The Role of Neutrophils in Multiple Sclerosis and Ischemic Stroke
Abstract
:1. Introduction
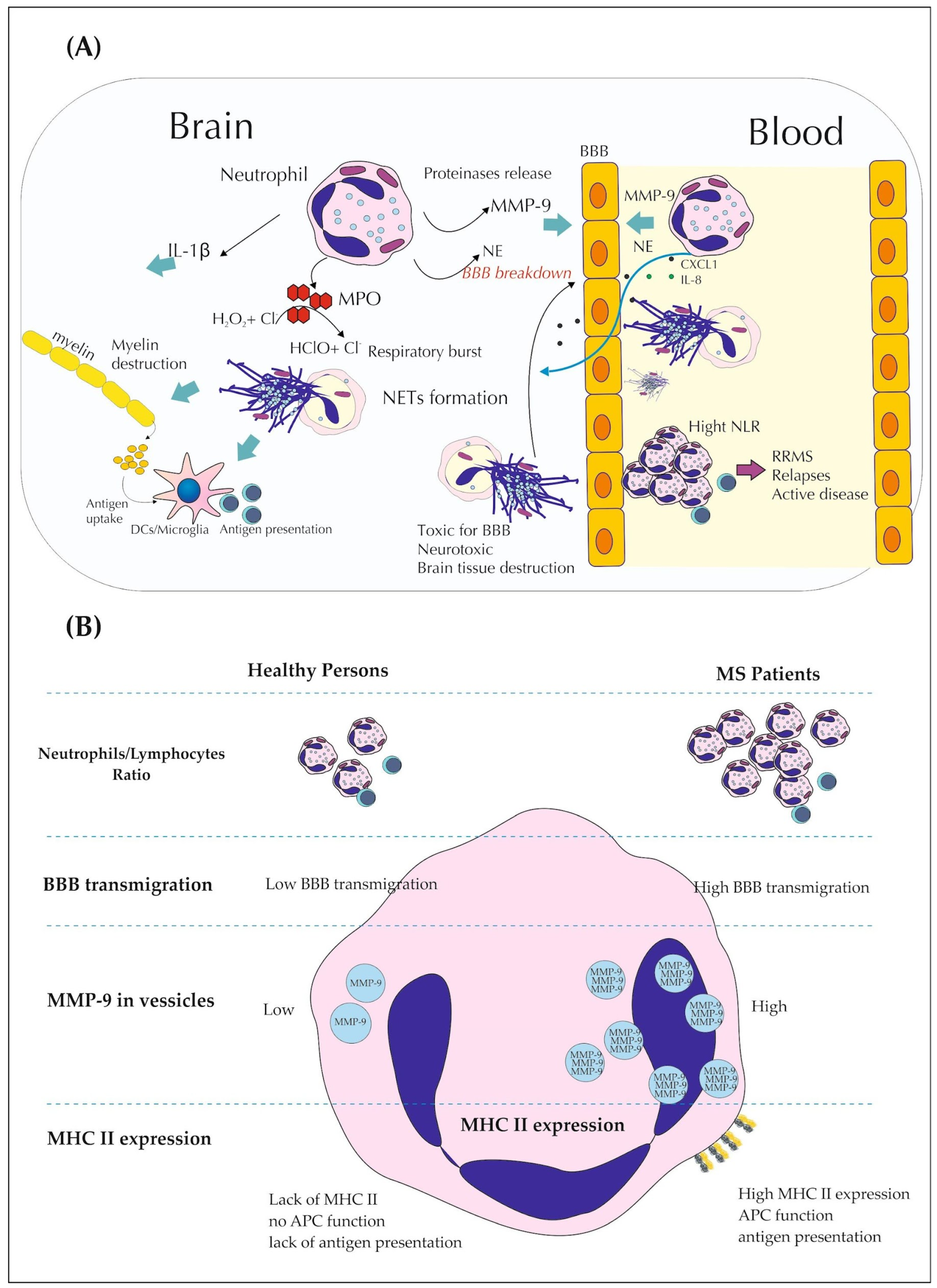
2. The Role of Neutrophils in Multiple Sclerosis
2.1. NLR as Promising Indicator of MS
2.2. Neutrophils Secretory Activity in MS
2.3. Role of PAD and CXCL1 in Demyelination and BBB Breakdown
2.4. Phenoplasticity of Neutrophils and MS
2.5. Neutrophils and DTM Drugs
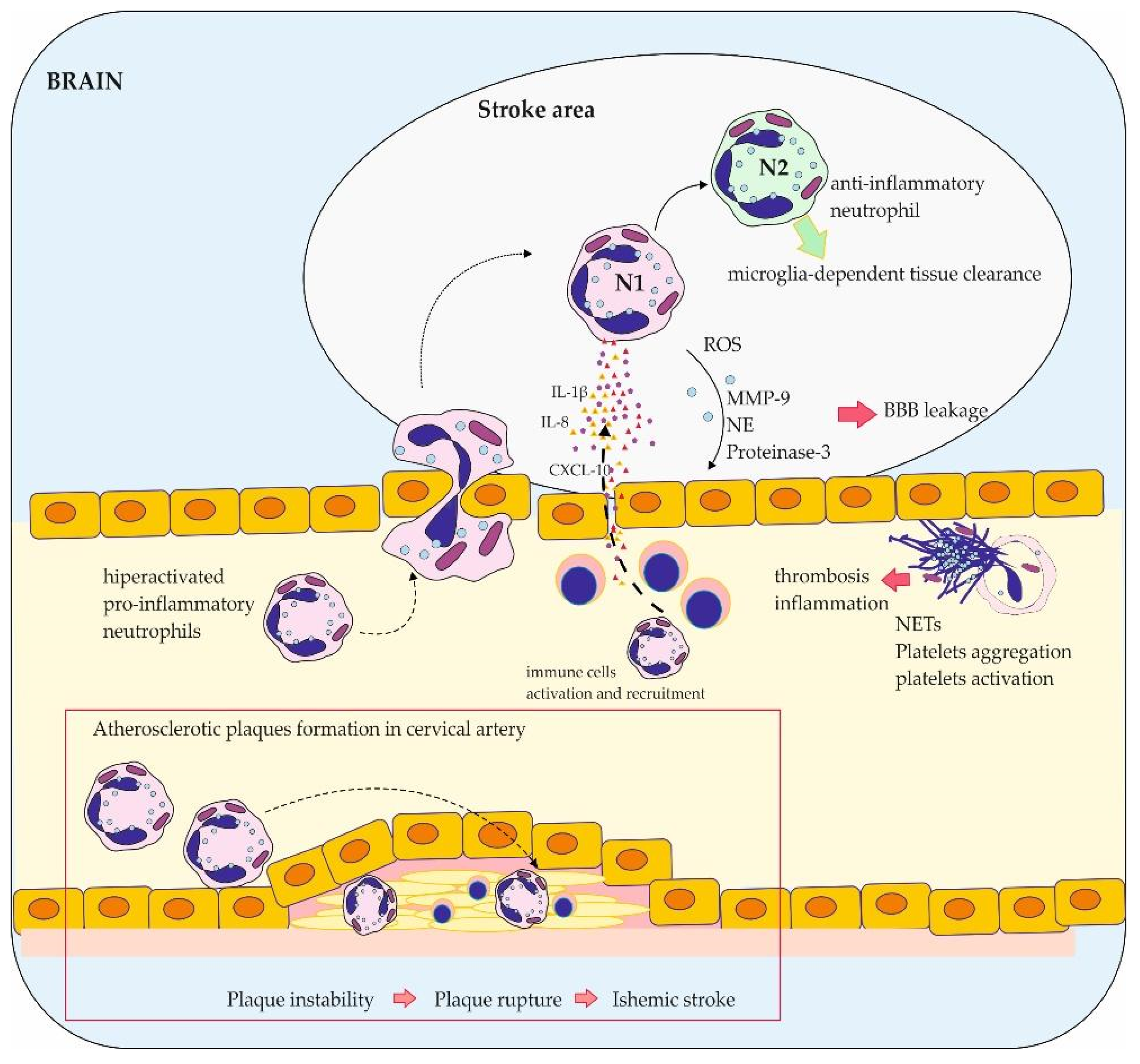
3. The Role of Neutrophils in Stroke
3.1. Phenotype Plasticity of Neutrophils and Their Role in Stroke
3.2. The Role of NETs in Thrombosis
3.3. The Impact of Neutrophils on BBB Function
3.4. NLR as Diagnostic and Prognostic Tool for Stroke
3.5. The Role of Neutrophils in Reperfusion Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The Devil Is in the Details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef]
- Grist, J.J.; Marro, B.; Lane, T.E. Neutrophils and Viral-Induced Neurologic Disease. Clin. Immunol. 2018, 189, 52–56. [Google Scholar] [CrossRef]
- Pillay, J.; Den Braber, I.; Vrisekoop, N.; Kwast, L.M.; De Boer, R.J.; Borghans, J.A.M.; Tesselaar, K.; Koenderman, L. In Vivo Labeling with 2H2O Reveals a Human Neutrophil Lifespan of 5.4 Days. Blood 2010, 116, 625–627. [Google Scholar] [CrossRef]
- Larochelle, C.; Alvarez, J.I.; Prat, A. How Do Immune Cells Overcome the Blood-Brain Barrier in Multiple Sclerosis? FEBS Lett. 2011, 585, 3770–3780. [Google Scholar] [CrossRef]
- De Bondt, M.; Hellings, N.; Opdenakker, G.; Struyf, S. Neutrophils: Underestimated Players in the Pathogenesis of Multiple Sclerosis (Ms). Int. J. Mol. Sci. 2020, 21, 4558. [Google Scholar] [CrossRef]
- Kambayashi, T.; Laufer, T.M. Atypical MHC Class II-Expressing Antigen-Presenting Cells: Can Anything Replace a Dendritic Cell? Nat. Rev. Immunol. 2014, 14, 719–730. [Google Scholar] [CrossRef]
- Steinbach, K.; Piedavent, M.; Bauer, S.; Neumann, J.T.; Friese, M.A. Neutrophils Amplify Autoimmune Central Nervous System Infiltrates by Maturing Local APCs. J. Immunol. 2013, 191, 4531–4539. [Google Scholar] [CrossRef]
- Kanashiro, A.; Hiroki, C.H.; da Fonseca, D.M.; Birbrair, A.; Ferreira, R.G.; Bassi, G.S.; Fonseca, M.D.; Kusuda, R.; Cebinelli, G.C.M.; da Silva, K.P.; et al. The Role of Neutrophils in Neuro-Immune Modulation. Pharmacol. Res. 2020, 151, 104580. [Google Scholar] [CrossRef]
- Cai, W.; Liu, S.; Hu, M.; Huang, F.; Zhu, Q.; Qiu, W.; Hu, X.; Colello, J.; Zheng, S.G.; Lu, Z. Functional Dynamics of Neutrophils After Ischemic Stroke. Transl. Stroke Res. 2020, 11, 108–121. [Google Scholar] [CrossRef]
- Gautier, S.; Ouk, T.; Tagzirt, M.; Lefebvre, C.; Laprais, M.; Pétrault, O.; Dupont, A.; Leys, D.; Bordet, R. Impact of the Neutrophil Response to Granulocyte Colony-Stimulating Factor on the Risk of Hemorrhage When Used in Combination with Tissue Plasminogen Activator during the Acute Phase of Experimental Stroke. J. Neuroinflamm. 2014, 11, 96. [Google Scholar] [CrossRef]
- Qu, J.; Jin, J.; Zhang, M.; Ng, L.G. Neutrophil Diversity and Plasticity: Implications for Organ Transplantation. Cell. Mol. Immunol. 2023, 20, 993–1001. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- Efendi, H. Clinically Isolated Syndromes: Clinical Characteristics, Differential Diagnosis, and Management. Noro Psikiyatr. Arsivi. 2015, 52, S1–S11. [Google Scholar] [CrossRef]
- Coyle, P.K. What Can We Learn from Sex Differences in MS? J. Pers. Med. 2021, 11, 1006. [Google Scholar] [CrossRef]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [CrossRef]
- Bisgaard, A.K.; Pihl-Jensen, G.; Frederiksen, J.L. The Neutrophil-to-Lymphocyte Ratio as Disease Actvity Marker in Multiple Sclerosis and Optic Neuritis. Mult. Scler. Relat. Disord. 2017, 18, 213–217. [Google Scholar] [CrossRef]
- Christy, A.L.; Walker, M.E.; Hessner, M.J.; Brown, M.A. Mast Cell Activation and Neutrophil Recruitment Promotes Early and Robust Inflammation in the Meninges in EAE. J. Autoimmun. 2013, 42, 50–61. [Google Scholar] [CrossRef]
- Wojkowska, D.W.; Szpakowski, P.; Ksiazek-Winiarek, D.; Leszczynski, M.; Glabinski, A. Interactions between Neutrophils, Th17 Cells, and Chemokines during the Initiation of Experimental Model of Multiple Sclerosis. Mediat. Inflamm. 2014, 2014, 590409. [Google Scholar] [CrossRef]
- Naegele, M.; Tillack, K.; Reinhardt, S.; Schippling, S.; Martin, R.; Sospedra, M. Neutrophils in Multiple Sclerosis Are Characterized by a Primed Phenotype. J. Neuroimmunol. 2012, 242, 60–71. [Google Scholar] [CrossRef]
- Hasselbalch, I.C.; Søndergaard, H.B.; Koch-Henriksen, N.; Olsson, A.; Ullum, H.; Sellebjerg, F.; Oturai, A.B. The Neutrophil-to-Lymphocyte Ratio Is Associated with Multiple Sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2018, 4, 2055217318813183. [Google Scholar] [CrossRef]
- Al-Hussain, F.; Alfallaj, M.M.; Alahmari, A.N.; Almazyad, A.N.; Alsaeed, T.K.; Abdurrahman, A.A.; Murtaza, G.; Bashir, S. Relationship between Neutrophiltolymphocyte Ratio and Stress in Multiple Sclerosis Patients. J. Clin. Diagn. Res. 2017, 11, CC01–CC04. [Google Scholar] [CrossRef]
- Huang, W.C.; Lin, H.C.; Yang, Y.H.; Hsu, C.W.; Chen, N.C.; Tsai, W.C.; Cheng, B.C.; Tsai, N.W. Neutrophil-to-Lymphocyte Ratio and Monocyte-to-Lymphocyte Ratio Are Associated with a 2-Year Relapse in Patients with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2022, 58, 103514. [Google Scholar] [CrossRef]
- D’amico, E.; Zanghì, A.; Romano, A.; Sciandra, M.; Palumbo, G.A.M.; Patti, F. The Neutrophil-to-Lymphocyte Ratio Is Related to Disease Activity in Relapsing Remitting Multiple Sclerosis. Cells 2019, 8, 1114. [Google Scholar] [CrossRef]
- Hemond, C.C.; Glanz, B.I.; Bakshi, R.; Chitnis, T.; Healy, B.C. The Neutrophil-to-Lymphocyte and Monocyte-to-Lymphocyte Ratios Are Independently Associated with Neurological Disability and Brain Atrophy in Multiple Sclerosis. BMC Neurol. 2019, 19, 23. [Google Scholar] [CrossRef]
- Guzel, I.; Mungan, S.; Oztekin, Z.N.; Ak, F. Is There an Association between the Expanded Disability Status Scale and Inflammatory Markers in Multiple Sclerosis? J. Chin. Med. Assoc. 2016, 79, 54–57. [Google Scholar] [CrossRef]
- Demirci, S.; Demirci, S.; Kutluhan, S.; Koyuncuoglu, H.R.; Yurekli, V.A. The Clinical Significance of the Neutrophil-to-Lymphocyte Ratio in Multiple Sclerosis. Int. J. Neurosci. 2016, 126, 700–706. [Google Scholar] [CrossRef]
- Kostic, M.; Dzopalic, T.; Zivanovic, S.; Zivkovic, N.; Cvetanovic, A.; Stojanovic, I.; Vojinovic, S.; Marjanovic, G.; Savic, V.; Colic, M. IL-17 and Glutamate Excitotoxicity in the Pathogenesis of Multiple Sclerosis. Scand. J. Immunol. 2014, 79, 181–186. [Google Scholar] [CrossRef]
- Rumble, J.M.; Huber, A.K.; Krishnamoorthy, G.; Srinivasan, A.; Giles, D.A.; Zhang, X.; Wang, L.; Segal, B.M. Neutrophil-Related Factors as Biomarkers in EAE and MS. J. Exp. Med. 2015, 212, 23–35. [Google Scholar] [CrossRef]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef]
- Wu, F.; Cao, W.; Yang, Y.; Liu, A. Extensive Infiltration of Neutrophils in the Acute Phase of Experimental Autoimmune Encephalomyelitis in C57BL/6 Mice. Histochem. Cell Biol. 2010, 133, 313–322. [Google Scholar] [CrossRef]
- Thiam, H.R.; Wong, S.L.; Wagner, D.D.; Waterman, C.M. Cellular Mechanisms of NETosis. Annu. Rev. Cell Dev. Biol. 2020, 36, 191–218. [Google Scholar] [CrossRef]
- Tillack, K.; Naegele, M.; Haueis, C.; Schippling, S.; Wandinger, K.P.; Martin, R.; Sospedra, M. Gender Differences in Circulating Levels of Neutrophil Extracellular Traps in Serum of Multiple Sclerosis Patients. J. Neuroimmunol. 2013, 261, 108–119. [Google Scholar] [CrossRef]
- Zhang, H.; Ray, A.; Miller, N.M.; Hartwig, D.; Pritchard, K.A.; Dittel, B.N. Inhibition of Myeloperoxidase at the Peak of Experimental Autoimmune Encephalomyelitis Restores Blood-Brain Barrier Integrity and Ameliorates Disease Severity. J. Neurochem. 2016, 136, 826–836. [Google Scholar] [CrossRef]
- Yu, G.; Zheng, S.; Zhang, H. Inhibition of Myeloperoxidase by N-Acetyl Lysyltyrosylcysteine Amide Reduces Experimental Autoimmune Encephalomyelitis-Induced Injury and Promotes Oligodendrocyte Regeneration and Neurogenesis in a Murine Model of Progressive Multiple Sclerosis. Neuroreport 2018, 29, 208–213. [Google Scholar] [CrossRef]
- Paré, A.; Mailhot, B.; Lévesque, S.A.; Lacroix, S. Involvement of the IL-1 System in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis: Breaking the Vicious Cycle between IL-1β and GM-CSF. Brain Behav. Immun. 2017, 62, 1–8. [Google Scholar] [CrossRef]
- Lin, C.-C.; Edelson, B.T. New Insights into the Role of IL-1β in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. J. Immunol. 2017, 198, 4553–4560. [Google Scholar] [CrossRef]
- Li, Q.; Powell, N.; Zhang, H.; Belevych, N.; Ching, S.; Chen, Q.; Sheridan, J.; Whitacre, C.; Quan, N. Endothelial IL-1R1 Is a Critical Mediator of EAE Pathogenesis. Brain Behav. Immun. 2011, 25, 160–167. [Google Scholar] [CrossRef]
- Monaghan, K.L.; Wan, E.C.K. The Role of Granulocyte-Macrophage Colony-Stimulating Factor in Murine Models of Multiple Sclerosis. Cells 2020, 9, 611. [Google Scholar] [CrossRef]
- Glennon-Alty, L.; Hackett, A.P.; Chapman, E.A.; Wright, H.L. Neutrophils and Redox Stress in the Pathogenesis of Autoimmune Disease. Free Radic. Biol. Med. 2018, 125, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, R.; Lu, H.; Butovsky, O.; Ohno, N.; Rietsch, A.M.; Cialic, R.; Wu, P.M.; Doykan, C.E.; Lin, J.; Cotleur, A.C.; et al. Differential Roles of Microglia and Monocytes in the Inflamed Central Nervous System. J. Exp. Med. 2014, 211, 1533–1549. [Google Scholar] [CrossRef] [PubMed]
- Aubé, B.; Lévesque, S.A.; Paré, A.; Chamma, É.; Kébir, H.; Gorina, R.; Lécuyer, M.-A.; Alvarez, J.I.; De Koninck, Y.; Engelhardt, B.; et al. Neutrophils Mediate Blood–Spinal Cord Barrier Disruption in Demyelinating Neuroinflammatory Diseases. J. Immunol. 2014, 193, 2438–2454. [Google Scholar] [CrossRef] [PubMed]
- Santos-Lima, B.; Pietronigro, E.C.; Terrabuio, E.; Zenaro, E.; Constantin, G. The Role of Neutrophils in the Dysfunction of Central Nervous System Barriers. Front. Aging Neurosci. 2022, 14, 965169. [Google Scholar] [CrossRef] [PubMed]
- Mirshafiey, A.; Asghari, B.; Ghalamfarsa, G.; Jadidi-Niaragh, F.; Azizi, G. The significance of matrix metalloproteinases in the immunopathogenesis and treatment of multiple sclerosis. Sultan Qaboos Univ. Med. J. 2014, 14, e13. [Google Scholar] [CrossRef]
- Agrawal, S.; Anderson, P.; Durbeej, M.; Van Rooijen, N.; Ivars, F.; Opdenakker, G.; Sorokin, L.M. Dystroglycan Is Selectively Cleaved at the Parenchymal Basement Membrane at Sites of Leukocyte Extravasation in Experimental Autoimmune Encephalomyelitis. J. Exp. Med. 2006, 203, 1007–1016. [Google Scholar] [CrossRef]
- Pun, P.B.L.; Lu, J.; Moochhala, S. Involvement of ROS in BBB Dysfunction. Free Radic. Res. 2009, 43, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Thompson, P.R. Protein Arginine Deiminases (PADs): Biochemistry and Chemical Biology of Protein Citrullination. Acc. Chem. Res. 2019, 52, 818–832. [Google Scholar] [CrossRef]
- Li, P.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y. PAD4 Is Essential for Antibacterial Innate Immunity Mediated by Neutrophil Extracellular Traps. J. Exp. Med. 2010, 207, 1853–1862. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, B.; Mittereder, N.; Chaerkady, R.; Strain, M.; An, L.L.; Rahman, S.; Ma, W.; Low, C.P.; Chan, D.; et al. Spontaneous Secretion of the Citrullination Enzyme PAD2 and Cell Surface Exposure of PAD4 by Neutrophils. Front. Immunol. 2017, 8, 1200. [Google Scholar] [CrossRef]
- Moscarello, M.A.; Lei, H.; Mastronardi, F.G.; Winer, S.; Tsui, H.; Li, Z.; Ackerley, C.; Zhang, L.; Raijmakers, R.; Wood, D.D. Inhibition of Peptidyl-Arginine Deiminases Reverses Protein- Hypercitrullination and Disease in Mouse Models of Multiple Sclerosis. DMM Dis. Models Mech. 2013, 6, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Bradford, C.M.; Ramos, I.; Cross, A.K.; Haddock, G.; McQuaid, S.; Nicholas, A.P.; Woodroofe, M.N. Localisation of Citrullinated Proteins in Normal Appearing White Matter and Lesions in the Central Nervous System in Multiple Sclerosis. J. Neuroimmunol. 2014, 273, 85–95. [Google Scholar] [CrossRef]
- Marro, B.S.; Grist, J.J.; Lane, T.E. Inducible Expression of CXCL1 within the Central Nervous System Amplifies Viral-Induced Demyelination. J. Immunol. 2016, 196, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Grist, J.J.; Marro, B.S.; Skinner, D.D.; Syage, A.R.; Worne, C.; Doty, D.J.; Fujinami, R.S.; Lane, T.E. Induced CNS Expression of CXCL1 Augments Neurologic Disease in a Murine Model of Multiple Sclerosis via Enhanced Neutrophil Recruitment. Eur. J. Immunol. 2018, 48, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Khaw, Y.M.; Cunningham, C.; Tierney, A.; Sivaguru, M.; Inoue, M. Neutrophil-Selective Deletion of Cxcr2 Protects against CNS Neurodegeneration in a Mouse Model of Multiple Sclerosis. J. Neuroinflamm. 2020, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Radsak, M.; Iking-Konert, C.; Stegmaier, S.; Andrassy, K.; Hänsch, G.M. Polymorphonuclear neutrophils as accessory cells for T-cell activation: Major histocompatibility complex class II restricted antigen-dependent induction of T-cell proliferation. Immunology 2000, 101, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Goverman, J. Autoimmune T Cell Responses in the Central Nervous System. Nat. Rev. Immunol. 2009, 9, 393–407. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Sansores-España, L.D.; Melgar-Rodríguez, S.; Vernal, R.; Carrillo-Ávila, B.A.; Martínez-Aguilar, V.M.; Díaz-Zúñiga, J. Neutrophil N1 and N2 Subsets and Their Possible Association with Periodontitis: A Scoping Review. Int. J. Mol. Sci. 2022, 23, 12068. [Google Scholar] [CrossRef] [PubMed]
- Woodfin, A.; Voisin, M.B.; Beyrau, M.; Colom, B.; Caille, D.; Diapouli, F.M.; Nash, G.B.; Chavakis, T.; Albelda, S.M.; Rainger, G.E.; et al. The Junctional Adhesion Molecule JAM-C Regulates Polarized Transendothelial Migration of Neutrophils in Vivo. Nat. Immunol. 2011, 12, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zeng, Y.; Fan, Y.; Wu, J.; Mulatibieke, T.; Ni, J.; Yu, G.; Wan, R.; Wang, X.; Hu, G. Reverse-Migrated Neutrophils Regulated by JAM-C Are Involved in Acute Pancreatitis-Associated Lung Injury. Sci. Rep. 2016, 6, 20545. [Google Scholar] [CrossRef]
- Luna, G.; Alping, P.; Burman, J.; Fink, K.; Fogdell-Hahn, A.; Gunnarsson, M.; Hillert, J.; Langer-Gould, A.; Lycke, J.; Nilsson, P.; et al. Infection Risks among Patients with Multiple Sclerosis Treated with Fingolimod, Natalizumab, Rituximab, and Injectable Therapies. JAMA Neurol. 2020, 77, 184–191. [Google Scholar] [CrossRef]
- Scutera, S.; Musso, T.; Cavalla, P.; Piersigilli, G.; Sparti, R.; Comini, S.; Vercellino, M.; Cuffini, A.M.; Banche, G.; Allizond, V. Inhibition of Human Neutrophil Functions in Vitro by Multiple Sclerosis Disease-Modifying Therapies. J. Clin. Med. 2020, 9, 3542. [Google Scholar] [CrossRef] [PubMed]
- Torgauten, H.M.; Myhr, K.M.; Wergeland, S.; Bø, L.; Aarseth, J.H.; Torkildsen, Ø. Safety and Efficacy of Rituximab as First- and Second Line Treatment in Multiple Sclerosis—A Cohort Study. Mult. Scler. J. Exp. Transl. Clin. 2021, 7, 2055217320973049. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Zielman, R.; Das Gupta, A.; Xi, J.; Stoneman, D.; Karlsson, G.; Robertson, D.; Cohen, J.A.; Kappos, L. Efficacy and Safety of Four-Year Ofatumumab Treatment in Relapsing Multiple Sclerosis: The ALITHIOS Open-Label Extension. Mult. Scler. J. 2023, 29, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics—2023 Update: A Report from the American Heart Association. Circulation 2023, 147, E93–E621. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, Á.; Meisel, A.; Planas, A.M.; Urra, X.; Van De Beek, D.; Veltkamp, R. The Immunology of Acute Stroke. Nat. Rev. Neurol. 2012, 8, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Anrather, J. The Immunology of Stroke: From Mechanisms to Translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Iadecola, C. Brain-immune interactions and ischemic stroke: Clinical implications. Arch. Neurol. 2012, 69, 576–581. [Google Scholar] [CrossRef]
- Wu, D.-M.; Liu, J.-P.; Liu, J.; Ge, W.-H.; Wu, S.-Z.; Zeng, C.-J.; Liang, J.; Liu, K.; Lin, Q.; Hong, X.-W.; et al. Immune Pathway Activation in Neurons Triggers Neural Damage after Stroke. Cell Rep. 2023, 42, 113368. [Google Scholar] [CrossRef] [PubMed]
- Buck, B.H.; Liebeskind, D.S.; Saver, J.L.; Bang, O.Y.; Yun, S.W.; Starkman, S.; Ali, L.K.; Kim, D.; Villablanca, J.P.; Salamon, N.; et al. Early Neutrophilia Is Associated with Volume of Ischemic Tissue in Acute Stroke. Stroke 2008, 39, 355–360. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, E364–E467. [Google Scholar] [CrossRef]
- Strong, K.; Mathers, C.; Bonita, R. Preventing stroke: Saving lives around the world. Lancet Neurol. 2007, 6, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; Khatri, P. Stroke. Lancet 2020, 396, 129–142. [Google Scholar] [CrossRef]
- Hendrix, P.; Sofoluke, N.; Adams, M.D.; Kunaprayoon, S.; Zand, R.; Kolinovsky, A.N.; Person, T.N.; Gupta, M.; Goren, O.; Schirmer, C.M.; et al. Risk Factors for Acute Ischemic Stroke Caused by Anterior Large Vessel Occlusion. Stroke 2019, 50, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Norrving, B.; Mensah, G.A. Global Burden of Stroke. Circ. Res. 2017, 120, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Nuñez, G. Sterile Inflammation: Sensing and Reacting to Damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, Á.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in Acute Stroke: Targeting Excitotoxicity, Oxidative and Nitrosative Stress, and Inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tang, X.N.; Yenari, M.A. The Inflammatory Response in Stroke. J. Neuroimmunol. 2007, 184, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Ruhnau, J.; Schulze, J.; Dressel, A.; Vogelgesang, A. Thrombosis, Neuroinflammation, and Poststroke Infection: The Multifaceted Role of Neutrophils in Stroke. J. Immunol. Res. 2017, 2017, 5140679. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.X.; Kim, H.A.; Lee, S.; Moore, J.P.; Chan, C.T.; Vinh, A.; Gelderblom, M.; Arumugam, T.V.; Broughton, B.R.; Drummond, G.R.; et al. Immune Cell Infiltration in Malignant Middle Cerebral Artery Infarction: Comparison with Transient Cerebral Ischemia. J. Cereb. Blood Flow Metab. 2014, 34, 450–459. [Google Scholar] [CrossRef]
- Allen, C.; Thornton, P.; Denes, A.; McColl, B.W.; Pierozynski, A.; Monestier, M.; Pinteaux, E.; Rothwell, N.J.; Allan, S.M. Neutrophil Cerebrovascular Transmigration Triggers Rapid Neurotoxicity through Release of Proteases Associated with Decondensed DNA. J. Immunol. 2012, 189, 381–392. [Google Scholar] [CrossRef]
- Stowe, A.M.; Adair-Kirk, T.L.; Gonzales, E.R.; Perez, R.S.; Shah, A.R.; Park, T.S.; Gidday, J.M. Neutrophil Elastase and Neurovascular Injury Following Focal Stroke and Reperfusion. Neurobiol. Dis. 2009, 35, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Del Zoppo, G.J.; Mabuchi, T. Cerebral Microvessel Responses to Focal Ischemia. J. Cereb. Blood Flow Metab. 2003, 23, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Del Zoppo, G.J.; Schmid-Schonbein, G.W.; Mori, E.; Copeland, B.R.; Chang, C.-M. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke 1991, 22, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Rosell, A.; Cuadrado, E.; Ortega-Aznar, A.; Hernández-Guillamon, M.; Lo, E.H.; Montaner, J. MMP-9-Positive Neutrophil Infiltration Is Associated to Blood-Brain Barrier Breakdown and Basal Lamina Type IV Collagen Degradation during Hemorrhagic Transformation after Human Ischemic Stroke. Stroke 2008, 39, 1121–1126. [Google Scholar] [CrossRef]
- Kim, J.; Song, T.J.; Park, J.H.; Lee, H.S.; Nam, C.M.; Nam, H.S.; Kim, Y.D.; Heo, J.H. Different Prognostic Value of White Blood Cell Subtypes in Patients with Acute Cerebral Infarction. Atherosclerosis 2012, 222, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, J.; Chang, J.Y.; Kim, S.H.; Lee, J.E. Inflammation after Ischemic Stroke: The Role of Leukocytes and Glial Cells. Exp. Neurobiol. 2016, 25, 241–251. [Google Scholar] [CrossRef]
- Jickling, G.C.; Liu, D.Z.; Ander, B.P.; Stamova, B.; Zhan, X.; Sharp, F.R. Targeting Neutrophils in Ischemic Stroke: Translational Insights from Experimental Studies. J. Cereb. Blood Flow Metab. 2015, 35, 888–901. [Google Scholar] [CrossRef]
- Cuartero, M.I.; Ballesteros, I.; Moraga, A.; Nombela, F.; Vivancos, J.; Hamilton, J.A.; Corbí, Á.L.; Lizasoain, I.; Moro, M.A. N2 Neutrophils, Novel Players in Brain Inflammation after Stroke: Modulation by the Pparγ Agonist Rosiglitazone. Stroke 2013, 44, 3498–3508. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Albelda, S.M. Tumor-Associated Neutrophils: Friend or Foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the Activation and Regulation of Innate and Adaptive Immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Jaillon, S.; Galdiero, M.R.; Del Prete, D.; Cassatella, M.A.; Garlanda, C.; Mantovani, A. Neutrophils in Innate and Adaptive Immunity. Semin. Immunopathol. 2013, 35, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Müller, I.; Munder, M.; Kropf, P.; Hänsch, G.M. Polymorphonuclear Neutrophils and T Lymphocytes: Strange Bedfellows or Brothers in Arms? Trends Immunol. 2009, 30, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell Biology of Ischemia/Reperfusion Injury. In International Review of Cell and Molecular Biology; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 298, pp. 229–317. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, H.; Gao, C. The Role of Neutrophil Extracellular Traps in Central Nervous System Diseases and Prospects for Clinical Application. Oxidative Med. Cell. Longev. 2021, 2021, 9931742. [Google Scholar] [CrossRef] [PubMed]
- Kollikowski, A.M.; Schuhmann, M.K.; Nieswandt, B.; Müllges, W.; Stoll, G.; Pham, M. Local Leukocyte Invasion during Hyperacute Human Ischemic Stroke. Ann. Neurol. 2020, 87, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Perez-de-Puig, I.; Miró-Mur, F.; Ferrer-Ferrer, M.; Gelpi, E.; Pedragosa, J.; Justicia, C.; Urra, X.; Chamorro, A.; Planas, A.M. Neutrophil Recruitment to the Brain in Mouse and Human Ischemic Stroke. Acta Neuropathol. 2015, 129, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Datsi, A.; Piotrowski, L.; Markou, M.; Köster, T.; Kohtz, I.; Lang, K.; Plöttner, S.; Käfferlein, H.U.; Pleger, B.; Martinez, R.; et al. Stroke-Derived Neutrophils Demonstrate Higher Formation Potential and Impaired Resolution of CD66b + Driven Neutrophil Extracellular Traps. BMC Neurol. 2022, 22, 186. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhao, X.; Li, J.; Liu, C.; Li, W.; Zhao, J.; Li, Z.; Wang, N.; Wang, F.; Dong, J.; et al. Neutrophil Extracellular Traps Mediated by Platelet Microvesicles Promote Thrombosis and Brain Injury in Acute Ischemic Stroke. Cell Commun. Signal. 2024, 22, 50. [Google Scholar] [CrossRef] [PubMed]
- Amantea, D.; Micieli, G.; Tassorelli, C.; Cuartero, M.I.; Ballesteros, I.; Certo, M.; Moro, M.A.; Lizasoain, I.; Bagetta, G. Rational Modulation of the Innate Immune System for Neuroprotection in Ischemic Stroke. Front. Neurosci. 2015, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Weisenburger-Lile, D.; Dong, Y.; Yger, M.; Weisenburger, G.; Polara, G.F.; Chaigneau, T.; Ochoa, R.Z.; Marro, B.; Lapergue, B.; Alamowitch, S.; et al. Harmful Neutrophil Subsets in Patients with Ischemic Stroke: Association with Disease Severity. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e571. [Google Scholar] [CrossRef]
- Colom, B.; Bodkin, J.V.; Beyrau, M.; Woodfin, A.; Ody, C.; Rourke, C.; Chavakis, T.; Brohi, K.; Imhof, B.A.; Nourshargh, S. Leukotriene B4-Neutrophil Elastase Axis Drives Neutrophil Reverse Transendothelial Cell Migration In Vivo. Immunity 2015, 42, 1075–1086. [Google Scholar] [CrossRef]
- Greenlee-Wacker, M.C. Clearance of Apoptotic Neutrophils and Resolution of Inflammation. Immunol. Rev. 2016, 273, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Pillay, J.; Kamp, V.M.; Van Hoffen, E.; Visser, T.; Tak, T.; Lammers, J.W.; Ulfman, L.H.; Leenen, L.P.; Pickkers, P.; Koenderman, L. A Subset of Neutrophils in Human Systemic Inflammation Inhibits T Cell Responses through Mac-1. J. Clin. Investig. 2012, 122, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Sauce, D.; Dong, Y.; Campillo-Gimenez, L.; Casulli, S.; Bayard, C.; Autran, B.; Boddaert, J.; Appay, V.; Elbim, C. Reduced Oxidative Burst by Primed Neutrophils in the Elderly Individuals Is Associated with Increased Levels of the CD16bright/CD62Ldim Immunosuppressive Subset. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019, 40, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Metzler, K.D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayannopoulos, V. Myeloperoxidase-Containing Complex Regulates Neutrophil Elastase Release and Actin Dynamics during NETosis. Cell Rep. 2014, 8, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Zychlinsky, A. Neutrophil Extracellular Traps: Is Immunity the Second Function of Chromatin? J. Cell Biol. 2012, 198, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Stakos, D.A.; Kambas, K.; Konstantinidis, T.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Tsironidou, V.; Giatromanolaki, A.; Skendros, P.; Konstantinides, S.; et al. Expression of Functional Tissue Factor by Neutrophil Extracellular Traps in Culprit Artery of Acute Myocardial Infarction. Eur. Heart J. 2015, 36, 1405–1414. [Google Scholar] [CrossRef]
- Mołek, P.; Ząbczyk, M.; Malinowski, K.P.; Natorska, J.; Undas, A. Markers of NET formation and stroke risk in patients with atrial fibrillation: Association with a prothrombotic state. Thromb. Res. 2022, 213, 1–7. [Google Scholar] [CrossRef]
- Staessens, S.; Denorme, F.; François, O.; Desender, L.; Dewaele, T.; Vanacker, P.; Deckmyn, H.; Vanhoorelbeke, K.; Andersson, T.; De Meyer, S.F. Structural Analysis of Ischemic Stroke Thrombi: Histological Indications for Therapy Resistance. Haematologica 2020, 105, 498–507. [Google Scholar] [CrossRef]
- Laridan, E.; Denorme, F.; Desender, L.; François, O.; Andersson, T.; Deckmyn, H.; Vanhoorelbeke, K.; De Meyer, S.F. Neutrophil Extracellular Traps in Ischemic Stroke Thrombi. Ann. Neurol. 2017, 82, 223–232. [Google Scholar] [CrossRef]
- Vallés, J.; Lago, A.; Santos, M.T.; Latorre, A.M.; Tembl, J.I.; Salom, J.B.; Nieves, C.; Moscardó, A. Neutrophil Extracellular Traps Are Increased in Patients with Acute Ischemic Stroke: Prognostic Significance. Thromb. Haemost. 2017, 117, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, M.; Hosseini, E. Platelet-Leukocyte Crosstalk: Linking Proinflammatory Responses to Procoagulant State. Thromb. Res. 2013, 131, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Etulain, J.; Martinod, K.; Wong, S.L.; Cifuni, S.M.; Schattner, M.; Wagner, D.D. P-Selectin Promotes Neutrophil Extracellular Trap Formation in Mice. Blood 2015, 126, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Frenette, P.S.; Denis, C.V.; Weiss, L.; Jurk, K.; Subbarao, S.; Kehrel, B.; Hartwig, J.H.; Vestweber, D.; Wagner, D.D. P-Selectin Glycoprotein Ligand 1 (PSGL-1) Is Expressed on Platelets and Can Mediate Platelet-Endothelial Interactions In Vivo. J. Exp. Med. 2000, 191, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.W.; Brown, C.A.; Valladolid, C.; Emebo, D.C.; Palzkill, T.G.; Cruz, M.A. The Vimentin Rod Domain Blocks P-Selectin- P-Selectin Glycoprotein Ligand 1 Interactions to Attenuate Leukocyte Adhesion to Inflamed Endothelium. PLoS ONE 2020, 15, e0240164. [Google Scholar] [CrossRef] [PubMed]
- Sreeramkumar, V.; Adrover, J.M.; Ballesteros, I.; Cuartero, M.I.; Rossaint, J.; Bilbao, I.; Nácher, M.; Pitaval, C.; Radovanovic, I.; Fukui, Y.; et al. Neutrophils Scan for Activated Platelets to Initiate Inflammation. Science 2014, 346, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, T.; Jin, J.; Liu, Y.; Li, B.; Sun, Q.; Tian, J.; Zhao, H.; Liu, Z.; Ma, S.; et al. Interactions between Neutrophil Extracellular Traps and Activated Platelets Enhance Procoagulant Activity in Acute Stroke Patients with ICA Occlusion. EBioMedicine 2020, 53, 102671. [Google Scholar] [CrossRef] [PubMed]
- Mangold, A.; Alias, S.; Scherz, T.; Hofbauer, T.; Jakowitsch, J.; Panzenböck, A.; Simon, D.; Laimer, D.; Bangert, C.; Kammerlander, A.; et al. Coronary Neutrophil Extracellular Trap Burden and Deoxyribonuclease Activity in ST-Elevation Acute Coronary Syndrome Are Predictors of ST-Segment Resolution and Infarct Size. Circ. Res. 2015, 116, 1182–1192. [Google Scholar] [CrossRef]
- Ducroux, C.; Di Meglio, L.; Loyau, S.; Delbosc, S.; Boisseau, W.; Deschildre, C.; Ben Maacha, M.; Blanc, R.; Redjem, H.; Ciccio, G.; et al. Thrombus Neutrophil Extracellular Traps Content Impair TPA-Induced Thrombolysis in Acute Ischemic Stroke. Stroke 2018, 49, 754–757. [Google Scholar] [CrossRef]
- Novotny, J.; Oberdieck, P.; Titova, A.; Pelisek, J.; Chandraratne, S.; Nicol, P.; Hapfelmeier, A.; Joner, M.; Maegdefessel, L.; Poppert, H.; et al. Thrombus NET Content Is Associated with Clinical Outcome in Stroke and Myocardial Infarction. Neurology 2020, 94, E2346–E2360. [Google Scholar] [CrossRef]
- Peña-Martínez, C.; Durán-Laforet, V.; García-Culebras, A.; Ostos, F.; Hernández-Jiménez, M.; Bravo-Ferrer, I.; Pérez-Ruiz, A.; Ballenilla, F.; Díaz-Guzmán, J.; Pradillo, J.M.; et al. Pharmacological Modulation of Neutrophil Extracellular Traps Reverses Thrombotic Stroke TPA (Tissue-Type Plasminogen Activator) Resistance. Stroke 2019, 50, 3228–3237. [Google Scholar] [CrossRef] [PubMed]
- Jabrah, D.; Rossi, R.; Molina, S.; Douglas, A.; Pandit, A.; McCarthy, R.; Gilvarry, M.; Ceder, E.; Fitzgerald, S.; Dunker, D.; et al. White Blood Cell Subtypes and Neutrophil Extracellular Traps Content as Biomarkers for Stroke Etiology in Acute Ischemic Stroke Clots Retrieved by Mechanical Thrombectomy. Thromb. Res. 2024, 234, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Essig, F.; Kollikowski, A.M.; Pham, M.; Solymosi, L.; Stoll, G.; Haeusler, K.G.; Kraft, P.; Schuhmann, M.K. Immunohistological Analysis of Neutrophils and Neutrophil Extracellular Traps in Human Thrombemboli Causing Acute Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7387. [Google Scholar] [CrossRef]
- Genchi, A.; Semerano, A.; Gullotta, G.S.; Strambo, D.; Schwarz, G.; Bergamaschi, A.; Panni, P.; Simionato, F.; Scomazzoni, F.; Michelozzi, C.; et al. Cerebral Thrombi of Cardioembolic Etiology Have an Increased Content of Neutrophil Extracellular Traps. J. Neurol. Sci. 2021, 423, 117355. [Google Scholar] [CrossRef]
- Langen, U.H.; Ayloo, S.; Gu, C. Development and Cell Biology of the Blood-Brain Barrier. Annu. Rev. Cell Dev. Biol. 2019, 35, 591–613. [Google Scholar] [CrossRef]
- Rossi, B.; Santos-Lima, B.; Terrabuio, E.; Zenaro, E.; Constantin, G. Common Peripheral Immunity Mechanisms in Multiple Sclerosis and Alzheimer’s Disease. Front. Immunol. 2021, 12, 639369. [Google Scholar] [CrossRef]
- Rossi, B.; Constantin, G.; Zenaro, E. The Emerging Role of Neutrophils in Neurodegeneration. Immunobiology 2020, 225, 151865. [Google Scholar] [CrossRef]
- Üllen, A.; Singewald, E.; Konya, V.; Fauler, G.; Reicher, H.; Nusshold, C.; Hammer, A.; Kratky, D.; Heinemann, A.; Holzer, P.; et al. Myeloperoxidase-Derived Oxidants Induce Blood-Brain Barrier Dysfunction In Vitro and In Vivo. PLoS ONE 2013, 8, e64034. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.J.; Sharp, F.R. Implications of MMP9 for Blood Brain Barrier Disruption and Hemorrhagic Transformation Following Ischemic Stroke. Front. Cell. Neurosci. 2016, 10, 56. [Google Scholar] [CrossRef]
- Reinhard, S.M.; Razak, K.; Ethell, I.M. A Delicate Balance: Role of MMP-9 in Brain Development and Pathophysiology of Neurodevelopmental Disorders. Front. Cell. Neurosci. 2015, 9, 280. [Google Scholar] [CrossRef]
- Akol, I.; Kalogeraki, E.; Pielecka-Fortuna, J.; Fricke, M.; Löwel, S. MMP2 and MMP9 Activity Is Crucial for Adult Visual Cortex Plasticity in Healthy and Stroke-Affected Mice. J. Neurosci. 2022, 42, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Uhl, B.; Vadlau, Y.; Zuchtriegel, G.; Nekolla, K.; Sharaf, K.; Gaertner, F.; Massberg, S.; Krombach, F.; Reichel, C.A. Aged Neutrophils Contribute to the First Line of Defense in the Acute Inflammatory Response. Blood 2016, 128, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, E.; Yalavarthi, S.; Berthier, C.C.; Hodgin, J.B.; Khandpur, R.; Lin, A.M.; Rubin, C.J.; Zhao, W.; Olsen, S.H.; Klinker, M.; et al. Netting Neutrophils Induce Endothelial Damage, Infiltrate Tissues, and Expose Immunostimulatory Molecules in Systemic Lupus Erythematosus. J. Immunol. 2011, 187, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Villalba, N.; Baby, S.; Cha, B.J.; Yuan, S.Y. Site-Specific Opening of the Blood-Brain Barrier by Extracellular Histones. J. Neuroinflamm. 2020, 17, 281. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Yu, H.; Yang, X.; Zhu, Y.; Bai, X.; Wang, R.; Cao, Y.; Xu, H.; Luo, H.; Lu, L.; et al. Neutrophil Extracellular Traps Released by Neutrophils Impair Revascularization and Vascular Remodeling after Stroke. Nat. Commun. 2020, 11, 2488. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Cui, Z.Y.; Huang, X.F.; Zhang, D.D.; Guo, R.J.; Han, M. Inflammation and Atherosclerosis: Signaling Pathways and Therapeutic Intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Horne, B.D.; Anderson, J.L.; John, J.M.; Weaver, A.; Bair, T.L.; Jensen, K.R.; Renlund, D.G.; Muhlestein, J.B. Which White Blood Cell Subtypes Predict Increased Cardiovascular Risk? J. Am. Coll. Cardiol. 2005, 45, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Afari, M.E.; Bhat, T. Neutrophil to Lymphocyte Ratio (NLR) and Cardiovascular Diseases: An Update. Expert Rev. Cardiovasc. Ther. 2016, 14, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Diem, S.; Schmid, S.; Krapf, M.; Flatz, L.; Born, D.; Jochum, W.; Templeton, A.J.; Früh, M. Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) as Prognostic Markers in Patients with Non-Small Cell Lung Cancer (NSCLC) Treated with Nivolumab. Lung Cancer 2017, 111, 176–181. [Google Scholar] [CrossRef]
- Adane, T.; Melku, M.; Worku, Y.B.; Fasil, A.; Aynalem, M.; Kelem, A.; Getawa, S. The Association between Neutrophil-to-Lymphocyte Ratio and Glycemic Control in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2023, 2023, 3117396. [Google Scholar] [CrossRef]
- Petrone, A.B.; Eisenman, R.D.; Steele, K.N.; Mosmiller, L.T.; Urhie, O.; Zdilla, M.J. Temporal Dynamics of Peripheral Neutrophil and Lymphocytes Following Acute Ischemic Stroke. Neurol. Sci. 2019, 40, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, Q.; Wang, C.; Wu, S.; Deng, L.; Li, Y.; Zheng, L.; Liu, M. Neutrophil to Lymphocyte Ratio Predicts Poor Outcomes after Acute Ischemic Stroke: A Cohort Study and Systematic Review. J. Neurol. Sci. 2019, 406, 116445. [Google Scholar] [CrossRef] [PubMed]
- Tokgoz, S.; Keskin, S.; Kayrak, M.; Seyithanoglu, A.; Ogmegul, A. Is Neutrophil/Lymphocyte Ratio Predict to Short-Term Mortality in Acute Cerebral Infarct Independently from Infarct Volume? J. Stroke Cerebrovasc. Dis. 2014, 23, 2163–2168. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, S.; Cagnetti, C.; Provinciali, L.; Silvestrini, M. Neutrophil-to-Lymphocyte Ratio and Neurological Deterioration Following Acute Cerebral Hemorrhage. Oncotarget 2017, 8, 57489–57494. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, L.; Jiang, T.-T.; Xia, J.-J.; Xu, F.; Shen, L.-J.; Kang, W.-H.; Ding, Y.; Mei, L.-X.; Ju, X.-F.; et al. Neutrophil-to-Lymphocyte Ratio Is an Independent Predictor of 30-Day Mortality of Intracerebral Hemorrhage Patients: A Validation Cohort Study. Neurotox. Res. 2018, 34, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cai, L.; Yi, T.; Yi, X.; Hu, Y. Neutrophil-to-Lymphocyte Ratio Is Associated with Stroke Progression and Functional Outcome in Patients with Ischemic Stroke. Brain Behav. 2023, 13, e3261. [Google Scholar] [CrossRef] [PubMed]
- Ertaş, G.; Sönmez, O.; Turfan, M.; Kul, Ş.; Erdoǧan, E.; Tasal, A.; Bacaksiz, A.; Vatankulu, M.A.; Altintaş, Ö.; Uyarel, H.; et al. Neutrophil/Lymphocyte Ratio Is Associated with Thromboembolic Stroke in Patients with Non-Valvular Atrial Fibrillation. J. Neurol. Sci. 2013, 324, 49–52. [Google Scholar] [CrossRef]
- Kocaturk, O.; Besli, F.; Gungoren, F.; Kocaturk, M.; Tanriverdi, Z. The Relationship among Neutrophil to Lymphocyte Ratio, Stroke Territory, and 3-Month Mortality in Patients with Acute Ischemic Stroke. Neurol. Sci. 2019, 40, 139–146. [Google Scholar] [CrossRef]
- Ying, A.N.; Cheng, Y.N.; Lin, Y.Y.; Yu, J.R.; Wu, X.Y.; Lin, Y.S. Dynamic Increase in Neutrophil Levels Predicts Parenchymal Hemorrhage and Function Outcome of Ischemic Stroke with R-TPA Thrombolysis. Neurol. Sci. 2020, 41, 2215–2223. [Google Scholar] [CrossRef]
- Song, S.Y.; Zhao, X.X.; Rajah, G.; Hua, C.; Kang, R.J.; Han, Y.P.; Ding, Y.C.; Meng, R. Clinical Significance of Baseline Neutrophil-to-Lymphocyte Ratio in Patients with Ischemic Stroke or Hemorrhagic Stroke: An Updated Meta-Analysis. Front. Neurol. 2019, 10, 1032. [Google Scholar] [CrossRef]
- Shekhar, S.; Cunningham, M.W.; Pabbidi, M.R.; Wang, S.; Booz, G.W.; Fan, F. Targeting Vascular Inflammation in Ischemic Stroke: Recent Developments on Novel Immunomodulatory Approaches. Eur. J. Pharmacol. 2018, 833, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.A.; Pandey, A.S.; Thompson, B.G.; Keep, R.F.; Hua, Y.; Xi, G. Injury Mechanisms in Acute Intracerebral Hemorrhage. Neuropharmacology 2018, 134, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Preclinical and Clinical Research on Inflammation after Intracerebral Hemorrhage. Prog. Neurobiol. 2010, 92, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Lux, D.; Alakbarzade, V.; Bridge, L.; Clark, C.N.; Clarke, B.; Zhang, L.; Khan, U.; Pereira, A.C. The Association of Neutrophil-Lymphocyte Ratio and Lymphocyte-Monocyte Ratio with 3-Month Clinical Outcome after Mechanical Thrombectomy Following Stroke. J. Neuroinflamm. 2020, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Peng, H.; You, S.; Liu, Y.; Xu, J.; Xu, Y.; Liu, H.; Shi, R.; Cao, Y.; Liu, C.F. Increase in Neutrophils after Recombinant Tissue Plasminogen Activator Thrombolysis Predicts Poor Functional Outcome of Ischaemic Stroke: A Longitudinal Study. Eur. J. Neurol. 2018, 25, 687.e45. [Google Scholar] [CrossRef]
- Zhong, C.; Zhu, Z.; Wang, A.; Xu, T.; Bu, X.; Peng, H.; Yang, J.; Han, L.; Chen, J.; Xu, T.; et al. Multiple Biomarkers Covering Distinct Pathways for Predicting Outcomes after Ischemic Stroke. Neurology 2019, 92, E295–E304. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, R.B.; Collard, C.D.; Gelman, S. Clinical Concepts and Commentary Pathophysiology, Clinical Manifestations, and Prevention of Ischemia-Reperfusion Injury. J. Am. Soc. Anesthesiol. 2001, 94, 1133–1138. Available online: www.anesthesiology.org (accessed on 22 April 2024).
- Ohab, J.J.; Fleming, S.; Blesch, A.; Carmichael, S.T. A Neurovascular Niche for Neurogenesis after Stroke. J. Neurosci. 2006, 26, 13007–13016. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiong, X.; Zhang, L.; Shen, J. Neurovascular Unit: A Critical Role in Ischemic Stroke. CNS Neurosci. Ther. 2021, 27, 7–16. [Google Scholar] [CrossRef]
- Pan, J.; Konstas, A.A.; Bateman, B.; Ortolano, G.A.; Pile-Spellman, J. Reperfusion Injury Following Cerebral Ischemia: Pathophysiology, MR Imaging, and Potential Therapies. Neuroradiology 2007, 49, 93–102. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Q.; Meng, H.; Duan, H.; Liu, X.; Wu, J.; Gao, F.; Wang, S.; Tan, R.; Yuan, J. Ischemia-Reperfusion Injury: Molecular Mechanisms and Therapeutic Targets. Signal Transduct. Target. Ther. 2024, 9, 12. [Google Scholar] [CrossRef]
- Powers, W.J.; Derdeyn, C.P.; Biller, J.; Coffey, C.S.; Hoh, B.L.; Jauch, E.C.; Johnston, K.C.; Johnston, S.C.; Khalessi, A.A.; Kidwell, C.S.; et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients with Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2015, 46, 3020–3035. [Google Scholar] [CrossRef] [PubMed]
- IST-3 Collaborative Group. The Benefi Ts and Harms of Intravenous Thrombolysis with Recombinant Tissue Plasminogen Activator within 6 h of Acute Ischaemic Stroke (the Third International Stroke Trial [IST-3]): A Randomised Controlled Trial. Lancet 2012, 379, 2352–2363. [Google Scholar] [CrossRef]
- Abbasi, M.; Arturo Larco, J.; Mereuta, M.O.; Liu, Y.; Fitzgerald, S.; Dai, D.; Kadirvel, R.; Savastano, L.; Kallmes, D.F.; Brinjikji, W. Diverse Thrombus Composition in Thrombectomy Stroke Patients with Longer Time to Recanalization. Thromb. Res. 2022, 209, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Jickling, G.C.; Liu, D.; Stamova, B.; Ander, B.P.; Zhan, X.; Lu, A.; Sharp, F.R. Hemorrhagic Transformation after Ischemic Stroke in Animals and Humans. J. Cereb. Blood Flow Metab. 2014, 34, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Watson, B.D.; Prado, R.; Veloso, A.; Brunschwig, J.P.; Dietrich, W.D. Cerebral Blood Flow Restoration and Reperfusion Injury after Ultraviolet Laser-Facilitated Middle Cerebral Artery Recanalization in Rat Thrombotic Stroke. Stroke 2002, 33, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Vandelanotte, S.; François, O.; Desender, L.; Staessens, S.; Vanhoorne, A.; Van Gool, F.; Tersteeg, C.; Vanhoorelbeke, K.; Vanacker, P.; Andersson, T.; et al. R-tPA Resistance Is Specific for Platelet-Rich Stroke Thrombi and Can Be Overcome by Targeting Nonfibrin Components. Stroke 2024, 55, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Desilles, J.P.; Di Meglio, L.; Delvoye, F.; Maïer, B.; Piotin, M.; Ho-Tin-Noé, B.; Mazighi, M. Composition and Organization of Acute Ischemic Stroke Thrombus: A Wealth of Information for Future Thrombolytic Strategies. Front. Neurol. 2022, 13, 870331. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; An, X.; Kong, Y.; Chen, Z. Predictive Model for the Risk of Hemorrhagic Transformation after Rt-PA Intravenous Thrombolysis in Patients with Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. Clin. Neurol. Neurosurg. 2024, 239, 108225. [Google Scholar] [CrossRef]
- Teekaput, C.; Thiankhaw, K.; Tanprawate, S.; Teekaput, K.; Chai-Adisaksopha, C. Outcomes of Asymptomatic Recombinant Tissue Plasminogen Activator Associated Intracranial Hemorrhage. PLoS ONE 2022, 17, e0272257. [Google Scholar] [CrossRef] [PubMed]
- Bagoly, Z. Hemorrhagic transformation after acute ischemic stroke thrombolysis treatment: Navigating the landscape of hemostasis genetic risk factors. J. Thromb. Haemost. 2024, 22, 919–921. [Google Scholar] [CrossRef]
- Yepes, M.; Sandkvist, M.; Moore, E.G.; Bugge, T.H.; Strickland, D.K.; Lawrence, D.A. Tissue-Type Plasminogen Activator Induces Opening of the Blood-Brain Barrier via the LDL Receptor-Related Protein. J. Clin. Investig. 2003, 112, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nagai, N.; Umemura, K. A Review of the Mechanisms of Blood-Brain Barrier Permeability by Tissue-Type Plasminogen Activator Treatment for Cerebral Ischemia. Front. Cell. Neurosci. 2016, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Zou, M.; Jia, D.M.; Shi, S.; Yang, X.; Liu, Q.; Dong, J.F.; Sheth, K.N.; Wang, X.; Shi, F.D. TPA Mobilizes Immune Cells That Exacerbate Hemorrhagic Transformation in Stroke. Circ. Res. 2021, 128, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Uhl, B.; Zuchtriegel, G.; Puhr-Westerheide, D.; Praetner, M.; Rehberg, M.; Fabritius, M.; Hessenauer, M.; Holzer, M.; Khandoga, A.; Fürst, R.; et al. Tissue Plasminogen Activator Promotes Postischemic Neutrophil Recruitment via Its Proteolytic and Nonproteolytic Properties. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Maestrini, I.; Strbian, D.; Gautier, S.; Haapaniemi, E.; Moulin, S.; Sairanen, T.; Dequatre-Ponchelle, N.; Sibolt, G.; Cordonnier, C.; Melkas, S.; et al. Higher Neutrophil Counts before Thrombolysis for Cerebral Ischemia Predict Worse Outcomes. Neurology 2015, 85, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.N.; Tong, M.S.; Sung, P.H.; Chen, Y.L.; Chen, C.H.; Tsai, N.W.; Huang, C.J.; Chang, Y.T.; Chen, S.F.; Chang, W.N.; et al. Higher Neutrophil Counts and Neutrophil-to-Lymphocyte Ratio Predict Prognostic Outcomes in Patients after Non-Atrial Fibrillation-Caused Ischemic Stroke. Biomed. J. 2017, 40, 154–162. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA Traps Promote Thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef]
- Daniel, C.; Leppkes, M.; Muñoz, L.E.; Schley, G.; Schett, G.; Herrmann, M. Extracellular DNA Traps in Inflammation, Injury and Healing. Nat. Rev. Nephrol. 2019, 15, 559–575. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Y.; Liu, Z.; Chang, L.; Bai, X.; Kang, L.; Cao, Y.; Yang, X.; Yu, H.; Shi, M.-J.; et al. Neutrophil Extracellular Traps Promote TPA-Induced Brain Hemorrhage via CGAS in Mice with Stroke. Blood 2021, 138, 91–103. [Google Scholar] [CrossRef]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and Tissue Factor–Enriched Neutrophil Extracellular Traps Are Key Drivers in COVID-19 Immunothrombosis. J. Clin. Investig. 2020, 130, 6151–6157. [Google Scholar] [CrossRef]
- Lim, H.H.; Jeong, I.H.; An, G.D.; Woo, K.S.; Kim, K.H.; Kim, J.M.; Yun, S.H.; Park, J.I.; Cha, J.K.; Kim, M.; et al. Evaluation of Neutrophil Extracellular Traps as the Circulating Marker for Patients with Acute Coronary Syndrome and Acute Ischemic Stroke. J. Clin. Lab. Anal. 2020, 34, e23190. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Fu, X.; Cai, J.; Sun, C.; Yu, M.; Peng, Y.; Zhuang, J.; Chen, J.; Chen, H.; Yu, Q.; et al. Neutrophil Extracellular Traps May Be a Potential Target for Treating Early Brain Injury in Subarachnoid Hemorrhage. Transl. Stroke Res. 2022, 13, 112–131. [Google Scholar] [CrossRef]
- Liaptsi, E.; Merkouris, E.; Polatidou, E.; Tsiptsios, D.; Gkantzios, A.; Kokkotis, C.; Petridis, F.; Christidi, F.; Karatzetzou, S.; Karaoglanis, C.; et al. Targeting Neutrophil Extracellular Traps for Stroke Prognosis: A Promising Path. Neurol. Int. 2023, 15, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Krams, M.; Lees, K.R.; Hacke, W.; Grieve, A.P.; Orgogozo, J.M.; Ford, G.A. Acute Stroke Therapy by Inhibition of Neutrophils (ASTIN): An Adaptive Dose-Response Study of UK-279,276 in Acute Ischemic Stroke. Stroke 2003, 34, 2543–2548. [Google Scholar] [CrossRef] [PubMed]
- El Amki, M.; Glück, C.; Binder, N.; Middleham, W.; Wyss, M.T.; Weiss, T.; Meister, H.; Luft, A.; Weller, M.; Weber, B.; et al. Neutrophils Obstructing Brain Capillaries Are a Major Cause of No-Reflow in Ischemic Stroke. Cell Rep. 2020, 33, 108260. [Google Scholar] [CrossRef] [PubMed]
- Veltkamp, R.; Gill, D. Clinical Trials of Immunomodulation in Ischemic Stroke. Neurotherapeutics 2016, 13, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Elkind, M.S.V.; Veltkamp, R.; Montaner, J.; Johnston, S.C.; Singhal, A.B.; Becker, K.; Lansberg, M.G.; Tang, W.; Kasliwal, R.; Elkins, J. Natalizumab in Acute Ischemic Stroke (ACTION II): A Randomized, Placebo-Controlled Trial. Neurology 2020, 95, E1091–E1104. [Google Scholar] [CrossRef] [PubMed]
- Elkins, J.; Veltkamp, R.; Montaner, J.; Johnston, S.C.; Singhal, A.B.; Becker, K.; Lansberg, M.G.; Tang, W.; Chang, I.; Muralidharan, K.; et al. Safety and Efficacy of Natalizumab in Patients with Acute Ischaemic Stroke (ACTION): A Randomised, Placebo-Controlled, Double-Blind Phase 2 Trial. Lancet Neurol. 2017, 16, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.J. Anti-Leukocyte Antibodies: LeukArrest (Hu23F2G) and Enlimomab (R6.5) in Acute Stroke. Curr. Med. Res. Opin. 2002, 18 (Suppl. S2), s18–s22. [Google Scholar] [CrossRef]
- Westman, J.; Grinstein, S.; Marques, P.E. Phagocytosis of Necrotic Debris at Sites of Injury and Inflammation. Front. Immunol. 2020, 10, 3030. [Google Scholar] [CrossRef]
- Jablonska, J.; Leschner, S.; Westphal, K.; Lienenklaus, S.; Weiss, S. Neutrophils Responsive to Endogenous IFN-β Regulate Tumor Angiogenesis and Growth in a Mouse Tumor Model. J. Clin. Investig. 2010, 120, 1151–1164. [Google Scholar] [CrossRef]
- García-Culebras, A.; Durán-Laforet, V.; Peña-Martínez, C.; Moraga, A.; Ballesteros, I.; Cuartero, M.I.; de la Parra, J.; Palma-Tortosa, S.; Hidalgo, A.; Corbí, A.L.; et al. Role of TLR4 (Toll-like Receptor 4) in N1/N2 Neutrophil Programming after Stroke. Stroke 2019, 50, 2922–2932. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Peri, G.; Delneste, Y.; Frémaux, I.; Doni, A.; Moalli, F.; Garlanda, C.; Romani, L.; Gascan, H.; Bellocchio, S.; et al. The Humoral Pattern Recognition Receptor PTX3 Is Stored in Neutrophil Granules and Localizes in Extracellular Traps. J. Exp. Med. 2007, 204, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Welin, A.; Amirbeagi, F.; Christenson, K.; Björkman, L.; Björnsdottir, H.; Forsman, H.; Dahlgren, C.; Karlsson, A.; Bylund, J. The human neutrophil subsets defined by the presence or absence of OLFM4 both transmigrate into tissue in vivo and give rise to distinct NETs in vitro. PLoS ONE 2013, 8, e69575. [Google Scholar] [CrossRef] [PubMed]
- Naess, A.; Nilssen, S.S.; Mo, R.; Eide, G.E.; Sjursen, H. Role of Neutrophil to Lymphocyte and Monocyte to Lymphocyte Ratios in the Diagnosis of Bacterial Infection in Patients with Fever. Infection 2017, 45, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Fu, Z.; Huang, W.; Huang, K. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Sepsis: A Meta-Analysis. Am. J. Emerg. Med. 2020, 38, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.H.; Wang, Z.M.; Chen, S.Y. Neutrophil to Lymphocyte Ratio Predict Mortality and Major Adverse Cardiac Events in Acute Coronary Syndrome: A Systematic Review and Meta-Analysis. Clin. Biochem. 2018, 52, 131–136. [Google Scholar] [CrossRef]
- Lee, M.A.; Palace, J.; Stabler, G.; Ford, J.; Gearing, A.; Miller, K. Serum Gelatinase B, TIMP-1 and TIMP-2 Levels in Multiple Sclerosis A Longitudinal Clinical and MRI Study. Brain 1999, 122, 191–197. [Google Scholar] [CrossRef]
- Chaturvedi, M.; Kaczmarek, L. MMP-9 Inhibition: A Therapeutic Strategy in Ischemic Stroke. Mol. Neurobiol. 2014, 49, 563–573. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, R.; Liu, C.; Zhou, P.; Li, J.; Wang, Y.; Zhao, X.; Zhao, H.; Song, L.; Yan, H. Associations of NETs with Inflammatory Risk and Atherosclerotic Severity in ST-Segment Elevation Myocardial Infarction. Thromb. Res. 2021, 203, 5–11. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowaczewska-Kuchta, A.; Ksiazek-Winiarek, D.; Szpakowski, P.; Glabinski, A. The Role of Neutrophils in Multiple Sclerosis and Ischemic Stroke. Brain Sci. 2024, 14, 423. https://doi.org/10.3390/brainsci14050423
Nowaczewska-Kuchta A, Ksiazek-Winiarek D, Szpakowski P, Glabinski A. The Role of Neutrophils in Multiple Sclerosis and Ischemic Stroke. Brain Sciences. 2024; 14(5):423. https://doi.org/10.3390/brainsci14050423
Chicago/Turabian StyleNowaczewska-Kuchta, Anna, Dominika Ksiazek-Winiarek, Piotr Szpakowski, and Andrzej Glabinski. 2024. "The Role of Neutrophils in Multiple Sclerosis and Ischemic Stroke" Brain Sciences 14, no. 5: 423. https://doi.org/10.3390/brainsci14050423
APA StyleNowaczewska-Kuchta, A., Ksiazek-Winiarek, D., Szpakowski, P., & Glabinski, A. (2024). The Role of Neutrophils in Multiple Sclerosis and Ischemic Stroke. Brain Sciences, 14(5), 423. https://doi.org/10.3390/brainsci14050423





