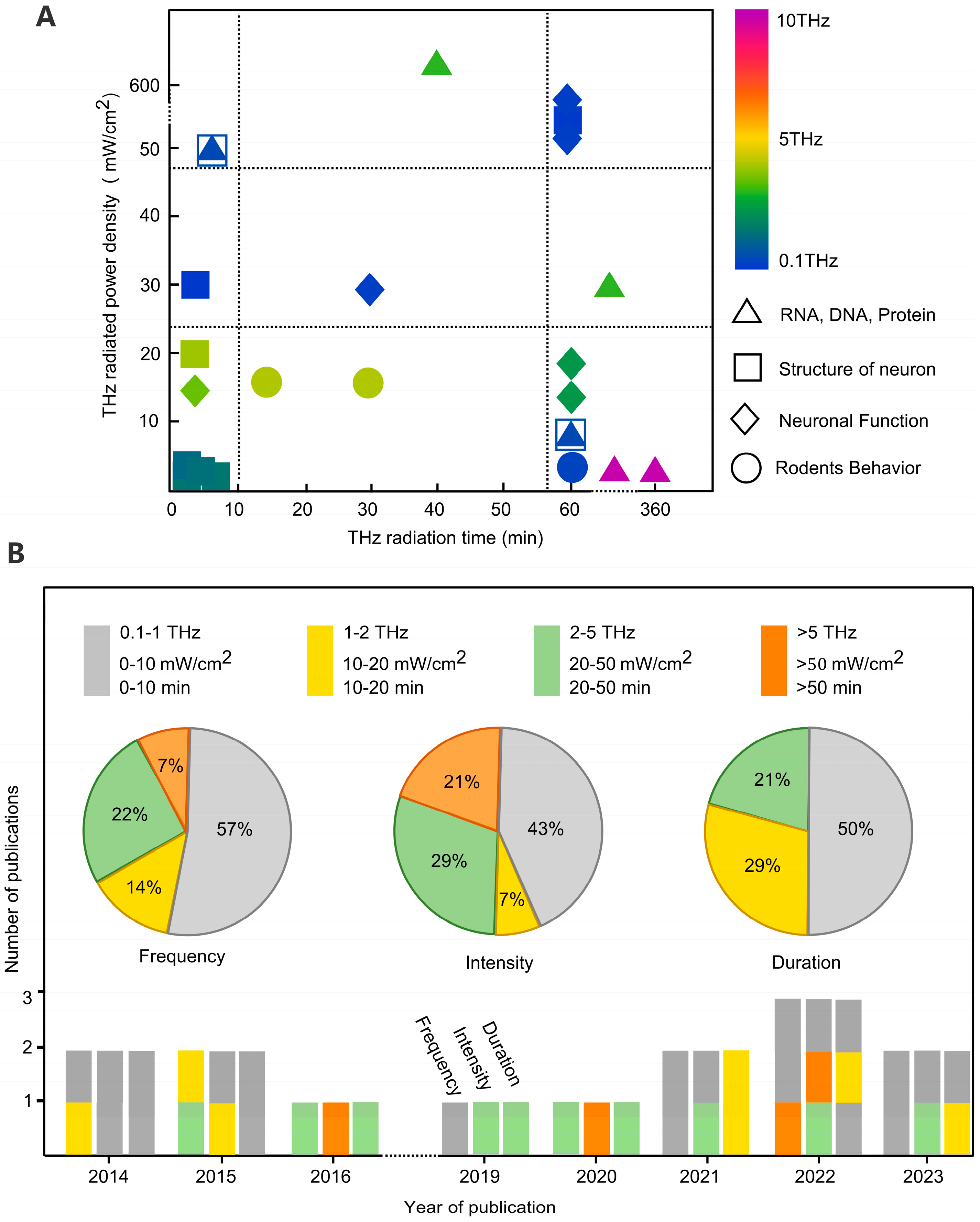
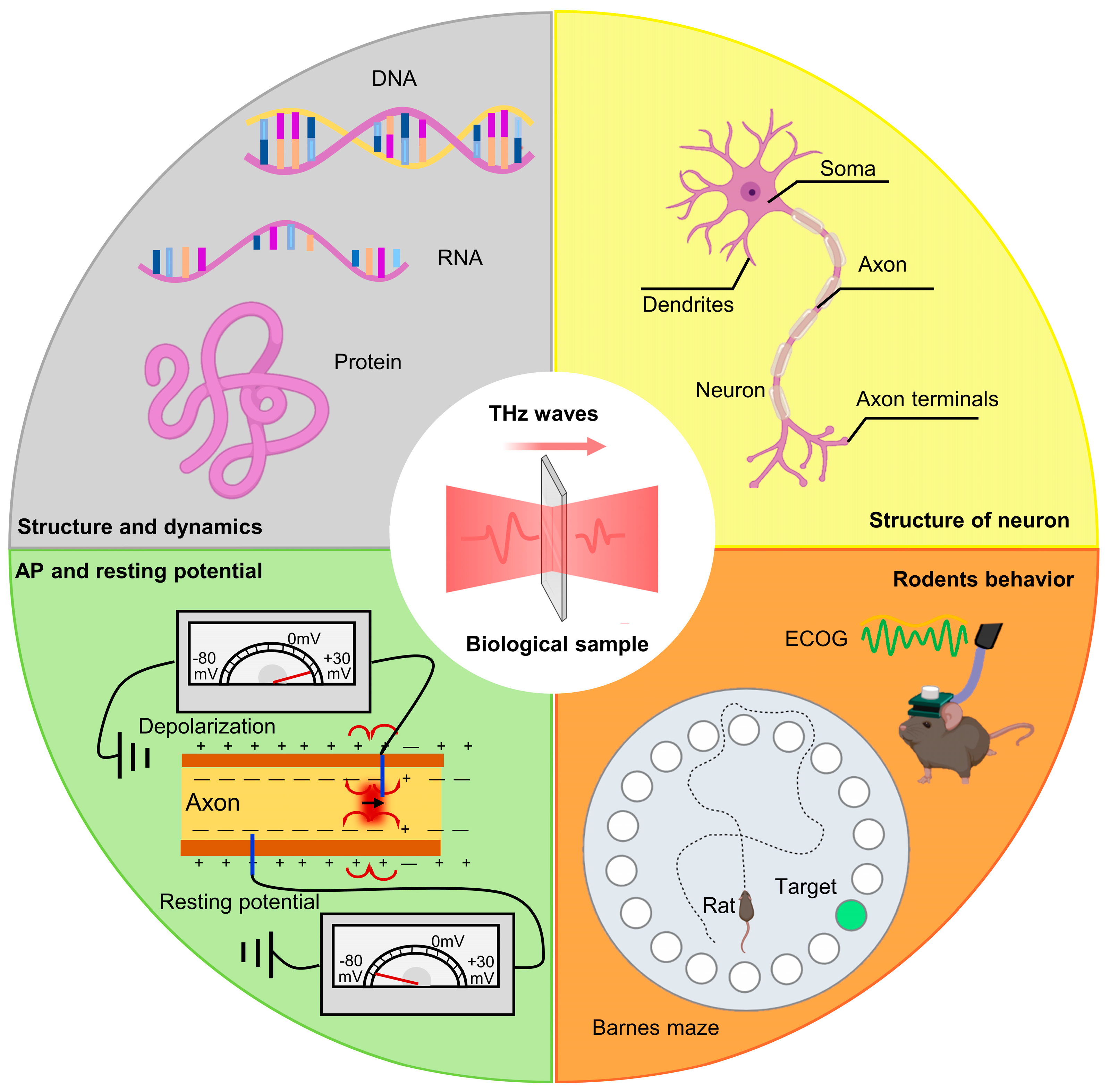
| Cell In Vitro Study | Frequency | Intensity | Exposure Time | Effect | Reference |
|---|---|---|---|---|---|
| Hippocampal neurons Cortical neurons | 0.16 THz; 0.17 THz | 10 mW; 50 mW | 6 min; 60 min | Will not adversely affect the development of neurons. | [66] |
| C57 mouse cortical neurons | not specified | 70 μW/cm2 | 15 min; 3 h | Facilitate the formation of neuronal synapses. | [67] |
| Mouse embryonic cancer cells | 0.094 THz | 3.1 kW/cm2; 7.8 kW/cm2; 18.6 kW/cm2 | 60 min | The structure of neuronal actin protein is compromised. | [71] |
| Xenopus laevis embryonic neurons | 0.094 THz | 310 mW/cm2 | 3 min | Enhancing the growth rate of neurons. | [72] |
| Chicken spinal cord neurons at 10–12 days | 0.05–1.2 THz | 0.23–11.6 μW/m2 | 3 min | Facilitating or inhibiting the growth of neuronal processes. | [58] |
| Chicken embryonic spinal cord neurons | 0.1–2 THz | 1.1 μW/cm2 | 3–5 min | Promoting the growth and development of neurons. | [77] |
| Adult rats | 0.09–0.16 THz | not specified | 1–20 min | Neuronal dehydration atrophy. | [68] |
| Neurons | 3.67 THz | 15–20 mW/cm2 | 60 min | Impeding the growth of neurons. | [43] |
| Chicken embryo sensory neurons | 0.05–2 THz | 0.5–50 μW/m2 | 3 min | Neuronal growth is associated with radiation power. | [57] |
| Cell In Vitro Study | Frequency | Intensity | Exposure Time | Effect | Reference |
|---|---|---|---|---|---|
| C57 mouse cortical Neurons | not specified | 70 μW/cm2 | 15 min; 3 h | Enhancing neuronal synaptic transmission and excitability. | [67] |
| Mouse embryonic cells | 0.094 THz | 3.1 kW/cm2; 7.8 kW/cm2; 18.6 kW/cm2 | 60 min | Increasing the number of Ca2+ peaks in neurons. | [71] |
| Neurons | 3.67 THz | 15–20 mW/cm2 | 60 min | Depolarization of neuronal membrane potential. | [43] |
| Mouse embryonic cells | 0.094 THz | 30 mW; 60 mW | 30 min; 60 min | Modulating the concentration of Ca2+ within neurons. | [88] |
| SD rat hippocampal neurons | 0.1 THz | 2.65 mW/cm2 | 15 min; 25 min | Augmenting the concentrations of Ca2+ and Na+ within neurons. | [64] |
| Mouse pyramidal neurons | 35–45 THz | 10 mW; 30 mW | 10–100 s | Augmenting the excitability of neurons. | [90] |
| Hippocampal CA1 | 0.138 THz | 2 mW | 60 min | Increased synaptic transmission efficiency. | [89] |
| Male albino rats | 0.15 THz | 0.2 mW/cm2 | 30 min; 60 min | Increased time for rats to pass through the maze, depression. | [91] |
| Male mice | 3.6 THz | 15 mW | 15 min; 30 min | Mice showed a significant increase in anxiety levels. | [92] |
| Male albino rats | 0.15 THz | 3 mW/cm2 | 60 min | Induce signs of depression. | [93] |
| Male rats | 0.167 THz; 0.144 THz | not specified | 5 days | Maintain normal ability to explore new things; Increased anxiety, reduced appetite and sleep time. | [94] |
| Eight-week-old female C57BL/6 mice | 0.14 THz | not specified | 20 min | Enhanced anxiolytic, antidepressant, and social interaction. | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Ding, P.; Zhou, Z.; Jin, H.; Li, X.; Li, Y. Terahertz Radiation Modulates Neuronal Morphology and Dynamics Properties. Brain Sci. 2024, 14, 279. https://doi.org/10.3390/brainsci14030279
Ma S, Ding P, Zhou Z, Jin H, Li X, Li Y. Terahertz Radiation Modulates Neuronal Morphology and Dynamics Properties. Brain Sciences. 2024; 14(3):279. https://doi.org/10.3390/brainsci14030279
Chicago/Turabian StyleMa, Shaoqing, Peng Ding, Zhengxuan Zhou, Huilong Jin, Xiaoli Li, and Yingwei Li. 2024. "Terahertz Radiation Modulates Neuronal Morphology and Dynamics Properties" Brain Sciences 14, no. 3: 279. https://doi.org/10.3390/brainsci14030279
APA StyleMa, S., Ding, P., Zhou, Z., Jin, H., Li, X., & Li, Y. (2024). Terahertz Radiation Modulates Neuronal Morphology and Dynamics Properties. Brain Sciences, 14(3), 279. https://doi.org/10.3390/brainsci14030279








