Abstract
Migraine, recognized as a severe headache disorder, is widely prevalent, significantly impacting the quality of life for those affected. This article aims to provide a comprehensive review of the application of animal model technologies in unraveling the pathomechanism of migraine and developing more effective therapies. It introduces a variety of animal experimental models used in migraine research, emphasizing their versatility and importance in simulating various aspects of the condition. It details the benefits arising from the utilization of these models, emphasizing their role in elucidating pain mechanisms, clarifying trigeminal activation, as well as replicating migraine symptoms and histological changes. In addition, the article consciously acknowledges the inherent limitations and challenges associated with the application of animal experimental models. Recognizing these constraints is a fundamental step toward fine-tuning and optimizing the models for a more accurate reflection of and translatability to the human environment. Overall, a detailed and comprehensive understanding of migraine animal models is crucial for navigating the complexity of the disease. These findings not only provide a deeper insight into the multifaceted nature of migraine but also serve as a foundation for developing effective therapeutic strategies that specifically address the unique challenges arising from migraine pathology.
1. Introduction
Migraine, as a neurological disorder, affects millions of people worldwide, imposing a significant burden on individuals and society alike. The condition is intricately linked to trigeminal activation, a key component of the pain-sensing system. Animal experimental models play a critical role in providing insights into the functioning of migraine and the trigeminal system. Through these models, various aspects of the disease’s pathomechanism can be examined, contributing to more precise diagnostics and the development of more effective therapies. This review offers an overview of the fundamental characteristics of migraine and trigeminal activation, the crucial role of animal experimental models, and their significance in advancing clinical treatments and therapeutic strategies for the condition.
2. The Multifaceted World of Migraine: Characteristics, Pathomechanisms, Associated Disorders, and Therapeutic Challenges
Migraine is a primary, episodic headache disorder distinguished by diverse manifestations of neurological, gastrointestinal, and autonomic alterations [1]. The ongoing Global Burden of Diseases, Injuries, and Risk Factors Study consistently recognizes migraine as a prominent contributor to global disability, especially among individuals under the age of 50 [2,3]. Moreover, conditions frequently occurring alongside migraine, such as neck pain, depression, and anxiety, rank among the top ten causes of global disability [4,5].
Migraine attacks are often preceded by warning signs (such as fatigue, euphoria, irritability, food cravings, or constipation) [6,7] and the phenomenon of aura [8], which is believed to originate in the hypothalamus, brainstem, and cortical areas [9,10]. The attacks are accompanied by severe, throbbing headaches typically affecting one side and intensifying with increased intracranial pressure, and their duration varies but generally lasts between 4–72 h. Migraine headaches often come with nausea and potential vomiting. Patients are frequently sensitive to light and sound during the attacks [10,11]. Furthermore, it may be accompanied by abnormal skin sensitivity (allodynia), muscle tenderness, and osmophobia [12]. Exacerbation of headache due to routine physical activity is an extremely sensitive characteristic of migraine headaches, occurring in over 95% of sufferers [13]. The symptoms observed from the prodromal stage to the headache phase of migraine indicate the dysfunction of several neuronal systems. According to The International Classification of Headache Disorders-3 beta (ICHD-3) criteria, the vestibular symptoms of migraine, such as vertigo and dizziness, are now identified with the diagnosis of migraine with brainstem aura, emphasizing that these symptoms likely reflect changes in neural activity in the brainstem and vestibular system rather than alterations in blood flow through the basilar artery [14].
Migraine is a complex headache condition that can be classified into different types. Episodic migraine (EM) implies that the patient experiences migraine attacks periodically, typically for up to 14 days a month. In contrast, chronic migraine (CM) involves more frequent headaches, occurring for more than 15 days a month [15]. EM can evolve into CM when the frequency of headaches gradually increases, leading to a persistent migrainous state [16]. Factors influencing progression include older age, female gender, Caucasian ethnicity, low educational level, low socioeconomic status, and genetics. Obese individuals and snorers are two to five times more likely to develop CM. Additional risk factors include head or neck injury, comorbid depression, stressful life events, asthma, and allergic rhinitis. Factors promoting remission include lower baseline headache frequency and the absence of cutaneous allodynia, especially thermal [17]. The regular and excessive use of migraine medications can result in medication-overuse headache (MOH). The ICHD-3 beta criteria conceptualize medication overuse as a behavior rather than being based on causality. MOH is characterized by a patient using triptans, ergot alkaloids, mixed analgesics, or opioids for ≥10 days per month; simple analgesics or nonsteroidal anti-inflammatory drugs (NSAIDs) for ≥15 days per month; or the concurrent use of multiple substances for more than 10 days per month [14].
Despite extensive investigation, our understanding of the pathogenesis of migraine disease remains limited. Several key factors, including vascular dysfunction, cortical spreading depression (CSD), trigeminovascular pathway activation, and inflammatory and oxidative conditions, have been implicated as fundamental components contributing to the development of migraine pain [18]. Several neurochemical changes play a role in the development of migraine, and one key element is calcitonin gene-related peptide (CGRP) and pituitary adenylate cyclase-activating polypeptide (PACAP). These peptide molecules are involved in triggering and sustaining headaches [19]. Neuropeptide release can lead to mast cell degranulation and plasma extravasation, initiating neurogenic inflammation (NI) [20]. Simultaneously, second-order neurons are activated in the caudal trigeminal nucleus (TNC), and their axons ascend to terminate in the thalamus, transmitting nociceptive information to the primary somatosensory cortex. Neuroimaging studies have identified additional regions of the central nervous system (CNS), such as the cerebellum, insula, and pulvinar, that may play a role in modulating the sensation of pain [21,22] (Figure 1).
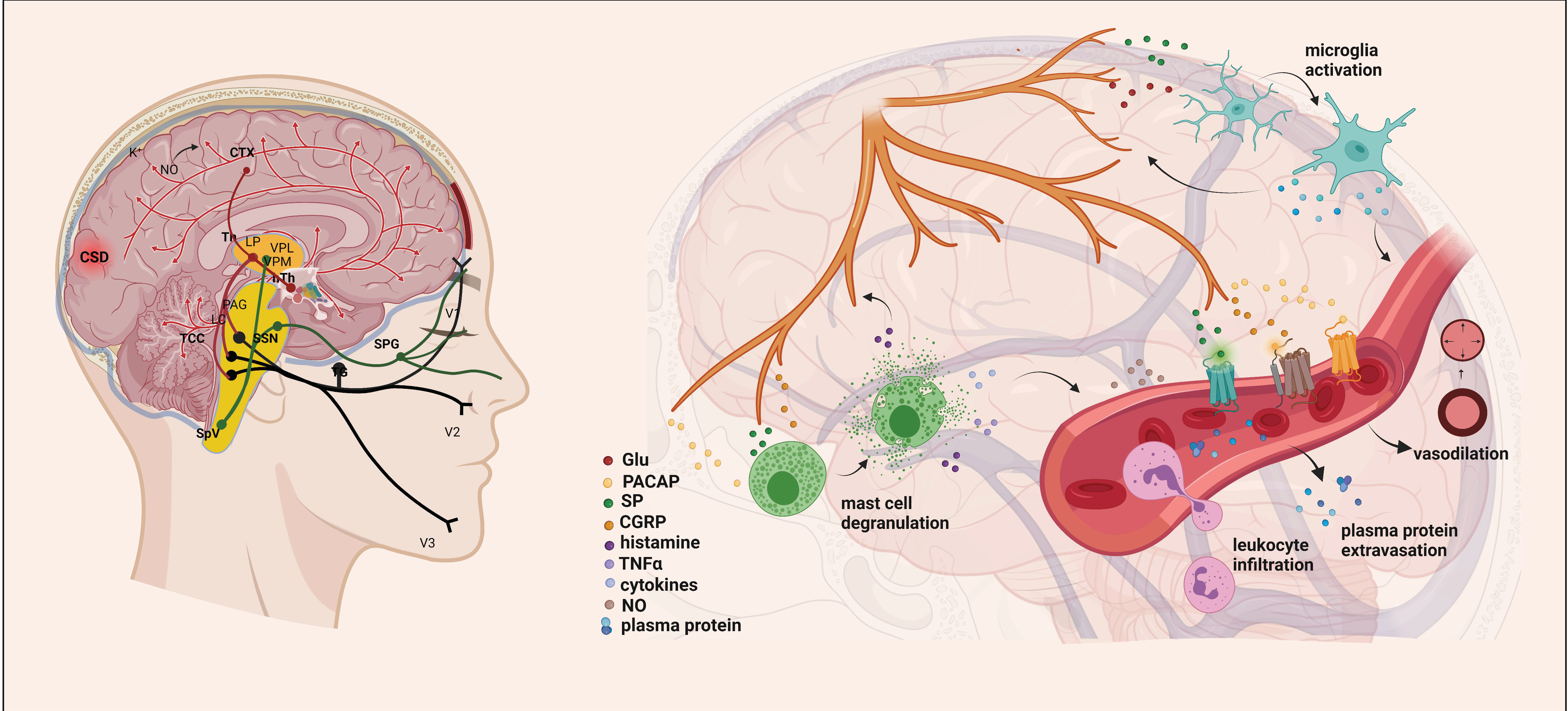
Figure 1.
Exploring the Mechanisms Underlying Migraine Attacks [23]. During migraines, various brain regions are affected, including the dorsolateral pons and dorsal midbrain: NRM, DR, LC, and PAG. These nuclei influence the activity of the trigeminocervical complex and play a role in pain transmission. The initiation and spread of migraine attacks are determined by significant increases in extracellular K+, NO, and glutamate concentrations. Cortical spreading depression (CSD) can activate sensory neurons in the trigeminal ganglion, releasing molecules like ATP, glutamate, K+, H+, AA, and NO locally, leading to their diffusion and activation of meningeal nociceptive neurons. This results in a localized increase in neuroactive inflammatory mediators and sensitization of brainstem regions relevant to pain. Stimulation of the trigeminal nerve causes the release of neuropeptides, initiating neurogenic inflammation with four main features: vasodilation, increased vascular permeability, leukocyte infiltration, and activation of glial cells, along with mast cell degranulation, leading to increased production of inflammatory mediators such as cytokines and chemokines. AA, arachidonic acid; CTX, cortex; NO, nitric oxide; CSD, cortical spreading depression; Th, thalamus; hTh, hypothalamus; LP, lateral posterior nucleus; VPM, ventral posteromedial nucleus; VPL, ventral posterolateral nucleus; PAG, periaqueductal grey matter; LC, locus coeruleus; TCC, trigeminocervical complex; SSN, superior salivatory nucleus; SpV, spinal trigeminal nucleus caudalis; TG, trigeminal ganglion; SPG, sphenopalatine ganglion; V1, ophthalmic nerve; V2, maxillary nerve; V3, mandibular nerve; Glu, glutamate; CGRP, calcitonin gene-related peptide; SP, substance P; PACAP, pituitary adenylate cyclase-activating polypeptide; TNFα, tumor necrosis factor alpha; NRM, nucleus raphe magnus; DR, nucleus raphe dorsalis.
Functional and structural changes occurring in various areas of the brain may contribute to the development of migraines. Various imaging techniques comparing migraineurs to control subjects reveal increased activation in specific areas, including the periaqueductal grey, red nucleus, substantia nigra, hypothalamus, posterior thalamus, cerebellum, insula, cingulate and prefrontal cortices, anterior temporal pole, and hippocampus. Conversely, diminished activation is observed in the somatosensory cortex, nucleus cuneiformis, caudate, putamen, and pallidum [24,25,26,27,28]. Among the structural changes are thickening of the somatosensory cortex, increased gray matter density in the caudate, and gray matter volume loss in various areas such as the superior temporal gyrus, inferior frontal gyrus, precentral gyrus, anterior cingulate cortex, amygdala, parietal operculum, middle and inferior frontal gyrus, inferior frontal gyrus, and bilateral insula. These changes may depend on the frequency of migraine attacks and other chronic pain conditions [29,30,31,32,33].
The genetic background of migraine is complex, and the role of genetic factors in the development of the condition is still partially unexplored. Family occurrences indicating the frequency of migraine attacks in certain families and the study of risks associated with specific gene variants suggest that genetics may play an important role in migraine predisposition. Family history suggests a genetic predisposition to migraine, initially observed in patients with familial hemiplegic migraine (FHM). Three genes linked to FHM regulate glutamate availability in the synapse, affecting neurotransmission and synaptic plasticity [34,35,36]. Large-scale genome-wide association studies have identified 13 susceptibility gene variants for migraine, with three influencing glutaminergic neurotransmission and two related to synaptic development and plasticity [37,38,39,40]. These findings provide insights into the generalized neuronal hyperexcitability observed in the migraine brain. However, it is important to note that genetic predisposition is just one factor in the development of migraines.
Several triggering factors are known, such as certain foods, stress, weather changes, hormonal influences, and certain habits like excessive caffeine consumption or lack of sleep [41,42,43,44,45].
The intricacy of migraines has hindered the comprehensive modeling of this disorder in animals, presenting a significant challenge that still needs to be addressed. With the appropriate diagnosis and treatment, the quality of life for those affected and the manageability of their condition can be significantly improved.
3. Exploring Animal Models in Migraine Research: Unraveling Complexity
Utilizing animal models to study human diseases has been immensely helpful, contributing to the enhanced understanding of brain disorders and the development of innovative therapeutic approaches. Various methods are usually employed in animal experimental research to study the role of age, such as comparing young and old animals, analyzing age-related changes in the frequency and severity of the disease, or investigating age-related biological processes. Although the subjective nature of migraine headache limits detailed investigations in animals, we would emphasize that such models remain valuable in understanding fundamental mechanisms. Behavioral and physiological markers are often employed in animal experimental studies to assess headache. These markers may include behavioral signs related to pain, such as head scratching or restlessness, as well as autonomic nervous system responses, such as changes in heart rate or elevation in stress hormone levels. Such markers can aid in estimating the degree and intensity of headache in the animal experimental environment. Although alternative methods such as in vitro models or computer simulations as well as advanced AI-based techniques are playing an increasingly significant role, they may not always be as effective in examining complex disease mechanisms as animal experiments.
Our understanding of migraine pathophysiology in recent decades is largely based on animal experiments. These experiments explore the nociceptive pathways of the trigeminovascular system and their ascending projections to the brainstem and diencephalic nuclei as well as the control these structures exert over nociceptive and other sensory processing pathways that lead to migraine symptoms. The existing migraine models, like models in other scientific fields, are essentially simplified representations of reality due to the constraints of available knowledge. Advancements in current models, alongside human research, have the potential to increase the translational efficacy of headache models. The expanding use of genetically modified animals and innovative methods may result in the development of novel, distinctive models (Figure 2).
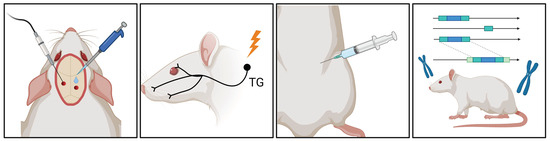
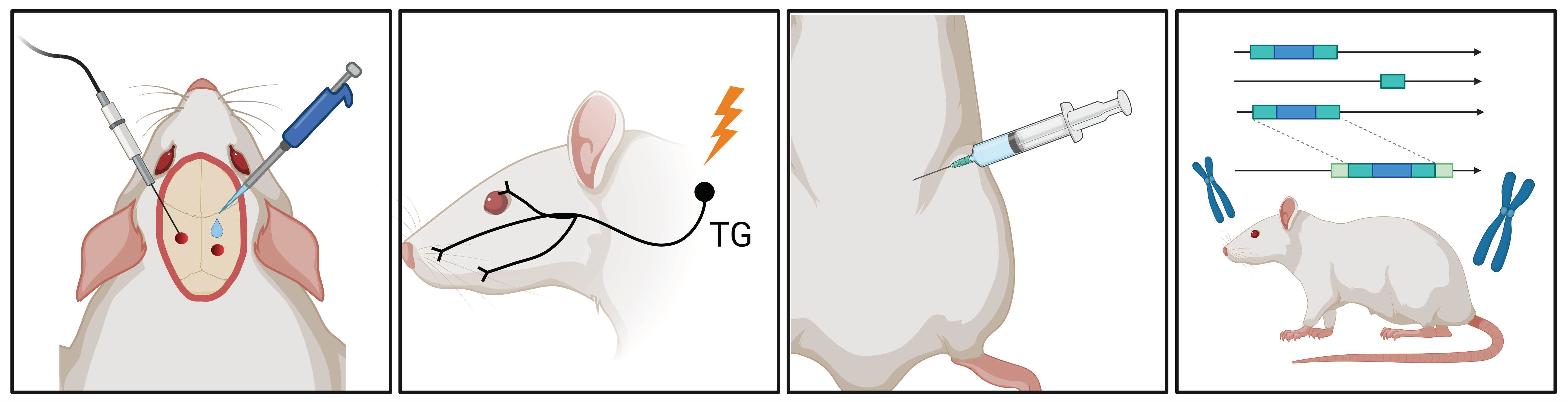
Figure 2.
Migraine Research Insights: Schematic Overview of Animal Models. In migraine research, various animal models are available, including electrical or chemical stimulation of the dura mater, electrical stimulation of the trigeminal ganglion, systemic administration of nitroglycerin (NTG), and the utilization of various genetic models. These models facilitate the study of diverse migraine-triggering mechanisms and pathophysiological processes in animal experiments. TG, trigeminal ganglion.
3.1. Cranial Stimulation Models: Investigating Migraine Mechanisms via Electrical Activation
The experimental model of migraine involves the electrical stimulation of the Gasserian ganglion, resulting in plasma extravasation within the dura mater [46,47]. The greatest advantage of electrical stimulation is that specifying stimulation parameters (current intensity: mA; voltage: V; pulse duration: ms–min; frequency: Hz) provides a controllable, reliable, monitorable, and repeatable method in contrast to mechanical, chemical, or magnetic stimulation. Furthermore, the use of electrical electrodes allows for precise and accurate stimulation [48]. Based on current research findings, electrical stimulation of the Gasserian ganglion leads to modifications in the distribution of CGRP content, impacting both the peripheral and central processes of primary sensory neurons. It is suggested that the decline in CGRP immunoreactivity indicates a depletion of this neuropeptide from the central terminals [49,50].
Under experimental conditions in one study, electrical stimulation of the trigeminal ganglion triggered plasma protein extravasation in the dura mater. However, stimulation of the trigeminal ganglion did not result in uniform brainstem activation in areas related to migraine, although it activated the pain modulatory system [51]. Additionally, this model allowed for the identification of a direct correlation between PACAP (another vasoactive neuropeptide) and the kynurenine pathway (KP) during trigeminal vascular system activation [52]. In other studies, repeated stimulation of the trigeminal ganglion led to allodynia, and it was demonstrated that the effect of sumatriptan in this model is primarily mediated through 5-hydroxytryptamine 1B/1D (5-HT1B/D) receptors [53]. It is important to note that a drawback of this model is the induction of inflammatory responses due to the insertion of electrodes into the brain parenchyma [54].
Stimulation of meningeal nerve terminals innervating the superior sagittal sinus (SSS) through electrical stimulation as well as the transverse sinus or middle meningeal arteries [55,56,57] serves as a preclinical model for studying migraines. The model has been employed to investigate the central effects of drugs such as ergotamine, sumatriptan, and acetylsalicylic acid, which inhibit the transmission of trigeminal nociceptive information in the brainstem. SSS stimulation allows for the analysis of c-Fos protein expression, a crucial marker of neuronal activity, revealing, for example, the role of glutamate in neurotransmission within the trigeminocervical complex [58,59,60,61]. Based on these findings, the SSS stimulation model proves to be a valuable tool for understanding migraine mechanisms and developing potential treatment strategies.
Electrical stimulation of the dura mater in rats, activating thin myelinated and unmyelinated nerve fibers, increases meningeal blood flow. This response can be diminished by 5-HT1 receptor agonists and eliminated by a CGRP receptor antagonist [62,63,64].
3.2. Migraine Models: Chemical Stimulation of the Dura Mater
One of the migraine models used in animal experiments involves the chemical stimulation of the dura mater. In this method, various irritants and inflammatory mediators (capsaicin, complete Freund’s adjuvant (CFA), and inflammatory soup (IS)) are applied to the dura mater. The application or infusion of inflammatory or algesic substances onto the dura mater or chemical stimulation of the dural receptive fields in rats induces heightened sensitivity to mechanical and thermal stimulation, accompanied by the direct activation of the trigeminal ganglion [65,66,67,68,69].
In animal experiments, capsaicin is used through either direct application to the exposed dura mater in a rat open cranial window preparation or intracisternal injection. This leads to the activation of trigeminovascular nociceptive afferents [70,71]. Triggering trigeminal nociceptive nerve fibers with capsaicin induces nociceptive behavior associated with face grooming [72,73,74]. Additionally, it brings about changes in blood flow within meningeal tissues and induces neurochemical alterations in second-order neurons of the trigeminal brainstem nuclei. Models utilizing capsaicin application are widely acknowledged as effective representations of meningeal nociception and headache [71].
Edvinsson et al. proposed that the persistent trigeminal activation caused by administering dural or temporomandibular CFA could serve as a model for the shift from episodic to chronic migraine [75]. Nociceptor activation leads to the release of CGRP and other neural mediators, initiating a local inflammatory response. This process likely triggers ongoing activation and sensitization of the trigeminal system, establishing a self-reinforcing mechanism [76,77,78]. This effect is comparable to the consequences of recurrent migraine attacks, potentially giving rise to continuous neurogenic inflammation, also known as neurogenic neuroinflammation [72]. Applying CFA to the dura or temporomandibular region may induce sustained activation in the trigeminal system, contributing to the onset of chronic migraine [68].
Oshinsky and Gomonchareonsiri administered IS treatment three times a week for up to four weeks, revealing that repeated IS infusions over weeks resulted in a sustained reduction in periorbital pressure thresholds [79]. A study by Lukács et al. revealed that CFA or IS application onto the dura induces changes in pERK1/2, IL-1β, and CGRP-positive nerve fibers in the TG [67]. Laborc et al. used IS or CFA topically on the dura, finding IS induced short-term c-Fos activation, while CFA showed no significant difference in c-Fos-positive cells [68]. Spekker et al. noted that IS induces sterile neurogenic inflammation in the dura mater, leading to an increase in the area covered by CGRP and transient receptor potential vanilloid type 1 (TRPV1) immunoreactive fibers as well as a rise in the count of neuronal nitric oxide synthase (nNOS)-positive cells in the TNC, which was modulated by sumatriptan, and kynurenic acid (KYNA) [80]. In another study by Spekker et al., behavioral results indicated that IS infusion enhances nociceptive responses. They found that the multiple administrations of IS on the dura mater can cause a significant decrease in mechanical pain thresholds in both the orofacial von Frey test and the hind-paw mechanical allodynia test. Furthermore, they observed increased facial grooming and scratching behavior [81]. Wieseler et al. reported IS administration leading to increased IL-1β, TNFα, and CD11b levels in TNC. IS also impacts animal behavior, inducing persistent changes in periorbital pressure thresholds, altered locomotor activity, and specific grooming behaviors [82]. Melo-Carrillo and colleagues noted increased resting and freezing behavior [83], while Malick et al. demonstrated reduced appetite in rats subjected to simultaneous chemical and mechanical dural stimulation [84]. In a large animal model, recurrent IS stimulation of the dura decreased locomotor behavior and induced pain-related behaviors, making it a relevant acute migraine animal model [85].
3.3. Exploring the Migraine-Inducing Properties of Nitroglycerin: Mechanisms, Neurological Impacts, and Experimental Models
Nitroglycerin (NTG) is a medication commonly used to treat angina by dilating blood vessels, reducing the workload on the heart, and improving blood flow. NTG was initially deployed in 1879 to address angina, and ever since, it has been integral to the management of relieving chest pain associated with angina [86]. Nevertheless, its ability to dilate blood vessels may result in headaches for certain individuals [87]. Moreover, it has been noted that among migraine sufferers, a form of seizure without aura can manifest hours after the headache subsides [88]. The effect of NTG has been extensively studied and thoroughly documented in animal migraine models, making it of fundamental importance in migraine research. Its ability to reliably induce migraine-like symptoms in animal models allows researchers to closely mimic the physiological and symptomatic aspects of migraine attacks, facilitating the investigation of underlying mechanisms and the development of potential therapeutic interventions.
NTG is a highly permeable and lipophilic organic nitrate, and its ability to donate nitric oxide (NO) is believed to be the primary mechanism underlying its migraine-inducing effects [89]. Initially, it was believed that the conversion of NTG to NO primarily occurred within vascular walls, influencing vascular tone exclusively. However, the discovery of NO’s presence in various tissues revealed its significant modulatory roles in the nervous system and inflammatory responses [90]. Due to its remarkable permeability and diffusion properties, NO acts as a pivotal neurotransmitter in the CNS, affecting nearby neural and glial structures [90].
Endogenously, the production of NO involves three distinct isoforms of NO synthase (NOS), which form dimers and catalyze the oxidation of l-arginine into l-citrulline. These isoforms, namely nNOS, inducible NOS (iNOS), and endothelial NOS (eNOS), were initially named based on the tissues in which they were identified but are extensively distributed throughout both the peripheral and CNS [91]. In the endothelium, eNOS produces NO in response to various stimuli, including shear stress from blood flow [92]. NO diffuses to nearby vascular smooth muscle cells, where it activates the enzyme soluble guanylyl cyclase (sGC), leading to increased levels of cyclic guanosine monophosphate (cGMP). Elevated cGMP levels cause relaxation of smooth muscle cells, leading to vasodilation and increased blood flow. This process helps regulate blood pressure and maintain cardiovascular health [92]. In the nervous system, NO acts as a neurotransmitter and neuromodulator. In neurons, nNOS synthesizes NO, which can diffuse across cell membranes and modulate the activity of neighboring cells. NO is involved in various neuronal functions, including synaptic plasticity, learning, and memory [93]. It also plays a role in regulating the release of other neurotransmitters such as glutamate and dopamine [94]. Beyond these, NO has antimicrobial properties and is involved in the immune response to pathogens. Macrophages and other immune cells express iNOS in response to inflammatory signals, leading to the production of large amounts of NO [95]. High levels of NO can help kill bacteria, viruses, and other pathogens by damaging their DNA or disrupting their metabolic processes [94]. However, excessive NO production can also contribute to tissue damage and inflammation in conditions such as septic shock [96]. Overall, NO is a versatile signaling molecule involved in numerous physiological processes in the human body, including cardiovascular regulation, neurotransmission, immune response, and smooth muscle function. Dysregulation of NO signaling is implicated in various diseases, including hypertension, atherosclerosis, neurodegenerative disorders, and erectile dysfunction.
The connection between NO, NTG, and headache has been recognized for a long time. Intravenous administration of NTG induces a mild-to-moderate bifrontal, throbbing headache within minutes, affecting both migraineurs and non-migraineurs, thus serving as a reliable model of vascular headache [97]. This headache is linked to NTG’s potent vasodilatory effects, believed to be mediated by its conversion into NO within the endothelial layer of the vascular wall [98,99]. However, while NTG can provoke a mild-to-moderate headache in non-migraineurs, it can trigger a headache attack resembling migraines exclusively in migraineurs [100,101] or individuals with a family history of migraines [102]. This migraine-like headache may onset within 45 min of NTG administration or be delayed by up to 4 to 5 h after the initial moderate headache subsides. Crucially, NTG also triggers the occurrence of common premonitory and associated symptoms [101]. The temporal pattern and clinical characteristics of these NTG-mediated migraine attacks suggest a downstream mechanism of action within the CNS. NTG has the ability to trigger the activation and sensitization in the trigeminal system of humans, a phenomenon observed in migraineurs, as well. Moreover, it stimulates various anatomical regions implicated in migraines, including the cervical spinal cord area, trigeminal nuclei, brainstem, and hypothalamus [9,103]. Migraine attack induced by NTG also increase levels of CGRP [104], a significant factor in migraine pathology [105,106]. Moreover, antimigraine agents like sumatriptan have been shown to diminish NTG-induced migraine attacks [107], thereby validating the model’s effectiveness.
On the other hand, numerous experiments, primarily conducted in rodents, have demonstrated that NTG can activate and sensitize the anatomical structures associated with migraines. The use of NTG in animal models is known to be both acute and chronic. Systemic administration of NTG as an acute model is able to increase the levels of c-Fos in rats [108], which indicates the activation of trigeminal system in many areas that correlate with migraine. Furthermore, NTG has also been shown to influence nNOS [76], CGRP [109], calcium/calmodulin-dependent protein kinase II alfa (CAMKIIα) [110], TRPV1 [111], nuclear factor kappa B (NF-κB) [112], and cyclooxygenase-2 (COX-2) [113] levels in the trigeminal system of rats. In addition to this, 5-HT and its transporter levels increased in the trigeminal system of rats after NTG administration [109,114]. On the other hand, administration of NTG led to a notable reduction in the ambulation distance observed in rats [115], which can be compared to the loss of movement seen in migraineurs. The use of NTG in animal studies can be justified by the fact that changes observed in human migraine patients have also been described in animals after NTG administration. An example of this observation is the involvement of the KP (kynurenine pathway) in migraine. KYNA, a crucial molecule in the KP, functions as an endogenous antagonist of N-methyl-D-aspartate (NMDA) receptors [116,117]. Within glutamate receptors, NMDA receptors have a central role in the pathomechanism of migraine [118]. Curto and her colleagues described abnormalities of various metabolites of the KP in the serum of patients with cluster headache and chronic migraine [119,120]. Similar alterations to these changes have been identified in rats, where the protein levels of several KP enzymes decreased four hours after systemic NTG administration [121]. In essence, NTG can induce alterations akin to those observed in human migraineurs.
In addition to the acute model of NTG, a chronic model is also known, which aims to investigate chronic migraine. By employing chronic intermittent administration of NTG, Pradhan and her colleagues devised a testing method that mimics the transition of migraine from an acute phase to a chronic condition [122]. In this chronic model, administering NTG every other day for nine days resulted in two different pain conditions: immediate hyperalgesia after each NTG injection and gradually increasing basal hypersensitivity [123]. Numerous animal studies support the possibility of repeated administration of NTG to induce activation and sensitization of the trigeminal system [123]. In addition to this, it has been described that chronic administration of NTG can trigger phenomena that are correlated to migraine, such as periorbital and perimasseter mechanical hyperalgesia [79,124], reduced locomotor activity [125], photophobia [126], and facial expressions of pain [125]. Besides these data, chronic administration of NTG can increase the CGRP and PACAP plasma levels in rats [127], suggesting the reliability of the model. PACAP, a member of the vasoactive intestinal peptide (VIP) family, has been shown to play a role in migraine [128].
Certainly, the NTG model possesses certain aspects that could be viewed unfavorably. It is crucial to emphasize that NTG does not induce migraine attacks in every individual; hence, care should be exercised when employing it in animal research. In the context of animal studies, it is worth mentioning that the commonly utilized dose of 5–10 mg/kg is significantly higher than the dosage administered in humans by orders of magnitude.
While NTG-induced models provide valuable insights, it is essential to recognize that migraine is a complex and multifactorial condition, and NTG may not fully replicate all aspects of migraine pathology. Findings from this experimental model can contribute to our understanding of migraine and potentially inform the development of new therapeutic approaches.
3.4. The Role of Genetic Factors in the Development of Migraine: Models and Research Approaches
Migraine shows a strong familial clustering, which is more pronounced in migraine with aura than in migraine without aura [129]. This allows us to conclude that genetic factors also play a role in the development of the disease, at least partially interacting with environmental factors. Many studies have been performed to search for the genetic background, such as genome-wide association studies (GWAS) and polygenic risk score (PRS) studies [130], which have identified many migraine-predisposing genes and confirmed that, from a genetic point of view, migraine is basically a disease with polygenic inheritance, which is caused by interaction between genetic and environmental factors.
At the same time, it is known that there are very rare but proven monogenic versions of migraine in which a single gene mutation can cause the disease. These have already given an opportunity to develop genetically modified animal models for migraine, which help better the understanding of the pathomechanism of migraine [131].
Among migraines with monogenic inheritance, the best known is the autosomal dominant FHM. Four subtypes are distinguished based on that in which gene the mutation exists: FHM1, FHM2, FHM3, and FHM4.
The FHM1 subtype includes migraineurs who carry a mutation affecting the CACNA1A gene located on chromosome 19p13.13 [132]. This gene encodes the alpha1 A subunit of the so-called CaV2.1 voltage-gated calcium channel, which plays, among others, an important role in the regulation of neurotransmitter release in neuronal synaptic nerve endings [133]. Several different mutations of this gene have been identified. The common feature of the mutations causing FHM1 is that they increase the function of the channel and therefore increase the influx of calcium into the cells, which leads to increased glutamate release and neuronal excitability in humans [134] and in mice, too [135]; these are so-called gain-of-function mutations.
There are transgenic mouse strains only for a few mutations among the many mutations affecting the CACNA1A gene identified in humans. The two knock-in mouse models are the mice with the milder R192Q and mice with the severer S218L missense mutation, which have similar visible symptoms as patients suffering from FHM1 carrying the same mutation [132,133,134,135,136,137,138]. These mice exhibit several differences compared to wild-type mice that can be paralleled with the known pathomechanism of migraine. Pain sensation is partially altered; i.e., the baseline facial pain is increased as measured by mouse grimace scale [139]. Transgenic animals exhibited photophobia-like behavior and several symptoms indicating spontaneous head pain induced by restraint and novelty stress. These behaviors were ipsilateral, had increased frequency, and showed sexual dimorphism and recovered dose-dependently by painkillers [140]. Interestingly, reactions to noxious thermal, mechanical, and chemical stimuli were unchanged in these animals [140]. Facilitated depolarization-evoked CGRP release from the trigeminal ganglion can be shown [130]. Moreover, there are some data suggesting the role of altered purinergic signaling induced by CGRP in changed pain-related molecular composition and facilitated signal transmission of pain in the trigeminal system [141]. In vitro and in vivo studies suggest that CSD susceptibility and underlying mechanisms such as release of excitatory neurotransmitters, synaptic transmission mediated by glutamatergic neurons, neuronal calcium concentrations, and the synaptic morphology are altered [142,143,144,145]. Changes related to synaptic plasticity can also be observed at the synaptic level in the cerebral cortex, brainstem, cerebellum, and hippocampus in in vitro and/or in vivo studies [136,142,143,146,147,148,149]. These animals showed changes in a number of substances that play an important role in the inflammatory process at protein and gene expression levels without triggering CSD and after CSD, which suggests that they are more susceptible to neuroinflammation [150,151,152]. Moreover, in the plasma of mutant mice, a changed concentration of some metabolites can be observed, which can be linked to compensatory mechanisms after excitation induced by CSD compared to wild-type animals [153]. At the cortical and subcortical level, CSD also induced alteration in the concentration of numerous metabolites and peptides [154]. The results on learning and memory are conflicting because learning and memory were impaired as measured by Morris in water maze and fear-conditioning experiments and a novel object recognition test, but increase of excitatory transmission and long-term potentiation (LTP) were measured in the hippocampus [149]. In relation to stress-induced migraine attacks, the restraint of stress enhanced the concentration of corticosterone in plasma of mice but did not affect CSD susceptibility, while corticosterone administered subcutaneously increased the frequency of CSD [155]. In line with the disrupted sleep observable in migraineurs, mutant mice have changed behavior related to sleep–wake rhythm, adapting faster to the light–dark cycle phase shifts and showing enhanced waking patterns in the active dark period after a 6 h sleep deprivation [156]. Differences related to sex can also be detected in mutant mice, where the CSD’s properties changed in unoperated females and in males that underwent an orchiectomy compared to male animals [157,158].
In the FHM2 subtype of migraine, the ATP1A2 gene carries mutations located on the 1q23.2 chromosome and encodes the alpha-2 subunit of Na+/K+ ATPase [35]. This pump is responsible for transporting Na+ out of the cell and K+ into the cell. Na+/K+ ATPase primarily is found on astrocytes in the nervous system and is involved in the regulation of neurotransmitter clearance from the synaptic cleft by removing its excess K+. Numerous and different types of mutations have been identified of the ATP1A2 gene in FHM2 patients, which result in loss-of-function of the encoded ATPase, causing increased K+ concentration in the synaptic cleft, due to which the glutamate uptake decreases, and neuronal excitability increases [159,160]. Transgenic mice have also been created to model this type of migraine, which carry either a knock-in loss-of-function missense mutation in heterozygous form (the homozygous form is lethal [161,162]), knock-out mutations also in heterozygous form [163], or mutations conditionally deleted [164]. Heterozygous mice do not show clinical symptoms of FHM2 [160,161], while episodic paralysis is observed in knock-out mice where the mutation is induced by programmed method [164]. Changes in the behavior of heterozygous mutant mice are more indicative of psychiatric diseases like anxiety, fear, depression, and increased immobility [161,162], and some of them showed sexual dimorphism, suggesting here again the role of sex hormones in the pathomechanism of migraine [162]. At the same time, the susceptibility to CSD is increased in all strains [161,162,163,164], which may be related to decreased clearance of K+ and glutamate by cortical astrocytes due to neuronal activation and the smaller density of GLT-1a glutamate transporters in the cortical presynaptic astrocytes [165]. Interestingly, endoplasmic reticulum retention and subsequent proteosomal degradation decreased the mutant type of Na+/K+ ATPase protein in brain lysates [161].
The FHM3 subtype of migraine is related to the SCN1A gene located on the 2q24.3 chromosome and its mutations. This gene encodes the α1 subunit of the voltage-dependent neuronal NaV1.1 sodium channel [36], which plays an important role in the generation and propagation of action potentials in cortical neurons and GABAergic inhibitory interneurons [166]. In migraineurs suffering from FHM3, some mainly missense mutations were identified as causing a gain of function of the NaV1.1 channel, with complex functional consequences [167,168]. Some transgenic mouse strains exist to model the FHM3 at a preclinical level [166,169,170]. The homozygous mice carrying a SCN1A-R1648H mutation exhibit spontaneous generalized seizures and premature death, while the heterozygous mice showed infrequent spontaneous generalized seizures and increased susceptibility for seizures induced by flurothyl and by hyperthermia, mimicking febrile seizures in which interneurons seem to play a major role leading to decreased inhibition [166]. The mutant mice expressing a SCN1A-L263V mutation demonstrated spontaneous CSDs that propagated from the visual to the motor cortex [169]. The third mutant mouse strain carries the mutation of SCN1A-L1649Q, where homozygous mice died prematurely, whereas heterozygous mice had a normal lifespan [170]. Heterozygous mice displayed a significantly enhanced susceptibility to CSD, which is assumed to be due to interneuron hyperactivity [170].
In FHM4, the gene encoding PRRT2 protein is mutated. It is located on the 16p11.2 chromosome and participates in the regulation of neurotransmitter release, affects the function of several types of ion channels, and influences the synaptogenesis. Mutations affecting the gene cause the loss of the function of protein, and the decreased amount of protein is not sufficient for complete function, leading to abnormal signaling between neurons, affecting the activity of ion channels and modifying the synaptogenesis. To better investigate the PRRT2 mutation’s effect, it is possible to use homozygous PRRT2 knock-out mice. These animals are viable but show paroxysmal movements and responses with abnormal motor behaviors to audiogenic stimuli and are more sensitive to the convulsive effects of pentylenetetrazol [171]. Related to movement abnormalities, high-frequency stimulation induced higher excitatory strength at parallel fiber–Purkinje cell synapses in cerebellar slices, indicating specific effects in the cerebellum [171]. Analysis of synaptic function showed changes detected at the level of excitatory neurons and inhibitory neurons that result in a state of heightened spontaneous and evoke activity at the network level, suggesting the network’s instability or hyperexcitability, which can be a possible basis of the paroxysmal phenotypes associated with PRRT2 mutations [172]. During synaptogenesis in cultured hippocampal neurons, morphology of the growth cones is altered in neurons derived from PRRT2 KO mice, which is associated with a selective alteration of the actin–cytoskeleton dynamics, supporting a key role of this protein in the regulation of growth cone morphology during neuronal development [173].
Based on numerous studies, the overall conclusion is that FHM is one of the main models of migraine aura, in which cortical spreading depression as a result of increased cortical excitability triggers the migraine headache (Figure 3).
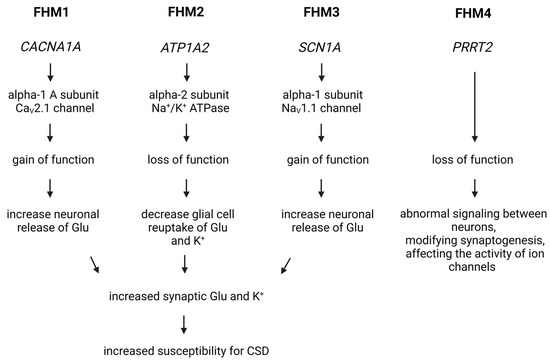
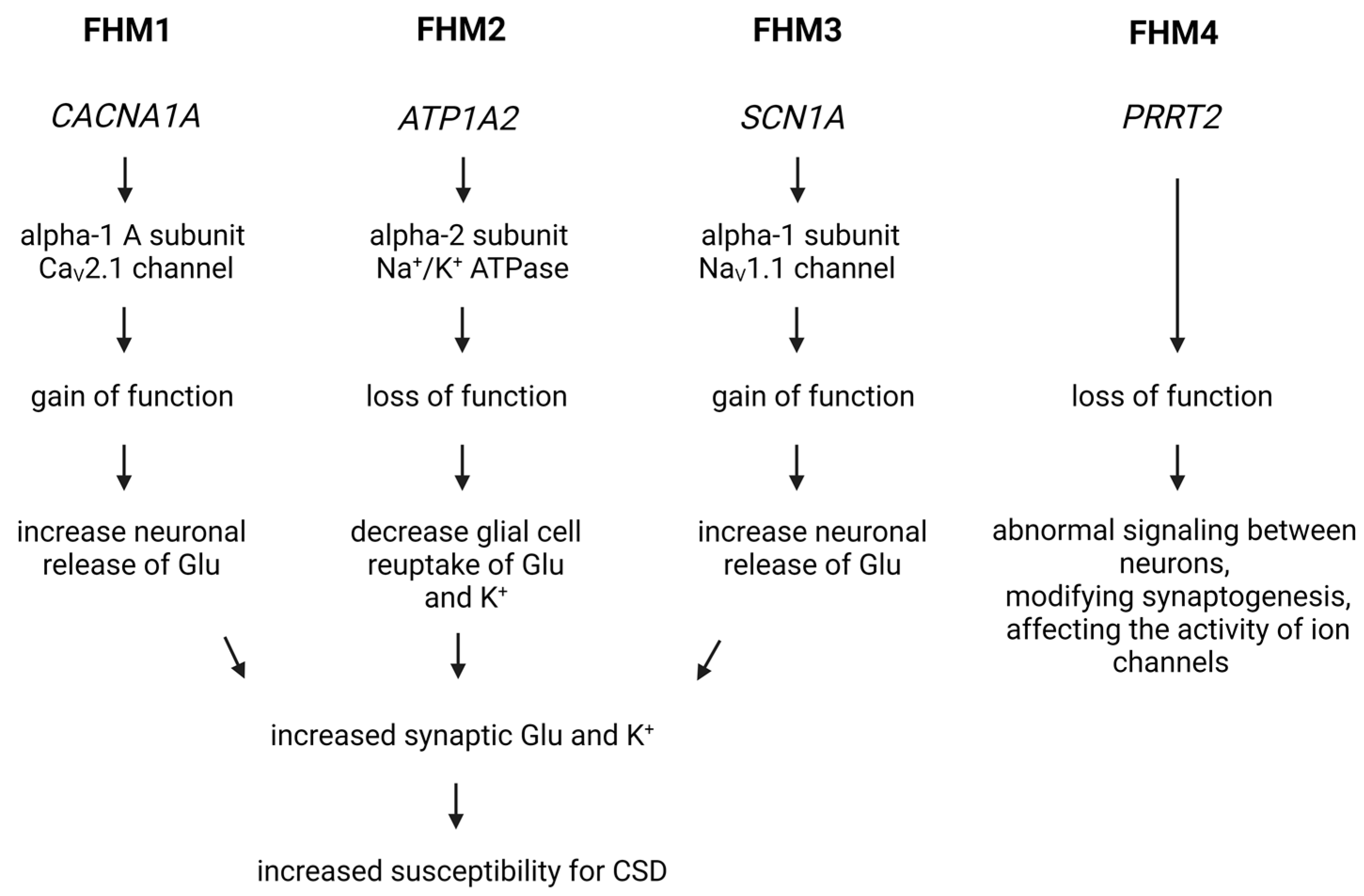
Figure 3.
Genetic Insights into Familial Hemiplegic Migraine and Migraine Mechanisms. In the case of FHM1, a mutation in the CACNA1A gene increases the activity of the CaV2.1 voltage-gated calcium channel, facilitating the influx of calcium into cells and leading to increased glutamate release and heightened excitability of nerve cells. For FHM2 and FHM3, mutations affect the function of the Na+/K+ ATPase and the NaV1.1 sodium channel, influencing the transmission of nerve impulses. In FHM4, a mutation in the PRRT2 gene impacts neurotransmitter release and ion channel function, contributing to migraine symptoms. Cortical spreading depression is a key mechanism in triggering migraine headaches in these models. Glu, glutamate; CSD, cortical spreading depression.
In addition to the mutations associated with FHM, there are also some other monogenic mutations that may be associated with migraine, such as KCNK18 mutations [173], CSNK1D mutations [174], and NOTCH3 mutations [175]. Results have been obtained in transgenic animal models of these mutations and suggest that they may indeed be associated with migraine, but this requires further research [156,174,176].
In conclusion, genetic models of migraine help us to understand certain aspects of the pathomechanism of migraine and can be a good tool for personalized medicine and biomarker and drug research. But it is not yet possible to create the perfect genetic migraine model with the knowledge we currently have because there is no consensus currently on the exact genes that clearly predispose to migraine, and it is problematic to create a polygenetic animal model. Unfortunately, it was also established by GWAS and PRS studies that genes associated with monogenic types of migraine probably do not play a stronger role in common migraine compared to other genes associated with migraine, increasing alone the overall risk for migraine only slightly [129]. However, in investigation of monogenic migraine, GWAS and PRS studies unanimously support the fact that the neurovascular unit plays a prominent role in migraine since a significant part of the proteins encoded by genes associated with migraine can be connected to the nervous or vascular systems.
4. Conclusions
Migraine, an extremely common primary headache type, has been a subject of scientific inquiry for a long time. Despite extensive research, the exact pathophysiological mechanism behind migraine remains elusive. However, in this field of investigation, animal experimental models have become of paramount importance, offering deeper insight into the triggering factors and biological foundations of migraines. In this article, we reviewed the significance of electrical and chemical stimulation models and delved into the mechanisms of migraine induced by nitroglycerin. Genetic modeling adds another layer to the picture, showcasing rare yet clinically important monogenic forms of migraine that help map out the genetic and biochemical foundations of the disease. The results from these models contribute not only to a better understanding of migraine pathophysiology but may also prove instrumental in identifying therapeutic targets and developing new treatment strategies.
Author Contributions
Conceptualization, E.S., G.N.-G. and A.F.-S.; writing—original draft preparation, E.S., G.N.-G. and A.F.-S.; writing—review and editing, E.S., G.N.-G. and A.F.-S.; visualization, E.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the Incubation Competence Centre of the Life Sciences Cluster of the Centre of Excellence for Interdisciplinary Research, Development, and Innovation of the University of Szeged and by SZTE SZAOK-KKA No: 2022/5S729. G.N-G. is a member of the Preventive Health Sciences Research Group.
Data Availability Statement
Not applicable.
Acknowledgments
All figures created with BioRender.com, accessed on 10 December 2023.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| 5-HT1B/D receptor | 5-hydroxytryptamine 1B/1D receptor |
| CAMKIIα | Calcium/calmodulin-dependent protein kinase II alfa |
| CFA | Complete Freund’s adjuvant |
| cGMP | Cyclic guanosine monophosphate |
| CGRP | Calcitonin gene-related peptide |
| CM | Chronic migraine |
| CNS | Central nervous system |
| COX-2 | Cyclooxygenase-2 |
| CSD | Cortical spreading depression |
| EM | Episodic migraine |
| eNOS | Endothelial nitric oxide synthase |
| FHM | Familial hemiplegic migraine |
| GWAS | Genome-wide association studies |
| ICHD-3 | The International Classification of Headache Disorders-3 beta |
| iNOS | Inducible nitric oxide synthase |
| IS | Inflammatory soup |
| KP | Kynurenine pathway |
| MOH | Medication-overuse headache |
| NF-κB | Nuclear factor kappa B |
| NI | Neurogenic inflammation |
| NMDA | N-methyl-D-aspartate |
| nNOS | Neuronal nitric oxide synthase |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| NTG | Nitroglycerin |
| PACAP | Pituitary adenylate cyclase-activating polypeptide |
| PRS | Polygenic risk score |
| sGC | enzyme-soluble guanylyl cyclase |
| SSS | Superior sagittal sinus |
| TNC | Caudal trigeminal nucleus |
| TRPV1 | Transient receptor potential vanilloid type 1 |
| VIP | Vasoactive intestinal peptide |
References
- Leonardi, M.; Steiner, T.J.; Scher, A.T.; Lipton, R.B. The global burden of migraine: Measuring disability in headache disorders with WHO’s Classification of Functioning, Disability and Health (ICF). J. Headache Pain 2005, 6, 429–440. [Google Scholar] [CrossRef]
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, S.; Adjepong, D.; Al-Shami, H.A.; Malik, B.H. Is There an Association between Migraine and Major Depressive Disorder? A Narrative Review. Cureus 2020, 12, e8551. [Google Scholar] [CrossRef] [PubMed]
- Al-Khazali, H.M.; Younis, S.; Al-Sayegh, Z.; Ashina, S.; Ashina, M.; Schytz, H.W. Prevalence of neck pain in migraine: A systematic review and meta-analysis. Cephalalgia 2022, 42, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Kelman, L. The premonitory symptoms (prodrome): A tertiary care study of 893 migraineurs. Headache 2004, 44, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Schoonman, G.G.; Evers, D.J.; Terwindt, G.M.; van Dijk, J.G.; Ferrari, M.D. The prevalence of premonitory symptoms in migraine: A questionnaire study in 461 patients. Cephalalgia 2006, 26, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Lipton, R.B.; Dodick, D.W.; Silberstein, S.D.; Saper, J.R.; Aurora, S.K.; Goadsby, P.J.; Charles, A. Migraine headache is present in the aura phase: A prospective study. Neurology 2012, 79, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Maniyar, F.H.; Sprenger, T.; Monteith, T.; Schankin, C.; Goadsby, P.J. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain 2014, 137 Pt 1, 232–241. [Google Scholar] [CrossRef]
- Burstein, R.; Noseda, R.; Borsook, D. Migraine: Multiple processes, complex pathophysiology. J. Neurosci. 2015, 35, 6619–6629. [Google Scholar] [CrossRef]
- Khan, J.; Asoom, L.I.A.; Sunni, A.A.; Rafique, N.; Latif, R.; Saif, S.A.; Almandil, N.B.; Almohazey, D.; AbdulAzeez, S.; Borgio, J.F. Genetics, pathophysiology, diagnosis, treatment, management, and prevention of migraine. Biomed. Pharmacother. 2021, 139, 111557. [Google Scholar] [CrossRef]
- Kelman, L.; Tanis, D. The relationship between migraine pain and other associated symptoms. Cephalalgia 2006, 26, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Bahra, A. Primary Headache Disorders: Focus on Migraine. Rev. Pain 2011, 5, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Katsarava, Z.; Buse, D.C.; Manack, A.N.; Lipton, R.B. Defining the differences between episodic migraine and chronic migraine. Curr. Pain Headache Rep. 2012, 16, 86–92. [Google Scholar] [CrossRef]
- Lipton, R.B. Tracing transformation: Chronic migraine classification, progression, and epidemiology. Neurology 2009, 72 (Suppl. S5), S3–S7. [Google Scholar] [CrossRef]
- Ashina, S.; Serrano, D.; Lipton, R.B.; Maizels, M.; Manack, A.N.; Turkel, C.C.; Reed, M.L.; Buse, D.C. Depression and risk of transformation of episodic to chronic migraine. J. Headache Pain 2012, 13, 615–624. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L. Tracing neural connections to pain pathways with relevance to primary headaches. Cephalalgia 2011, 31, 737–747. [Google Scholar] [CrossRef]
- Fusco, B.M.; Barzoi, G.; Agrò, F. Repeated intranasal capsaicin applications to treat chronic migraine. Br. J. Anaesth. 2003, 90, 812. [Google Scholar] [CrossRef]
- Matharu, M.S.; Bartsch, T.; Ward, N.; Frackowiak, R.S.; Weiner, R.; Goadsby, P.J. Central neuromodulation in chronic migraine patients with suboccipital stimulators: A PET study. Brain 2004, 127, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Hadjikhani, N. The cerebellum and migraine. Headache 2007, 47, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Spekker, E.; Nagy-Grócz, G.; Vécsei, L. Ion Channel Disturbances in Migraine Headache: Exploring the Potential Role of the Kynurenine System in the Context of the Trigeminovascular System. Int. J. Mol. Sci. 2023, 24, 16574. [Google Scholar] [CrossRef]
- Weiller, C.; May, A.; Limmroth, V.; Jüptner, M.; Kaube, H.; Schayck, R.V.; Coenen, H.H.; Diener, H.C. Brain stem activation in spontaneous human migraine attacks. Nat. Med. 1995, 1, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Burstein, R.; Jakubowski, M.; Garcia-Nicas, E.; Kainz, V.; Bajwa, Z.; Hargreaves, R.; Becerra, L.; Borsook, D. Thalamic sensitization transforms localized pain into widespread allodynia. Ann. Neurol. 2010, 68, 81–91. [Google Scholar] [CrossRef]
- Moulton, E.A.; Becerra, L.; Maleki, N.; Pendse, G.; Tully, S.; Hargreaves, R.; Burstein, R.; Borsook, D. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine States. Cereb. Cortex 2011, 21, 435–448. [Google Scholar] [CrossRef]
- Maleki, N.; Becerra, L.; Nutile, L.; Pendse, G.; Brawn, J.; Bigal, M.; Burstein, R.; Borsook, D. Migraine attacks the Basal Ganglia. Mol. Pain 2011, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, A.; Russo, A.; Esposito, F.; Giordano, A.; Taglialatela, G.; De Micco, R.; Cirillo, M.; Conte, F.; d’Onofrio, F.; Cirillo, S.; et al. Interictal cortical reorganization in episodic migraine without aura: An event-related fMRI study during parametric trigeminal nociceptive stimulation. Neurol. Sci. 2011, 32 (Suppl. S1), S165–S167. [Google Scholar] [CrossRef]
- DaSilva, A.F.; Granziera, C.; Snyder, J.; Hadjikhani, N. Thickening in the somatosensory cortex of patients with migraine. Neurology 2007, 69, 1990–1995. [Google Scholar] [CrossRef]
- Hadjikhani, N. Relevance of cortical thickness in migraine sufferers. Expert. Rev. Neurother. 2008, 8, 327–329. [Google Scholar] [CrossRef]
- Valfrè, W.; Rainero, I.; Bergui, M.; Pinessi, L. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache 2008, 48, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Raecke, R.; Niemeier, A.; Ihle, K.; Ruether, W.; May, A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J. Neurosci. 2009, 29, 13746–13750. [Google Scholar] [CrossRef] [PubMed]
- Baliki, M.N.; Schnitzer, T.J.; Bauer, W.R.; Apkarian, A.V. Brain morphological signatures for chronic pain. PLoS ONE 2011, 6, e26010. [Google Scholar] [CrossRef]
- Ducros, A.; Denier, C.; Joutel, A.; Cecillon, M.; Lescoat, C.; Vahedi, K.; Darcel, F.; Vicaut, E.; Bousser, M.G.; Tournier-Lasserve, E. The clinical spectrum of familial hemiplegic migraine associated with mutations in a neuronal calcium channel. N. Engl. J. Med. 2001, 345, 17–24. [Google Scholar] [CrossRef] [PubMed]
- De Fusco, M.; Marconi, R.; Silvestri, L.; Atorino, L.; Rampoldi, L.; Morgante, L.; Ballabio, A.; Aridon, P.; Casari, G. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump α2 subunit associated with familial hemiplegic migraine type 2. Nat. Genet. 2003, 33, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Dichgans, M.; Freilinger, T.; Eckstein, G.; Babini, E.; Lorenz-Depiereux, B.; Biskup, S.; Ferrari, M.D.; Herzog, J.; van den Maagdenberg, A.M.; Pusch, M.; et al. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet 2005, 366, 371–377. [Google Scholar] [CrossRef]
- The International Headache Genetics Consortium. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22.1. Nat. Genet. 2010, 42, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Chasman, D.I.; Schürks, M.; Anttila, V.; de Vries, B.; Schminke, U.; Launer, L.J.; Terwindt, G.M.; van den Maagdenberg, A.M.; Fendrich, K.; Völzke, H.; et al. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat. Genet. 2011, 43, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Freilinger, T.; Anttila, V.; de Vries, B.; Malik, R.; Kallela, M.; Terwindt, G.M.; Pozo-Rosich, P.; Winsvold, B.; Nyholt, D.R.; van Oosterhout, W.P.; et al. Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat. Genet. 2012, 44, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, H.G.; Albury, C.L.; Griffiths, L.R. Advances in genetics of migraine. J. Headache Pain 2019, 20, 72. [Google Scholar] [CrossRef]
- Peroutka, S.J. What turns on a migraine? A systematic review of migraine precipitating factors. Curr. Pain Headache Rep. 2014, 18, 454. [Google Scholar] [CrossRef]
- Pavlovic, J.M.; Buse, D.C.; Sollars, C.M.; Haut, S.; Lipton, R.B. Trigger factors and premonitory features of migraine attacks: Summary of studies. Headache 2014, 54, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Marmura, M.J. Triggers, Protectors, and Predictors in Episodic Migraine. Curr. Pain Headache Rep. 2018, 22, 81. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.; Pavlović, J.M. Sleep Disorders and Migraine: Review of Literature and Potential Pathophysiology Mechanisms. Headache 2018, 58, 1030–1039. [Google Scholar] [CrossRef]
- Martinelli, D.; Pocora, M.M.; De Icco, R.; Putortì, A.; Tassorelli, C. Triggers of migraine: Where do we stand? Curr. Opin. Neurol. 2022, 35, 360–366. [Google Scholar] [CrossRef]
- Buzzi, M.G.; Moskowitz, M.A. The trigemino-vascular system and migraine. Pathol. Biol. 1992, 40, 313–317. [Google Scholar]
- Buzzi, M.G.; Bonamini, M.; Moskowitz, M.A. Neurogenic model of migraine. Cephalalgia 1995, 15, 277–280. [Google Scholar] [CrossRef]
- Knyihár-Csillik, E.; Tajti, J.; Samsam, M.; Sáry, G.; Buzás, P.; Vécsei, L. Depletion of calcitonin gene-related peptide from the caudal trigeminal nucleus of the rat after electrical stimulation of the Gasserian ganglion. Exp. Brain Res. 1998, 118, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Csillik, B.; Knyihár-Csillik, E.; Kreutzberg, G.W.; Tajti, L.; Kereszturi, A.; Kovács, T. Calcitonin gene-related peptide is released from cholinergic synapses. Ann. N. Y. Acad. Sci. 1992, 657, 466–468. [Google Scholar] [CrossRef]
- Csillik, B.; Tajti, L.; Kovács, T.; Kukla, E.; Rakic, P.; Knyihár-Csillik, E. Distribution of calcitonin gene-related peptide in vertebrate neuromuscular junctions: Relationship to the acetylcholine receptor. J. Histochem. Cytochem. 1993, 41, 1547–1555. [Google Scholar] [CrossRef]
- Bohár, Z.; Fejes-Szabó, A.; Tar, L.; Varga, H.; Tajti, J.; Párdutz, Á.; Vécsei, L. Evaluation of c-Fos immunoreactivity in the rat brainstem nuclei relevant in migraine pathogenesis after electrical stimulation of the trigeminal ganglion. Neurol. Sci. 2013, 34, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Körtési, T.; Tuka, B.; Tajti, J.; Bagoly, T.; Fülöp, F.; Helyes, Z.; Vécsei, L. Kynurenic Acid Inhibits the Electrical Stimulation Induced Elevated Pituitary Adenylate Cyclase-Activating Polypeptide Expression in the TNC. Front. Neurol. 2018, 8, 745. [Google Scholar] [CrossRef] [PubMed]
- Buzzi, M.G.; Carter, W.B.; Shimizu, T.; Heath, H.; Moskowitz, M.A. Dihydroergotamine and sumatriptan attenuate levels of CGRP in plasma in rat superior sagittal sinus during electrical stimulation of the trigeminal ganglion. Neuropharmacology 1991, 30, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Demartini, C.; De Icco, R.; Martinelli, D.; Putortì, A.; Tassorelli, C. Migraine neuroscience: From experimental models to target therapy. Neurol. Sci. 2020, 41 (Suppl. S2), 351–361. [Google Scholar] [CrossRef] [PubMed]
- Zagami, A.S.; Goadsby, P.J.; Edvinsson, L. Stimulation of the superior sagittal sinus in the cat causes release of vasoactive peptides. Neuropeptides 1990, 16, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Holland, P.R.; Akerman, S.; Andreou, A.P.; Karsan, N.; Wemmie, J.A.; Goadsby, P.J. Acid-sensing ion channel 1: A novel therapeutic target for migraine with aura. Ann. Neurol. 2012, 72, 559–563. [Google Scholar] [CrossRef]
- Robert, C.; Bourgeais, L.; Arreto, C.D.; Condes-Lara, M.; Noseda, R.; Jay, T.; Villanueva, L. Paraventricular hypothalamic regulation of trigeminovascular mechanisms involved in headaches. J. Neurosci. 2013, 33, 8827–8840. [Google Scholar] [CrossRef] [PubMed]
- Kaube, H.; Keay, K.A.; Hoskin, K.L.; Bandler, R.; Goadsby, P.J. Expression of c-Fos-like immunoreactivity in the caudal medulla and upper cervical spinal cord following stimulation of the superior sagittal sinus in the cat. Brain Res. 1993, 629, 95–102. [Google Scholar] [CrossRef]
- Kaube, H.; Hoskin, K.L.; Goadsby, P.J. Acetylsalicylic acid inhibits cerebral cortical vasodilatation caused by superior sagittal sinus stimulation in the cat. Eur. J. Neurol. 1994, 1, 141–146. [Google Scholar] [CrossRef]
- Hoskin, K.L.; Goadsby, P.J. Comparison of more and less lipophilic serotonin (5HT1B/1D) agonists in a model of trigeminovascular nociception in cat. Exp. Neurol. 1998, 150, 45–51. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Hoskin, K.L. Differential effects of low dose CP122,288 and eletriptan on fos expression due to stimulation of the superior sagittal sinus in cat. Pain 1999, 82, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Strassman, A.; Mason, P.; Moskowitz, M.; Maciewicz, R. Response of brainstem trigeminal neurons to electrical stimulation of the dura. Brain Res. 1986, 379, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, M.; Messlinger, K.; Pawlak, M.; Schmidt, R.F. Increase of meningeal blood flow after electrical stimulation of rat dura mater encephali: Mediation by calcitonin gene-related peptide. Br. J. Pharmacol. 1995, 114, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Messlinger, K.; Hotta, H.; Pawlak, M.; Schmidt, R.F. Effects of the 5-HT1 receptor agonists, sumatriptan and CP 93,129, on dural arterial flow in the rat. Eur. J. Pharmacol. 1997, 332, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Phebus, L.A.; Johnson, K.W. Dural inflammation model of migraine pain. Curr. Protoc. Neurosci. 2001. [Google Scholar] [CrossRef]
- Burstein, R. Deconstructing migraine headache into peripheral and central sensitization. Pain 2001, 89, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Lukács, M.; Haanes, K.A.; Majláth, Z.; Tajti, J.; Vécsei, L.; Warfvinge, K.; Edvinsson, L. Dural administration of inflammatory soup or Complete Freund’s Adjuvant induces activation and inflammatory response in the rat trigeminal ganglion. J. Headache Pain 2015, 16, 564. [Google Scholar] [CrossRef] [PubMed]
- Laborc, K.F.; Spekker, E.; Bohár, Z.; Szűcs, M.; Nagy-Grócz, G.; Fejes-Szabó, A.; Vécsei, L.; Párdutz, Á. Trigeminal activation patterns evoked by chemical stimulation of the dura mater in rats. J. Headache Pain 2020, 21, 101. [Google Scholar] [CrossRef] [PubMed]
- Spekker, E.; Tanaka, M.; Szabó, Á.; Vécsei, L. Neurogenic Inflammation: The Participant in Migraine and Recent Advancements in Translational Research. Biomedicines 2021, 10, 76. [Google Scholar] [CrossRef]
- Cutrer, F.M.; Mitsikostas, D.D.; Ayata, G.; Sanchez del Rio, M. Attenuation by butalbital of capsaicin-induced c-fos-like immunoreactivity in trigeminal nucleus caudalis. Headache 1999, 39, 697–704. [Google Scholar] [CrossRef]
- Dux, M.; Sántha, P.; Jancsó, G. Capsaicin-sensitive neurogenic sensory vasodilatation in the dura mater of the rat. J. Physiol. 2003, 552 Pt 3, 859–867. [Google Scholar] [CrossRef]
- Jancsó, G. Intracisternal capsaicin: Selective degeneration of chemosensitive primary sensory afferents in the adult rat. Neurosci. Lett. 1981, 27, 41–45. [Google Scholar] [CrossRef]
- Szolcsányi, J. Antidromic vasodilatation and neurogenic inflammation. Agents Actions. 1988, 23, 4–11. [Google Scholar] [CrossRef]
- Kwon, Y.B.; Jeong, Y.C.; Kwon, J.K.; Son, J.S.; Kim, K.W. The Antinociceptive Effect of Sigma-1 Receptor Antagonist, BD1047, in a Capsaicin Induced Headache Model in Rats. Korean J. Physiol. Pharmacol. 2009, 13, 425–429. [Google Scholar] [CrossRef][Green Version]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K. Does inflammation have a role in migraine? Nat. Rev. Neurol. 2019, 15, 483–490. [Google Scholar] [CrossRef]
- Pardutz, A.; Krizbai, I.; Multon, S.; Vecsei, L.; Schoenen, J. Systemic nitroglycerin increases nNOS levels in rat trigeminal nucleus caudalis. Neuroreport 2000, 11, 3071–3075. [Google Scholar] [CrossRef]
- Geppetti, P.; Capone, J.G.; Trevisani, M.; Nicoletti, P.; Zagli, G.; Tola, M.R. CGRP and migraine: Neurogenic inflammation revisited. J. Headache Pain 2005, 6, 61–70. [Google Scholar] [CrossRef]
- Haanes, K.A.; Edvinsson, L. Pathophysiological Mechanisms in Migraine and the Identification of New Therapeutic Targets. CNS Drugs 2019, 33, 525–537. [Google Scholar] [CrossRef]
- Oshinsky, M.L.; Gomonchareonsiri, S. Episodic dural stimulation in awake rats: A model for recurrent headache. Headache 2007, 47, 1026–1036. [Google Scholar] [CrossRef]
- Spekker, E.; Laborc, K.F.; Bohár, Z.; Nagy-Grócz, G.; Fejes-Szabó, A.; Szűcs, M.; Vécsei, L.; Párdutz, Á. Effect of dural inflammatory soup application on activation and sensitization markers in the caudal trigeminal nucleus of the rat and the modulatory effects of sumatriptan and kynurenic acid. J. Headache Pain 2021, 22, 17. [Google Scholar] [CrossRef]
- Spekker, E.; Bohár, Z.; Fejes-Szabó, A.; Szűcs, M.; Vécsei, L.; Párdutz, Á. Estradiol Treatment Enhances Behavioral and Molecular Changes Induced by Repetitive Trigeminal Activation in a Rat Model of Migraine. Biomedicines 2022, 10, 3175. [Google Scholar] [CrossRef]
- Wieseler, J.; Ellis, A.; McFadden, A.; Stone, K.; Brown, K.; Cady, S.; Bastos, L.F.; Sprunger, D.; Rezvani, N.; Johnson, K.; et al. Supradural inflammatory soup in awake and freely moving rats induces facial allodynia that is blocked by putative immune modulators. Brain Res. 2017, 1664, 87–94. [Google Scholar] [CrossRef]
- Melo-Carrillo, A.; Lopez-Avila, A. A chronic animal model of migraine, induced by repeated meningeal nociception, characterized by a behavioral and pharmacological approach. Cephalalgia 2013, 33, 1096–1105. [Google Scholar] [CrossRef]
- Malick, A.; Jakubowski, M.; Elmquist, J.K.; Saper, C.B.; Burstein, R. A neurohistochemical blueprint for pain-induced loss of appetite. Proc. Natl. Acad. Sci. USA 2001, 98, 9930–9935. [Google Scholar] [CrossRef]
- Chen, N.; Su, W.; Cui, S.H.; Guo, J.; Duan, J.C.; Li, H.X.; He, L. A novel large animal model of recurrent migraine established by repeated administration of inflammatory soup into the dura mater of the rhesus monkey. Neural Regen. Res. 2019, 14, 100–106. [Google Scholar] [CrossRef]
- Parratt, J.R. Nitroglycerin-the first one hundred years: New facts about an old drug. J. Pharm. Pharmacol. 1979, 31, 801–809. [Google Scholar]
- Bektas, F.; Soyuncu, S. Nitroglycerin-induced migraine type headache: Bilaterally visible temporal arteries. Int. J. Emerg. Med. 2010, 3, 463–464. [Google Scholar] [CrossRef]
- Sicuteri, F.; Del Bene, E.; Poggioni, M.; Bonazzi, A. Unmasking latent dysnociception in healthy subjects. Headache 1987, 27, 180–185. [Google Scholar] [CrossRef]
- Sureda-Gibert, P.; Romero-Reyes, M.; Akerman, S. Nitroglycerin as a model of migraine: Clinical and preclinical review. Neurobiol. Pain 2022, 12, 100105. [Google Scholar] [CrossRef]
- Moncada, S. Adventures in vascular biology: A tale of two mediators. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 735–759. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357 Pt 3, 593–615. [Google Scholar] [CrossRef]
- Tousoulis, D.; Kampoli, A.M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef]
- Dominguez, J.M.; Muschamp, J.W.; Schmich, J.M.; Hull, E.M. Nitric oxide mediates glutamate-evoked dopamine release in the medial preoptic area. Neuroscience 2004, 125, 203–210. [Google Scholar] [CrossRef]
- Palmieri, E.M.; McGinity, C.; Wink, D.A.; McVicar, D.W. Nitric Oxide in Macrophage Immunometabolism: Hiding in Plain Sight. Metabolites 2020, 10, 429. [Google Scholar] [CrossRef]
- Kirkebøen, K.A.; Strand, O.A. The role of nitric oxide in sepsis—An overview. Acta Anaesthesiol. Scand. 1999, 43, 275–288. [Google Scholar] [CrossRef]
- Olesen, J.; Iversen, H.K.; Thomsen, L.L. Nitric oxide supersensitivity: A possible molecular mechanism of migraine pain. Neuroreport 1993, 4, 1027–1030. [Google Scholar] [CrossRef]
- Tassorelli, C.; Joseph, S.A.; Buzzi, M.G.; Nappi, G. The effects on the central nervous system of nitroglycerin—Putative mechanisms and mediators. Prog. Neurobiol. 1999, 57, 607–624. [Google Scholar] [CrossRef]
- Tassorelli, C.; Joseph, S.A.; Nappi, G. Reciprocal circuits involved in nitroglycerin-induced neuronal activation of autonomic regions and pain pathways: A double immunolabeling and tract-tracing study. Brain Res. 1999, 842, 294–310. [Google Scholar] [CrossRef]
- Iversen, H.K.; Olesen, J.; Tfelt-Hansen, P. Intravenous nitroglycerin as an experimental model of vascular headache. Basic characteristics. Pain 1989, 38, 17–24. [Google Scholar] [CrossRef]
- Afridi, K.S.; Kaube, H.; Goadsby, J.P. Glyceryl trinitrate triggers premonitory symptoms in migraineurs. Pain 2004, 110, 675–680. [Google Scholar] [CrossRef]
- Sances, G.; Tassorelli, C.; Pucci, E.; Ghiotto, N.; Sandrini, G.; Nappi, G. Reliability of the nitroglycerin provocative test in the diagnosis of neurovascular headaches. Cephalalgia 2004, 24, 110–119. [Google Scholar] [CrossRef]
- Bahra, A.; Matharu, M.S.; Buchel, C.; Frackowiak, R.S.; Goadsby, P.J. Brainstem activation specific to migraine headache. Lancet 2001, 357, 1016–1017. [Google Scholar] [CrossRef]
- Juhasz, G.; Zsombok, T.; Modos, E.A.; Olajos, S.; Jakab, B.; Nemeth, J.; Szolcsanyi, J.; Vitrai, J.; Bagdy, G. NO-induced migraine attack: Strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. Pain 2003, 106, 461–470. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann. Neurol. 1988, 23, 193–196. [Google Scholar] [CrossRef]
- Ashina, H.; Schytz, H.W.; Ashina, M. CGRP in human models of primary headaches. Cephalalgia 2018, 38, 353–360. [Google Scholar] [CrossRef]
- Juhasz, G.; Zsombok, T.; Jakab, B.; Nemeth, J.; Szolcsanyi, J.; Bagdy, G. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia 2005, 25, 179–183. [Google Scholar] [CrossRef]
- Tassorelli, C.; Joseph, S.A.; Nappi, G. Central effects of nitroglycerin in the rat: New perspectives in migraine research. Funct. Neurol. 1996, 11, 219–223, 227–235. [Google Scholar]
- Pardutz, A.; Multon, S.; Malgrange, B.; Parducz, A.; Vecsei, L.; Schoenen, J. Effect of systemic nitroglycerin on CGRP and 5-HT afferents to rat caudal spinal trigeminal nucleus and its modulation by estrogen. Eur. J. Neurosci. 2002, 15, 1803–1809. [Google Scholar] [CrossRef]
- Pardutz, A.; Hoyk, Z.; Varga, H.; Vecsei, L.; Schoenen, J. Oestrogen-modulated increase of calmodulin-dependent protein kinase II (CamKII) in rat spinal trigeminal nucleus after systemic nitroglycerin. Cephalalgia 2007, 27, 46–53. [Google Scholar] [CrossRef]
- Nagy-Grócz, G.; Tar, L.; Bohár, Z.; Fejes-Szabó, A.; Laborc, K.F.; Spekker, E.; Vécsei, L.; Párdutz, Á. The modulatory effect of anandamide on nitroglycerin-induced sensitization in the trigeminal system of the rat. Cephalalgia 2016, 36, 849–861. [Google Scholar] [CrossRef]
- Greco, R.; Tassorelli, C.; Cappelletti, D.; Sandrini, G.; Nappi, G. Activation of the transcription factor NF-kappaB in the nucleus trigeminalis caudalis in an animal model of migraine. Neurotoxicology 2005, 26, 795–800. [Google Scholar] [CrossRef]
- Varga, H.; Pardutz, A.; Vamos, E.; Bohar, Z.; Bago, F.; Tajti, J.; Bari, F.; Vecsei, L. Selective inhibition of cyclooxygenase-2 attenuates nitroglycerin-induced calmodulin-dependent protein kinase II alpha in rat trigeminal nucleus caudalis. Neurosci. Lett. 2009, 451, 170–173. [Google Scholar] [CrossRef]
- Nagy-Grócz, G.; Bohár, Z.; Fejes-Szabó, A.; Laborc, K.F.; Spekker, E.; Tar, L.; Vécsei, L.; Párdutz, Á. Nitroglycerin increases serotonin transporter expression in rat spinal cord but anandamide modulated this effect. J. Chem. Neuroanat. 2017, 85, 13–20. [Google Scholar] [CrossRef]
- Fejes-Szabó, A.; Bohár, Z.; Vámos, E.; Nagy-Grócz, G.; Tar, L.; Veres, G.; Zádori, D.; Szentirmai, M.; Tajti, J.; Szatmári, I.; et al. Pre-treatment with new kynurenic acid amide dose-dependently prevents the nitroglycerine-induced neuronal activation and sensitization in cervical part of trigemino-cervical complex. J. Neural Transm. 2014, 121, 725–738. [Google Scholar] [CrossRef]
- Birch, P.J.; Grossman, C.J.; Hayes, A.G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur. J. Pharmacol. 1988, 154, 85–87. [Google Scholar] [CrossRef]
- Kessler, M.; Terramani, T.; Lynch, G.; Baudry, M. A glycine site associated with N-methyl-D-aspartic acid receptors: Characterization and identification of a new class of antagonists. J. Neurochem. 1989, 52, 1319–1328. [Google Scholar] [CrossRef]
- Curto, M.; Lionetto, L.; Negro, A.; Capi, M.; Perugino, F.; Fazio, F.; Giamberardino, M.A.; Simmaco, M.; Nicoletti, F.; Martelletti, P. Altered serum levels of kynurenine metabolites in patients affected by cluster headache. J. Headache Pain 2015, 17, 27. [Google Scholar] [CrossRef]
- Curto, M.; Lionetto, L.; Negro, A.; Capi, M.; Fazio, F.; Giamberardino, M.A.; Simmaco, M.; Nicoletti, F.; Martelletti, P. Altered kynurenine pathway metabolites in serum of chronic migraine patients. J. Headache Pain 2015, 17, 47. [Google Scholar] [CrossRef]
- Nagy-Grócz, G.; Laborc, K.F.; Veres, G.; Bajtai, A.; Bohár, Z.; Zádori, D.; Fejes-Szabó, A.; Spekker, E.; Vécsei, L.; Párdutz, Á. The Effect of Systemic Nitroglycerin Administration on the Kynurenine Pathway in the Rat. Front. Neurol. 2017, 8, 278, Erratum in Front. Neurol. 2020, 11, 1049. [Google Scholar] [CrossRef]
- Pradhan, A.A.; Smith, M.L.; McGuire, B.; Tarash, I.; Evans, C.J.; Charles, A. Characterization of a novel model of chronic migraine. Pain 2014, 155, 269–274. [Google Scholar] [CrossRef]
- Greco, R.; Demartini, C.; Zanaboni, A.M.; Tassorelli, C. Chronic and intermittent administration of systemic nitroglycerin in the rat induces an increase in the gene expression of CGRP in central areas: Potential contribution to pain processing. J. Headache Pain 2018, 19, 51. [Google Scholar] [CrossRef]
- Tipton, A.F.; Tarash, I.; McGuire, B.; Charles, A.; Pradhan, A.A. The effects of acute and preventive migraine therapies in a mouse model of chronic migraine. Cephalalgia 2016, 36, 1048–1056. [Google Scholar] [CrossRef]
- Sufka, K.J.; Staszko, S.M.; Johnson, A.P.; Davis, M.E.; Davis, R.E.; Smitherman, T.A. Clinically relevant behavioral endpoints in a recurrent nitroglycerin migraine model in rats. J. Headache Pain 2016, 17, 40. [Google Scholar] [CrossRef]
- Harris, H.M.; Carpenter, J.M.; Black, J.R.; Smitherman, T.A.; Sufka, K.J. The effects of repeated nitroglycerin administrations in rats; modeling migraine-related endpoints and chronification. J. Neurosci. Methods 2017, 284, 63–70. [Google Scholar] [CrossRef]
- Mahmoudi, J.; Mohaddes, G.; Erfani, M.; Sadigh-Eteghad, S.; Karimi, P.; Rajabi, M.; Reyhani-Rad, S.; Farajdokht, F. Cerebrolysin attenuates hyperalgesia, photophobia, and neuroinflammation in a nitroglycerin-induced migraine model in rats. Brain Res. Bull. 2018, 140, 197–204. [Google Scholar] [CrossRef]
- Tuka, B.; Helyes, Z.; Markovics, A.; Bagoly, T.; Szolcsányi, J.; Szabó, N.; Tóth, E.; Kincses, Z.T.; Vécsei, L.; Tajti, J. Alterations in PACAP-38-like immunoreactivity in the plasma during ictal and interictal periods of migraine patients. Cephalalgia 2013, 33, 1085–1095. [Google Scholar] [CrossRef]
- Russell, M.B.; Hilden, J.; Sørensen, S.A.; Olesen, J. Familial occurrence of migraine without aura and migraine with aura. Neurology 1993, 43, 1369–1373. [Google Scholar] [CrossRef]
- Grangeon, L.; Lange, K.S.; Waliszewska-Prosół, M.; Onan, D.; Marschollek, K.; Wiels, W.; Mikulenka, P.; Farham, F.; Gollion, C.; on behalf of the European Headache Federation School of Advanced Studies (EHF-SAS). Genetics of migraine: Where are we now? J. Headache Pain 2023, 24, 12. [Google Scholar] [CrossRef]
- Dehghani, A.; Karatas, H. Mouse Models of Familial Hemiplegic Migraine for Studying Migraine Pathophysiology. Curr. Neuropharmacol. 2019, 17, 961–973. [Google Scholar] [CrossRef]
- Ophoff, R.A.; Terwindt, G.M.; Vergouwe, M.N.; van Eijk, R.; Oefner, P.J.; Hoffman, S.M.; Lamerdin, J.E.; Mohrenweiser, H.W.; Bulman, D.E.; Ferrari, M.; et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 1996, 87, 543–552. [Google Scholar] [CrossRef]
- Catterall, W.A. Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium. 1998, 24, 307–323. [Google Scholar] [CrossRef]
- Tottene, A.; Fellin, T.; Pagnutti, S.; Luvisetto, S.; Striessnig, J.; Fletcher, C.; Pietrobon, D. Familial hemiplegic migraine mutations increase Ca2+ influx through single human CaV2.1 channels and decrease maximal CaV2.1 current density in neurons. Proc. Natl. Acad. Sci. USA 2002, 99, 13284–13289. [Google Scholar] [CrossRef]
- van den Maagdenberg, A.M.; Pietrobon, D.; Pizzorusso, T.; Kaja, S.; Broos, L.A.; Cesetti, T.; van de Ven, R.C.; Tottene, A.; van der Kaa, J.; Plomp, J.J.; et al. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron 2004, 41, 701–710. [Google Scholar] [CrossRef]
- Adams, P.J.; Rungta, R.L.; Garcia, E.; van den Maagdenberg, A.M.; MacVicar, B.A.; Snutch, T.P. Contribution of calcium-dependent facilitation to synaptic plasticity revealed by migraine mutations in the P/Q-type calcium channel. Proc. Natl. Acad. Sci. USA 2010, 107, 18694–18699. [Google Scholar] [CrossRef]
- Stam, A.H.; Luijckx, G.J.; Poll-Thé, B.T.; Ginjaar, I.B.; Frants, R.R.; Haan, J.; Ferrari, M.D.; Terwindt, G.M.; van den Maagdenberg, A.M. Early seizures and cerebral oedema after trivial head trauma associated with the CACNA1A S218L mutation. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1125–1129. [Google Scholar] [CrossRef]
- Eikermann-Haerter, K.; Dileköz, E.; Kudo, C.; Savitz, S.I.; Waeber, C.; Baum, M.J.; Ferrari, M.D.; van den Maagdenberg, A.M.; Moskowitz, M.A.; Ayata, C. Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J. Clin. Investig. 2009, 119, 99–109. [Google Scholar] [CrossRef]
- Langford, D.J.; Bailey, A.L.; Chanda, M.L.; Clarke, S.E.; Drummond, T.E.; Echols, S.; Glick, S.; Ingrao, J.; Klassen-Ross, T.; Lacroix-Fralish, M.L.; et al. Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 2010, 7, 447–449. [Google Scholar] [CrossRef]
- Chanda, M.L.; Tuttle, A.H.; Baran, I.; Atlin, C.; Guindi, D.; Hathaway, G.; Israelian, N.; Levenstadt, J.; Low, D.; Macrae, L.; et al. Behavioral evidence for photophobia and stress-related ipsilateral head pain in transgenic Cacna1a mutant mice. Pain 2013, 154, 1254–1262. [Google Scholar] [CrossRef]
- Fioretti, B.; Catacuzzeno, L.; Sforna, L.; Gerke-Duncan, M.B.; van den Maagdenberg, A.M.; Franciolini, F.; Connor, M.; Pietrobon, D. Trigeminal ganglion neuron subtype-specific alterations of Ca(V)2.1 calcium current and excitability in a Cacna1a mouse model of migraine. J. Physiol. 2011, 589 Pt 23, 5879–5895. [Google Scholar] [CrossRef]
- Tottene, A.; Conti, R.; Fabbro, A.; Vecchia, D.; Shapovalova, M.; Santello, M.; van den Maagdenberg, A.M.; Ferrari, M.D.; Pietrobon, D. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in Ca(v)2.1 knockin migraine mice. Neuron 2009, 61, 762–773. [Google Scholar] [CrossRef]
- Vecchia, D.; Tottene, A.; van den Maagdenberg, A.M.; Pietrobon, D. Mechanism underlying unaltered cortical inhibitory synaptic transmission in contrast with enhanced excitatory transmission in CaV2.1 knockin migraine mice. Neurobiol. Dis. 2014, 69, 225–234. [Google Scholar] [CrossRef]
- Vecchia, D.; Tottene, A.; van den Maagdenberg, A.M.; Pietrobon, D. Abnormal cortical synaptic transmission in CaV2.1 knockin mice with the S218L missense mutation which causes a severe familial hemiplegic migraine syndrome in humans. Front. Cell Neurosci. 2015, 9, 8. [Google Scholar] [CrossRef]
- Loonen, I.C.M.; Voskuyl, R.A.; Schenke, M.; van Heiningen, S.H.; van den Maagdenberg, A.M.J.M.; Tolner, E.A. Spontaneous and optogenetically induced cortical spreading depolarization in familial hemiplegic migraine type 1 mutant mice. Neurobiol. Dis. 2024, 192, 106405. [Google Scholar] [CrossRef]
- Klychnikov, O.I.; Li, K.W.; Sidorov, I.A.; Loos, M.; Spijker, S.; Broos, L.A.; Frants, R.R.; Ferrari, M.D.; Mayboroda, O.A.; Deelder, A.M.; et al. Quantitative cortical synapse proteomics of a transgenic migraine mouse model with mutated Ca(v)2.1 calcium channels. Proteomics 2010, 10, 2531–2535. [Google Scholar] [CrossRef]
- Di Guilmi, M.N.; Wang, T.; Inchauspe, C.G.; Forsythe, I.D.; Ferrari, M.D.; van den Maagdenberg, A.M.; Borst, J.G.; Uchitel, O.D. Synaptic gain-of-function effects of mutant Cav2.1 channels in a mouse model of familial hemiplegic migraine are due to increased basal [Ca2+]i. J. Neurosci. 2014, 34, 7047–7058. [Google Scholar] [CrossRef]
- Inchauspe, C.G.; Urbano, F.J.; Di Guilmi, M.N.; Ferrari, M.D.; van den Maagdenberg, A.M.; Forsythe, I.D.; Uchitel, O.D. Presynaptic CaV2.1 calcium channels carrying familial hemiplegic migraine mutation R192Q allow faster recovery from synaptic depression in mouse calyx of Held. J. Neurophysiol. 2012, 108, 2967–2976. [Google Scholar] [CrossRef][Green Version]
- Dilekoz, E.; Houben, T.; Eikermann-Haerter, K.; Balkaya, M.; Lenselink, A.M.; Whalen, M.J.; Spijker, S.; Ferrari, M.D.; van den Maagdenberg, A.M.; Ayata, C. Migraine mutations impair hippocampal learning despite enhanced long-term potentiation. J. Neurosci. 2015, 35, 3397–3402. [Google Scholar] [CrossRef][Green Version]
- Hullugundi, S.K.; Ansuini, A.; Ferrari, M.D.; van den Maagdenberg, A.M.; Nistri, A. A hyperexcitability phenotype in mouse trigeminal sensory neurons expressing the R192Q Cacna1a missense mutation of familial hemiplegic migraine type-1. Neuroscience 2014, 266, 244–254. [Google Scholar] [CrossRef]
- Franceschini, A.; Vilotti, S.; Ferrari, M.D.; van den Maagdenberg, A.M.; Nistri, A.; Fabbretti, E. TNFα levels and macrophages expression reflect an inflammatory potential of trigeminal ganglia in a mouse model of familial hemiplegic migraine. PLoS ONE 2013, 8, e52394. [Google Scholar] [CrossRef]
- Eising, E.; Shyti, R.; ‘t Hoen, P.A.C.; Vijfhuizen, L.S.; Huisman, S.M.H.; Broos, L.A.M.; Mahfouz, A.; Reinders, M.J.T.; Ferrari, M.D.; Tolner, E.A.; et al. Cortical Spreading Depression Causes Unique Dysregulation of Inflammatory Pathways in a Transgenic Mouse Model of Migraine. Mol. Neurobiol. 2017, 54, 2986–2996. [Google Scholar] [CrossRef]
- Shyti, R.; Eikermann-Haerter, K.; van Heiningen, S.H.; Meijer, O.C.; Ayata, C.; Joëls, M.; Ferrari, M.D.; van den Maagdenberg, A.M.; Tolner, E.A. Stress hormone corticosterone enhances susceptibility to cortical spreading depression in familial hemiplegic migraine type 1 mutant mice. Exp. Neurol. 2015, 263, 214–220. [Google Scholar] [CrossRef]
- Carreira, R.J.; Shyti, R.; Balluff, B.; Abdelmoula, W.M.; van Heiningen, S.H.; van Zeijl, R.J.; Dijkstra, J.; Ferrari, M.D.; Tolner, E.A.; McDonnell, L.A.; et al. Large-scale mass spectrometry imaging investigation of consequences of cortical spreading depression in a transgenic mouse model of migraine. J. Am. Soc. Mass. Spectrom. 2015, 26, 853–861. [Google Scholar] [CrossRef][Green Version]
- Shyti, R.; Kohler, I.; Schoenmaker, B.; Derks, R.J.; Ferrari, M.D.; Tolner, E.A.; Mayboroda, O.A.; van den Maagdenberg, A.M. Plasma metabolic profiling after cortical spreading depression in a transgenic mouse model of hemiplegic migraine by capillary electrophoresis–mass spectrometry. Mol. Biosyst. 2015, 11, 1462–1471. [Google Scholar] [CrossRef]
- Deboer, T.; van Diepen, H.C.; Ferrari, M.D.; van den Maagdenberg, A.M.; Meijer, J.H. Reduced sleep and low adenosinergic sensitivity in cacna1a R192Q mutant mice. Sleep 2013, 36, 127–136. [Google Scholar] [CrossRef]
- Eikermann-Haerter, K.; Yuzawa, I.; Dilekoz, E.; Joutel, A.; Moskowitz, M.A.; Ayata, C. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy syndrome mutations increase susceptibility to spreading depression. Ann. Neurol. 2011, 69, 413–418. [Google Scholar] [CrossRef]
- Eikermann-Haerter, K.; Baum, M.J.; Ferrari, M.D.; van den Maagdenberg, A.M.; Moskowitz, M.A.; Ayata, C. Androgenic suppression of spreading depression in familial hemiplegic migraine type 1 mutant mice. Ann. Neurol. 2009, 66, 564–568. [Google Scholar] [CrossRef]
- Tavraz, N.N.; Friedrich, T.; Dürr, K.L.; Koenderink, J.B.; Bamberg, E.; Freilinger, T.; Dichgans, M. Diverse functional consequences of mutations in the Na+/K+-ATPase α2-subunit causing familial hemiplegic migraine type 2. J. Biol. Chem. 2008, 283, 31097–31106. [Google Scholar] [CrossRef]
- Pisano, T.; Spiller, S.; Mei, D.; Guerrini, R.; Cianchetti, C.; Friedrich, T.; Pruna, D. Functional characterization of a novel C-terminal ATP1A2 mutation causing hemiplegic migraine and epilepsy. Cephalalgia 2013, 33, 1302–1310. [Google Scholar] [CrossRef]
- Leo, L.; Gherardini, L.; Barone, V.; De Fusco, M.; Pietrobon, D.; Pizzorusso, T.; Casari, G. Increased susceptibility to cortical spreading depression in the mouse model of familial hemiplegic migraine type 2. PLoS Genet. 2011, 7, e1002129. [Google Scholar] [CrossRef]
- Bøttger, P.; Glerup, S.; Gesslein, B.; Illarionova, N.B.; Isaksen, T.J.; Heuck, A.; Clausen, B.H.; Füchtbauer, E.M.; Gramsbergen, J.B.; Gunnarson, E.; et al. Glutamate-system defects behind psychiatric manifestations in a familial hemiplegic migraine type 2 disease-mutation mouse model. Sci. Rep. 2016, 6, 22047. [Google Scholar] [CrossRef]
- Unekawa, M.; Ikeda, K.; Tomita, Y.; Kawakami, K.; Suzuki, N. Enhanced susceptibility to cortical spreading depression in two types of Na+,K+-ATPase α2 subunit-deficient mice as a model of familial hemiplegic migraine 2. Cephalalgia 2018, 38, 1515–1524. [Google Scholar] [CrossRef]
- Smith, S.E.; Chen, X.; Brier, L.M.; Bumstead, J.R.; Rensing, N.R.; Ringel, A.E.; Shin, H.; Oldenborg, A.; Crowley, J.R.; Bice, A.R.; et al. Astrocyte deletion of α2-Na/K ATPase triggers episodic motor paralysis in mice via a metabolic pathway. Nat. Commun. 2020, 11, 6164. [Google Scholar] [CrossRef]
- Capuani, C.; Melone, M.; Tottene, A.; Bragina, L.; Crivellaro, G.; Santello, M.; Casari, G.; Conti, F.; Pietrobon, D. Defective glutamate and K+ clearance by cortical astrocytes in familial hemiplegic migraine type 2. EMBO Mol. Med. 2016, 8, 967–986. [Google Scholar] [CrossRef]
- Martin, M.S.; Dutt, K.; Papale, L.A.; Dubé, C.M.; Dutton, S.B.; de Haan, G.; Shankar, A.; Tufik, S.; Meisler, M.H.; Baram, T.Z.; et al. Altered function of the SCN1A voltage-gated sodium channel leads to gamma-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J. Biol. Chem. 2010, 285, 9823–9834. [Google Scholar] [CrossRef]
- Cestèle, S.; Scalmani, P.; Rusconi, R.; Terragni, B.; Franceschetti, S.; Mantegazza, M. Self-limited hyperexcitability: Functional effect of a familial hemiplegic migraine mutation of the Nav1.1 (SCN1A) Na+ channel. J. Neurosci. 2008, 28, 7273–7283. [Google Scholar] [CrossRef]
- Bertelli, S.; Barbieri, R.; Pusch, M.; Gavazzo, P. Gain of function of sporadic/familial hemiplegic migraine-causing SCN1A mutations: Use of an optimized cDNA. Cephalalgia 2019, 39, 477–488. [Google Scholar] [CrossRef]
- Jansen, N.A.; Dehghani, A.; Linssen, M.M.L.; Breukel, C.; Tolner, E.A.; van den Maagdenberg, A.M.J.M. First FHM3 mouse model shows spontaneous cortical spreading depolarizations. Ann. Clin. Transl. Neurol. 2020, 7, 132–138. [Google Scholar] [CrossRef]
- Auffenberg, E.; Hedrich, U.B.; Barbieri, R.; Miely, D.; Groschup, B.; Wuttke, T.V.; Vogel, N.; Lührs, P.; Zanardi, I.; Bertelli, S.; et al. Hyperexcitable interneurons trigger cortical spreading depression in an Scn1a migraine model. J. Clin. Investig. 2021, 131, e142202. [Google Scholar] [CrossRef]
- Michetti, C.; Castroflorio, E.; Marchionni, I.; Forte, N.; Sterlini, B.; Binda, F.; Fruscione, F.; Baldelli, P.; Valtorta, F.; Zara, F.; et al. The PRRT2 knockout mouse recapitulates the neurological diseases associated with PRRT2 mutations. Neurobiol. Dis. 2017, 99, 66–83. [Google Scholar] [CrossRef]
- Valente, P.; Romei, A.; Fadda, M.; Sterlini, B.; Lonardoni, D.; Forte, N.; Fruscione, F.; Castroflorio, E.; Michetti, C.; Giansante, G.; et al. Constitutive Inactivation of the PRRT2 Gene Alters Short-Term Synaptic Plasticity and Promotes Network Hyperexcitability in Hippocampal Neurons. Cereb. Cortex 2019, 29, 2010–2033. [Google Scholar] [CrossRef]
- Savino, E.; Guarnieri, F.C.; Tsai, J.W.; Corradi, A.; Benfenati, F.; Valtorta, F. An Emerging Role of PRRT2 in Regulating Growth Cone Morphology. Cells 2021, 10, 2666. [Google Scholar] [CrossRef]
- Lafrenière, R.G.; Cader, M.Z.; Poulin, J.F.; Andres-Enguix, I.; Simoneau, M.; Gupta, N.; Boisvert, K.; Lafrenière, F.; McLaughlan, S.; Dubé, M.P.; et al. A dominant-negative mutation in the TRESK potassium channel is linked to familial migraine with aura. Nat. Med. 2010, 16, 1157–1160. [Google Scholar] [CrossRef]
- Brennan, K.C.; Bates, E.A.; Shapiro, R.E.; Zyuzin, J.; Hallows, W.C.; Huang, Y.; Lee, H.Y.; Jones, C.R.; Fu, Y.H.; Charles, A.C.; et al. Casein kinase iδ mutations in familial migraine and advanced sleep phase. Sci. Transl. Med. 2013, 5, 183ra56. [Google Scholar] [CrossRef]
- Tan, R.Y.; Markus, H.S. CADASIL: Migraine, Encephalopathy, Stroke and Their Inter-Relationships. PLoS ONE 2016, 11, e0157613. [Google Scholar] [CrossRef]
- Weir, G.A.; Pettingill, P.; Wu, Y.; Duggal, G.; Ilie, A.S.; Akerman, C.J.; Cader, M.Z. The Role of TRESK in Discrete Sensory Neuron Populations and Somatosensory Processing. Front. Mol. Neurosci. 2019, 12, 170. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).