Cerebrospinal Fluid Dynamics and Partial Pressure of Carbon Dioxide as Prognostic Indicators in Hypoxic–Ischemic Brain Injury Following Cardiac Arrest
Abstract
1. Introduction
2. Materials and Methods
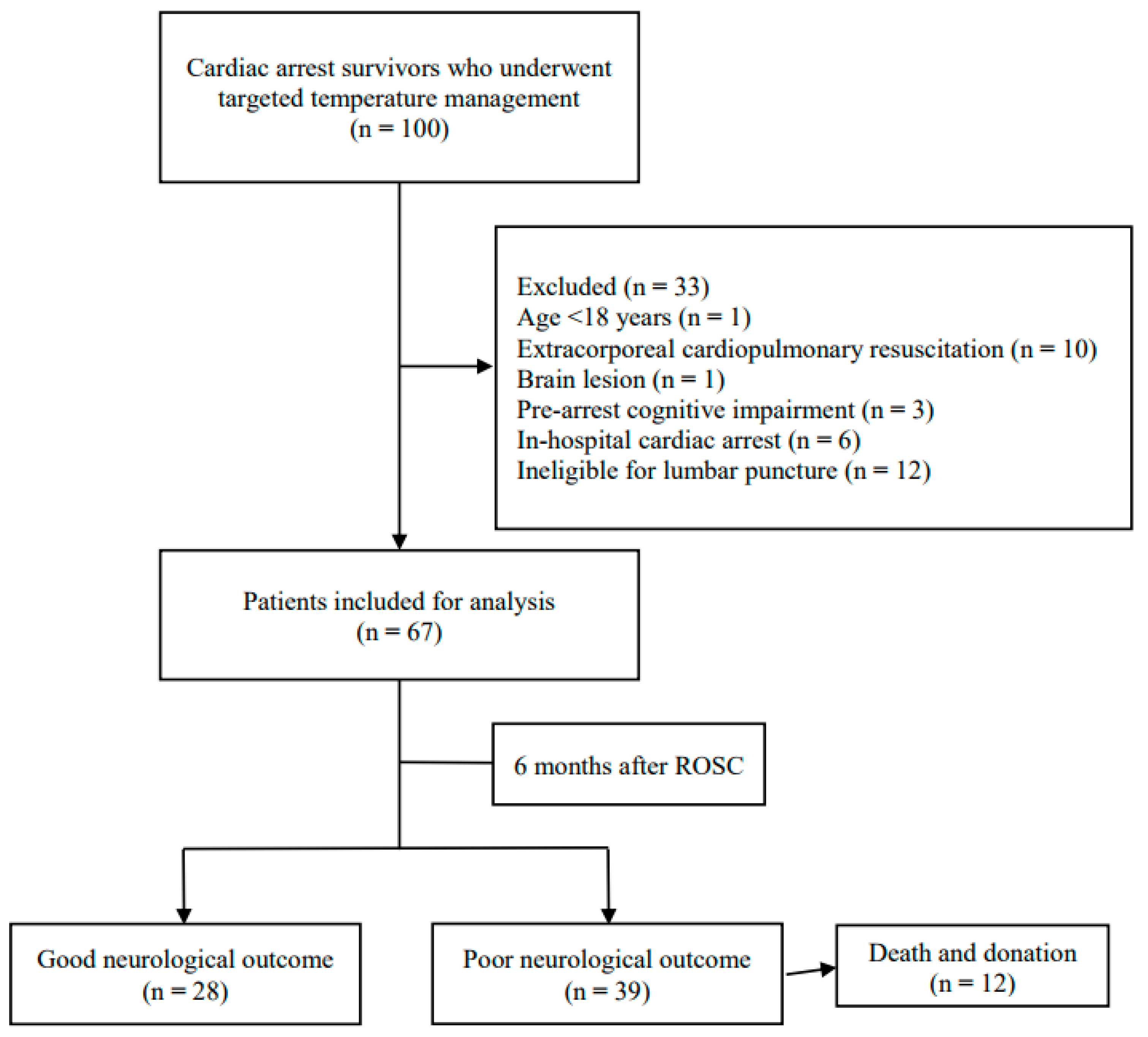
2.1. Study Design and Patients
2.2. TTM Protocol
2.3. CSF and Arterial Blood Gas Analyses
2.4. Data Collection
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Comparison of CSF and Arterial Blood Gas Analyses between Groups
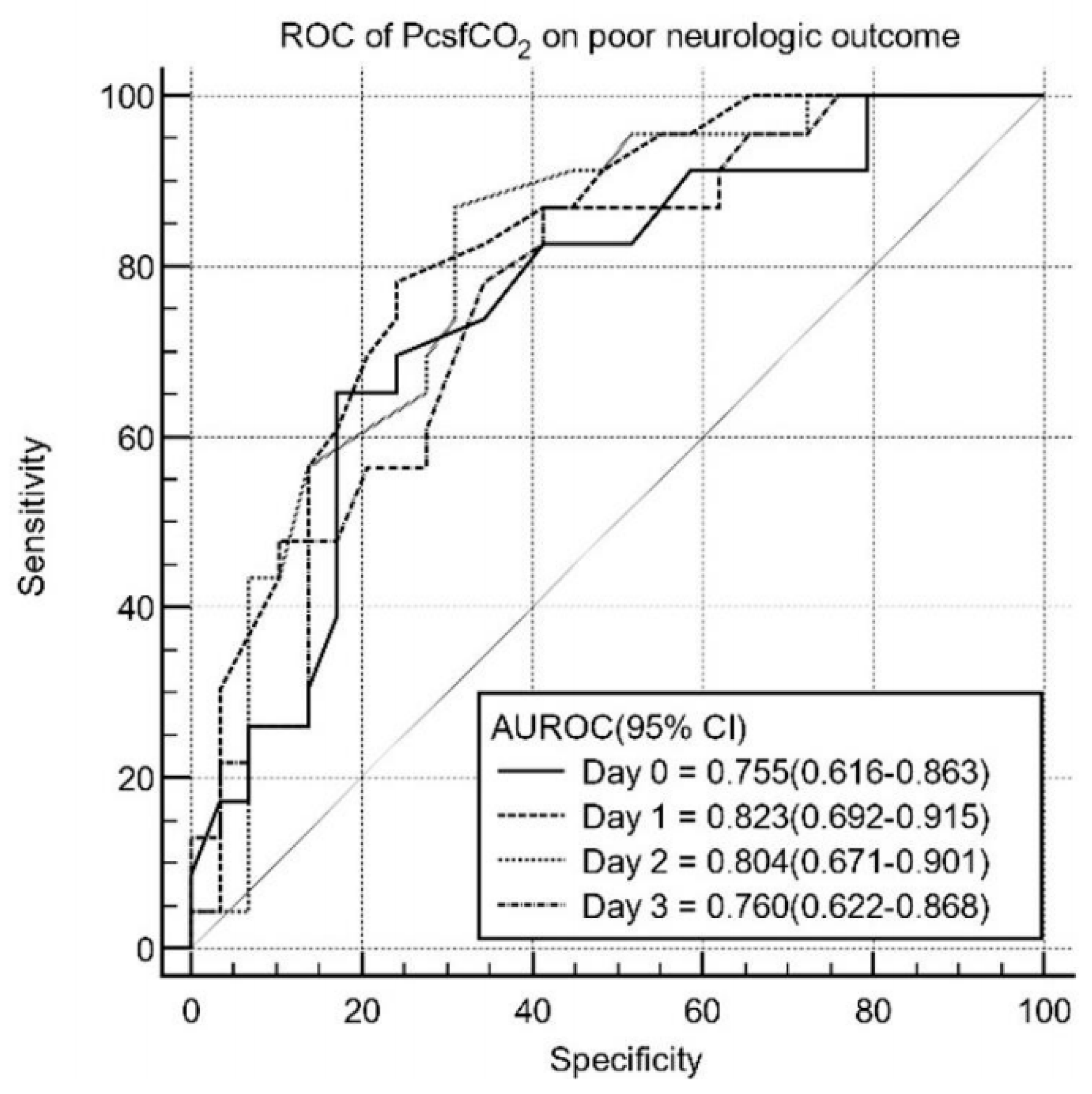
3.3. Prognostic Performance of PcsfCO2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johanson, C.E.; Duncan, J.A.; Klinge, P.M.; Brinker, T.; Stopa, E.G.; Silverberg, G.D. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008, 5, 10. [Google Scholar] [CrossRef]
- Johanson, C.E. Choroid Plexus–Cerebrospinal Fluid Circulatory Dynamics: Impact on Brain Growth, Metabolism, and Repair. In Neuroscience in Medicine; Conn, P.M., Ed.; Humana Press: Totowa, NJ, USA, 2008; pp. 173–200. ISBN 978-1-60327-455-5. [Google Scholar]
- Hladky, S.B.; Barrand, M.A. Fluid and ion transfer across the blood–brain and blood–cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS 2016, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.H.; Thomas, J.H. Cerebrospinal Fluid Flow. Annu. Rev. Fluid Mech. 2023, 55, 237–264. [Google Scholar] [CrossRef]
- Redzic, Z.B.; Preston, J.E.; Duncan, J.A.; Chodobski, A.; Szmydynger-Chodobska, J. The choroid plexus-cerebrospinal fluid system: From development to aging. Curr. Top. Dev. Biol. 2005, 71, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, G.D.; Heit, G.; Huhn, S.; Jaffe, R.A.; Chang, S.D.; Bronte-Stewart, H.; Rubenstein, E.; Possin, K.; Saul, T.A. The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurology 2001, 57, 1763–1766. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D.; Yao, Y.; Rokach, J.; Mayo, M.; Silverberg, G.G.; McGuire, D. Reduction of brain lipid peroxidation by CSF drainage in Alzheimer’s disease patients. J. Alzheimers Dis. JAD 2004, 6, 385–389. [Google Scholar] [CrossRef]
- Garton, M.J.; Keir, G.; Lakshmi, M.V.; Thompson, E.J. Age-related changes in cerebrospinal fluid protein concentrations. J. Neurol. Sci. 1991, 104, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H. Flow rate of cerebrospinal fluid (CSF)—A concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J. Neurol. Sci. 1994, 122, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J. Alzheimer’s disease is a result of loss of full brain buoyancy. Med. Hypotheses 2022, 164, 110857. [Google Scholar] [CrossRef]
- Bothwell, S.W.; Janigro, D.; Patabendige, A. Cerebrospinal fluid dynamics and intracranial pressure elevation in neurological diseases. Fluids Barriers CNS 2019, 16, 9. [Google Scholar] [CrossRef]
- Simon, M.J.; Iliff, J.J. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim. Biophys. Acta 2016, 1862, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Huang, X.; Huang, X.; Mai, H.; Li, J.; Jiang, T.; Wang, X.; Lü, T. Aquaporin-4 and Alzheimer’s Disease. J. Alzheimers Dis. JAD 2016, 52, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Sur, S.; Liu, P.; Li, Y.; Jiang, D.; Hou, X.; Darrow, J.; Pillai, J.J.; Yasar, S.; Rosenberg, P.; et al. Blood–Brain Barrier Breakdown in Relationship to Alzheimer and Vascular Disease. Ann. Neurol. 2021, 90, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, E. Relationship of senescence of cerebrospinal fluid circulatory system to dementias of the aged. Lancet Lond. Engl. 1998, 351, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; You, Y.; Ahn, H.J.; Min, J.H.; Jeong, W.; Yoo, I.; Cho, Y.; Ryu, S.; Lee, J.; Kim, S.; et al. Cerebrospinal fluid lactate dehydrogenase as a potential predictor of neurologic outcomes in cardiac arrest survivors who underwent target temperature management. J. Crit. Care 2020, 57, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Jung, S.; You, Y.; Park, J.S.; Min, J.H.; Jeong, W.; Kang, C.; Yoo, I.; Cho, Y.; Ryu, S.; et al. The value of cerebrospinal fluid ubiquitin C-terminal hydrolase-L1 protein as a prognostic predictor of neurologic outcome in post-cardiac arrest patients treated with targeted temperature management. Resuscitation 2020, 151, 50–58. [Google Scholar] [CrossRef]
- Park, J.S.; You, Y.; Min, J.H.; Yoo, I.; Jeong, W.; Cho, Y.; Ryu, S.; Lee, J.; Kim, S.W.; Cho, S.U.; et al. Study on the timing of severe blood-brain barrier disruption using cerebrospinal fluid-serum albumin quotient in post cardiac arrest patients treated with targeted temperature management. Resuscitation 2019, 135, 118–123. [Google Scholar] [CrossRef]
- Paul, M.; Benghanem, S.; Merceron, S.; Bellut, H.; Dumas, F.; Henry, A.; Bruneel, F.; Bedos, J.-P.; Cariou, A.; Legriel, S. Cerebrospinal fluid features in comatose survivors of cardiac arrest: A pilot study. PLoS ONE 2022, 17, e0270954. [Google Scholar] [CrossRef]
- Trillo-Contreras, J.L.; Ramírez-Lorca, R.; Hiraldo-González, L.; Sánchez-Gomar, I.; Galán-Cobo, A.; Suárez-Luna, N.; Sánchez de Rojas-de Pedro, E.; Toledo-Aral, J.J.; Villadiego, J.; Echevarría, M. Combined effects of aquaporin-4 and hypoxia produce age-related hydrocephalus. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2018, 1864, 3515–3526. [Google Scholar] [CrossRef]
- Michenkova, M.; Taki, S.; Blosser, M.C.; Hwang, H.J.; Kowatz, T.; Moss, F.J.; Occhipinti, R.; Qin, X.; Sen, S.; Shinn, E.; et al. Carbon dioxide transport across membranes. Interface Focus 2021, 11, 20200090. [Google Scholar] [CrossRef]
- Gutknecht, J.; Bisson, M.A.; Tosteson, F.C. Diffusion of carbon dioxide through lipid bilayer membranes: Effects of carbonic anhydrase, bicarbonate, and unstirred layers. J. Gen. Physiol. 1977, 69, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Endeward, V.; Al-Samir, S.; Itel, F.; Gros, G. How does carbon dioxide permeate cell membranes? A discussion of concepts, results and methods. Front. Physiol. 2014, 4, 72383. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Park, J.; Min, J.; Yoo, I.; Jeong, W.; Cho, Y.; Ryu, S.; Lee, J.; Kim, S.; Cho, S.; et al. Relationship between time related serum albumin concentration, optic nerve sheath diameter, cerebrospinal fluid pressure, and neurological prognosis in cardiac arrest survivors. Resuscitation 2018, 131, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Roales-Buján, R.; Páez, P.; Guerra, M.; Rodríguez, S.; Vío, K.; Ho-Plagaro, A.; García-Bonilla, M.; Rodríguez-Pérez, L.-M.; Domínguez-Pinos, M.-D.; Rodríguez, E.-M.; et al. Astrocytes acquire morphological and functional characteristics of ependymal cells following disruption of ependyma in hydrocephalus. Acta Neuropathol. 2012, 124, 531–546. [Google Scholar] [CrossRef]
- Akaishi, T.; Onishi, E.; Abe, M.; Toyama, H.; Ishizawa, K.; Kumagai, M.; Kubo, R.; Nakashima, I.; Aoki, M.; Yamauchi, M.; et al. The human central nervous system discharges carbon dioxide and lactic acid into the cerebrospinal fluid. Fluids Barriers CNS 2019, 16, 8. [Google Scholar] [CrossRef]
- Son, S.H.; In, Y.N.; Park, J.S.; You, Y.; Min, J.H.; Yoo, I.; Cho, Y.C.; Jeong, W.; Ahn, H.J.; Lee, B.K. Cerebrospinal Fluid Lactate Levels, Brain Lactate Metabolism and Neurologic Outcome in Patients with Out-of-Hospital Cardiac Arrest. Neurocrit. Care 2021, 35, 262–270. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Cohort (N = 67) | Good Outcome (n = 28) | Poor Outcome (n = 39) | p-Value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, years (IQR) | 60.0 (41.0–70.0) | 58.5 (43.5–69.6) | 56.8 (38.0–72.0) | 0.652 |
| Sex, male, n (%) | 49 (73.1) | 23 (82.1) | 26 (66.7) | 0.177 |
| Cardiac arrest characteristics | ||||
| Witnessed arrest, n (%) | 38 (56.7) | 23 (82.1) | 15 (39.5) | <0.001 * |
| Bystander CPR, n (%) | 47 (70.1) | 23 (82.1) | 24 (61.5) | 0.104 |
| Shockable rhythm, n (%) | 19 (28.4) | 16 (57.1) | 3 (7.7) | <0.001 * |
| Cardiac etiology, n (%) | 27 (40.3) | 18 (64.3) | 9 (23.1) | <0.001 * |
| No flow time, min, median (IQR) | 1.0 (0.0–13.5) | 1.0 (0.0–1.3) | 9.5 (0.0–25.5) | 0.002 * |
| Low flow time, min, median (IQR) | 20.0 (9.0–28.3) | 10.0 (8.0–19.0) | 26.0 (19.0–42.0) | <0.001 * |
| ROSC to LP time, h(IQR) | 4.5(3.2–6.0) | 4.1(3.0–5.9) | 4.7(4.0–6.0) | 0.150 |
| Origin of Sample | Cohort (N = 67) | Good Outcome (n = 28) | Poor Outcome (n = 39) | p-Value |
|---|---|---|---|---|
| CSF pH median (IQR) | ||||
| Day 0 | 7.43 (7.39–7.49) | 7.45 (7.40–7.52) | 7.41 (7.385–7.48) | 0.207 |
| Day 1 | 7.47 (7.41–7.69) | 7.49 (7.45–7.92) | 7.46 (7.39–7.64) | 0.251 |
| Day 2 | 7.45 (7.38–7.91) | 7.62 (7.42–8.05) | 7.43 (7.37–7.68) | 0.130 |
| Day 3 | 7.53 (7.41–7.88) | 7.60 (7.42–7.80) | 7.47 (7.41–7.82) | 0.654 |
| CSF PcsfO2 median (IQR) | ||||
| Day 0 | 156.0 (116.0–190.0) | 153.5 (127.8–165.0) | 148.0 (131.0–158.0) | 0.703 |
| Day 1 | 138.0 (120.0–159.0) | 151.0 (121.0–170.1) | 149.0 (122.5–170.3) | 0.940 |
| Day 2 | 123.5 (106.5–136.3) | 132.0 (122.5–165.0) | 141.0 (125.0–163.0) | 0.713 |
| Day 3 | 125.0 (104.5–143.5) | 143.0 (122.4–170.0) | 151.0 (127.5–166.5) | 0.699 |
| CSF PcsfCO2 median (IQR) | ||||
| Day 0 | 37.4 (31.0–43.5) | 33.2 (26.8–38.0) | 27.0 (24.0–33.0) | 0.006 * |
| Day 1 | 38.0 (35.0–43.5) | 34.5 (30.0–37.2) | 26.5 (21.2–30.0) | <0.001 * |
| Day 2 | 42.0 (36.8–45.3) | 35.0 (31.9–39.2) | 28.0 (19.0–33.0) | <0.001 * |
| Day 3 | 42.0 (38.1–45.6) | 35.0 (32.0–39.3) | 27.0 (18.5–34.0) | <0.001 * |
| CSF HCO3 median (IQR) | ||||
| Day 0 | 20.4 (18.5–23.3) | 22.2 (20.0–23.7) | 19.4 (15.5–20.9) | 0.003 * |
| Day 1 | 22.0 (19.9–25.0) | 23.8 (21.4–27.4) | 21.2 (18.6–23.2) | 0.019 |
| Day 2 | 22.2 (20.2–34.3) | 24.3 (21.8–27.6) | 20.6 (18.2–22.5) | 0.001 * |
| Day 3 | 23.5 (21.5–26.3) | 24.6 (22.7–36.4) | 22.3 (18.5–26.4) | 0.117 |
| Origin of Sample | Cohort (N = 67) | Good Outcome (n = 28) | Poor Outcome (n = 39) | p-Value |
|---|---|---|---|---|
| Arterial pH median (IQR) | ||||
| Day 0 | 7.30 (7.25–7.40) | 7.33 (7.29–7.40) | 7.27 (7.20–7.37) | 0.067 |
| Day 1 | 7.35 (7.28–7.40) | 7.37 (7.30–7.41) | 7.32 (7.27–7.40) | 0.173 |
| Day 2 | 7.39 (7.35–7.42) | 7.41 (7.38–7.43) | 7.38 (7.33–7.40) | 0.013 * |
| Day 3 | 7.40 (7.35–7.44) | 7.41 (7.34–7.45) | 7.39 (7.35–7.44) | 0.535 |
| Arterial PO2 median (IQR) | ||||
| Day 0 | 156.0 (116.0–190.0) | 151.5 (123.8–193.0) | 157.0 (104.0–189.0) | 0.844 |
| Day 1 | 138.0 (120.0–159.0) | 139.0 (114.8–153.5) | 138.0 (122.0–159.0) | 0.760 |
| Day 2 | 123.5 (106.5–136.3) | 127.0 (113.5–138.3) | 115.0 (102.0–134.8) | 0.159 |
| Day 3 | 125.0 (104.5–143.5) | 123.0 (106.0–154.0) | 125.5 (104.0–135.5) | 0.766 |
| Arterial PCO2 median (IQR) | ||||
| Day 0 | 37.4 (31.0–43.5) | 36.5 (31.3–40.0) | 38.0 (31.0–48.0) | 0.277 |
| Day 1 | 38.0 (35.0–43.5) | 38.9 (35.0–44.0) | 38.0 (35.0–42.0) | 0.670 |
| Day 2 | 42.0 (36.8–45.3) | 42.3 (36.0–46.1) | 42.0 (37.8–45.3) | 0.907 |
| Day 3 | 42.0 (36.8–45.3) | 40.0 (38.0–45.3) | 42.0 (37.8–45.9) | 0.856 |
| Arterial HCO3 median (IQR) | ||||
| Day 0 | 19.8 (15.7–22.0) | 21.1 (17.6–22.8) | 18.6 (15.0–20.8) | 0.095 |
| Day 1 | 22.7 (19.6–24.4) | 23.7 (21.2–24.7) | 21.7 (17.9–24.4) | 0.028 * |
| Day 2 | 25.3 (22.8–26.7) | 25.7 (23.9–27.3) | 24.5 (22.0–26.0) | 0.037 * |
| Day 3 | 25.6 (23.9–27.7) | 26.3 (24.5–28.3) | 24.6 (23.6–26.6) | 0.055 |
| Sample | Time | AUC (95% CI) | p Value | Cutoff | Sensitivity (95% CI) | Specificity (95% CI) | PPV/NPV |
|---|---|---|---|---|---|---|---|
| PcsfCO2 | Day 0 | 0.755 (0.616–0.863) | 0.002 * | 31 (mg/dL) | 60.7 (40.6–78.5) | 74.4 (57.9–87.0) | 68.0/57.8 |
| Day 1 | 0.823 (0.692–0.915) | <0.001 * | 30 (mg/dL) | 76.9 (56.4–91.0) | 78.9 (62.7–90.4) | 82.2/71.3 | |
| Day 2 | 0.804 (0.671–0.901) | <0.001 * | 30 (mg/dL) | 88.0 (68.8–97.5) | 67.7 (47.8–80.9) | 91.0/80.4 | |
| Day 3 | 0.760 (0.622–0.806) | <0.001 * | 29 (mg/dL) | 87.0 (66.4–97.2) | 58.6 (38.9–76.5) | 90.2/76.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.Y.; Ahn, H.J.; Kang, C.; You, Y.; Park, J.S.; Min, J.H.; Jeong, W.; In, Y.N. Cerebrospinal Fluid Dynamics and Partial Pressure of Carbon Dioxide as Prognostic Indicators in Hypoxic–Ischemic Brain Injury Following Cardiac Arrest. Brain Sci. 2024, 14, 297. https://doi.org/10.3390/brainsci14030297
Jeon SY, Ahn HJ, Kang C, You Y, Park JS, Min JH, Jeong W, In YN. Cerebrospinal Fluid Dynamics and Partial Pressure of Carbon Dioxide as Prognostic Indicators in Hypoxic–Ischemic Brain Injury Following Cardiac Arrest. Brain Sciences. 2024; 14(3):297. https://doi.org/10.3390/brainsci14030297
Chicago/Turabian StyleJeon, So Young, Hong Joon Ahn, Changshin Kang, Yeonho You, Jung Soo Park, Jin Hong Min, Wonjoon Jeong, and Yong Nam In. 2024. "Cerebrospinal Fluid Dynamics and Partial Pressure of Carbon Dioxide as Prognostic Indicators in Hypoxic–Ischemic Brain Injury Following Cardiac Arrest" Brain Sciences 14, no. 3: 297. https://doi.org/10.3390/brainsci14030297
APA StyleJeon, S. Y., Ahn, H. J., Kang, C., You, Y., Park, J. S., Min, J. H., Jeong, W., & In, Y. N. (2024). Cerebrospinal Fluid Dynamics and Partial Pressure of Carbon Dioxide as Prognostic Indicators in Hypoxic–Ischemic Brain Injury Following Cardiac Arrest. Brain Sciences, 14(3), 297. https://doi.org/10.3390/brainsci14030297






