Subtle Patterns of Altered Responsiveness to Delayed Auditory Feedback during Finger Tapping in People Who Stutter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
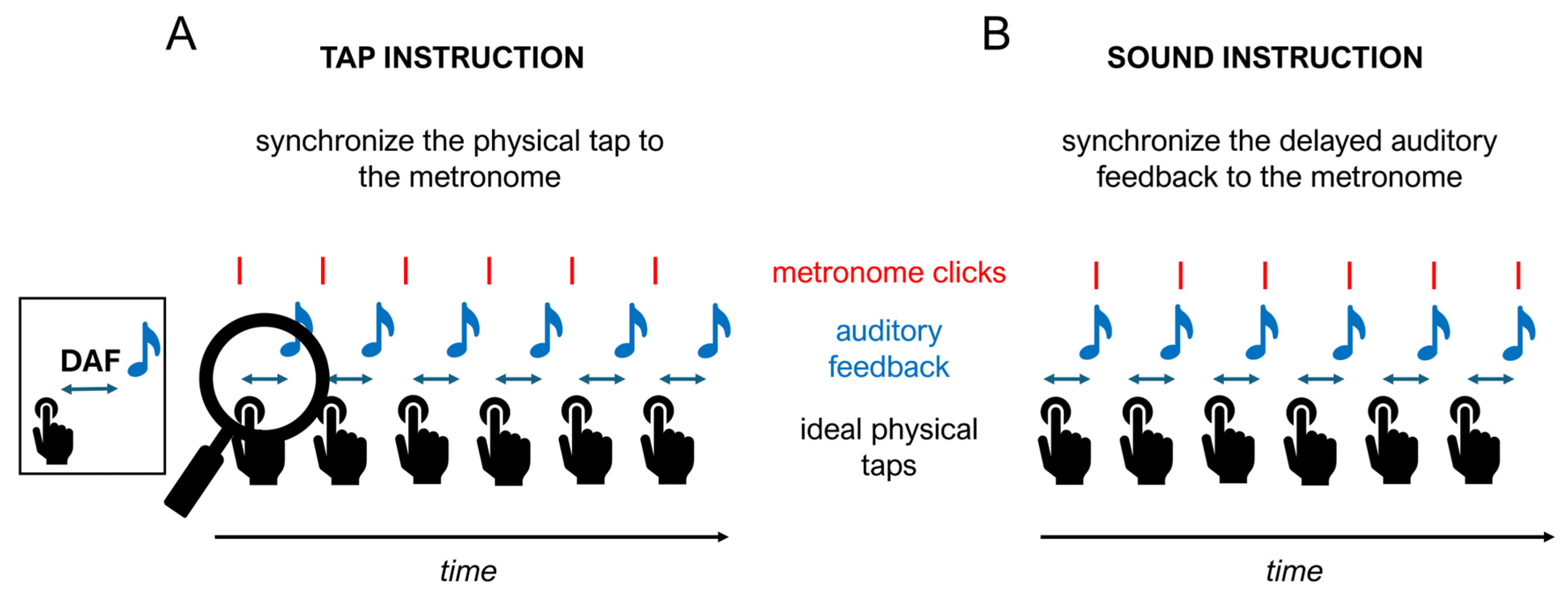
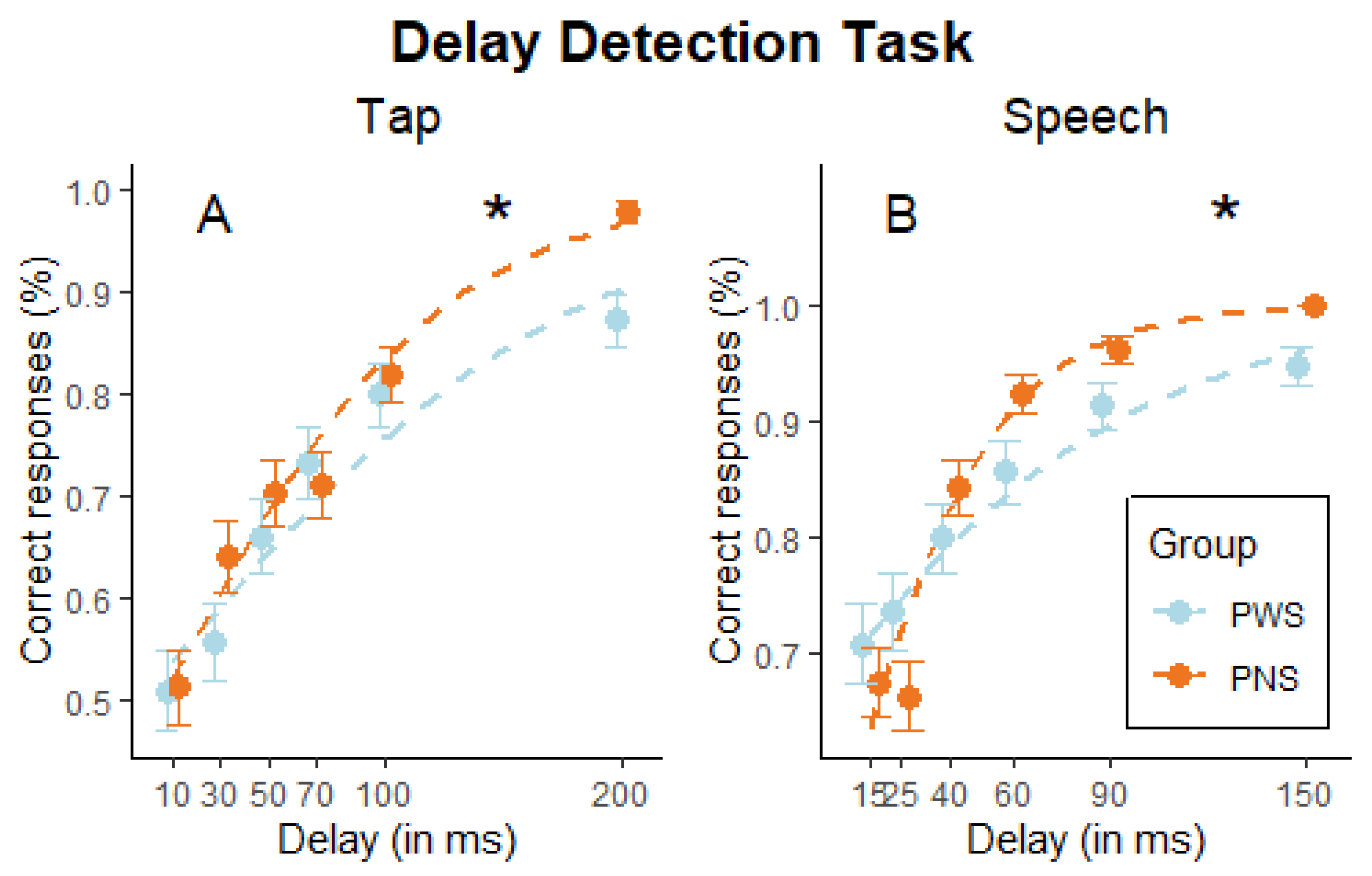
2.2. Design and Procedure
2.3. Materials
2.4. Data Analysis
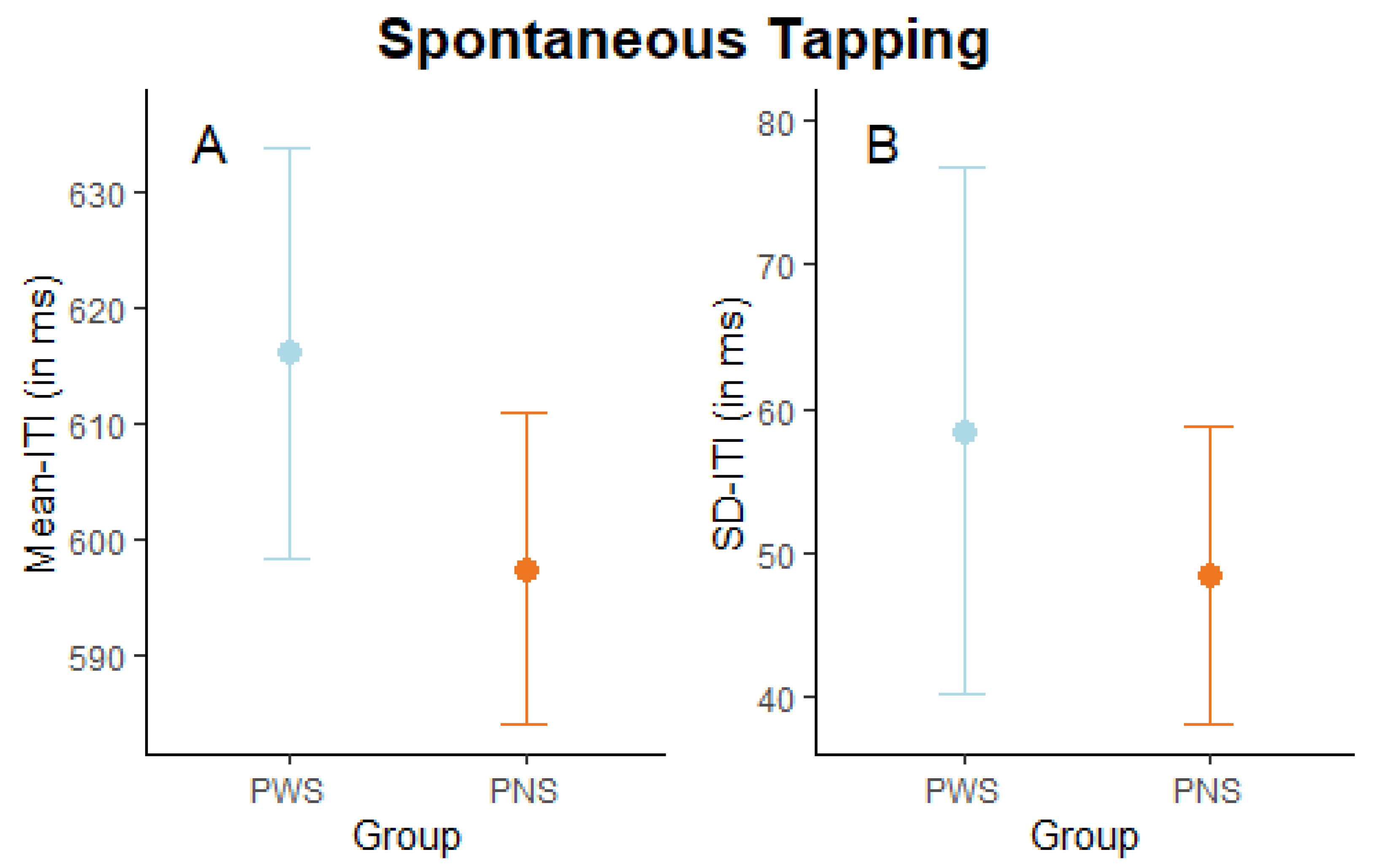
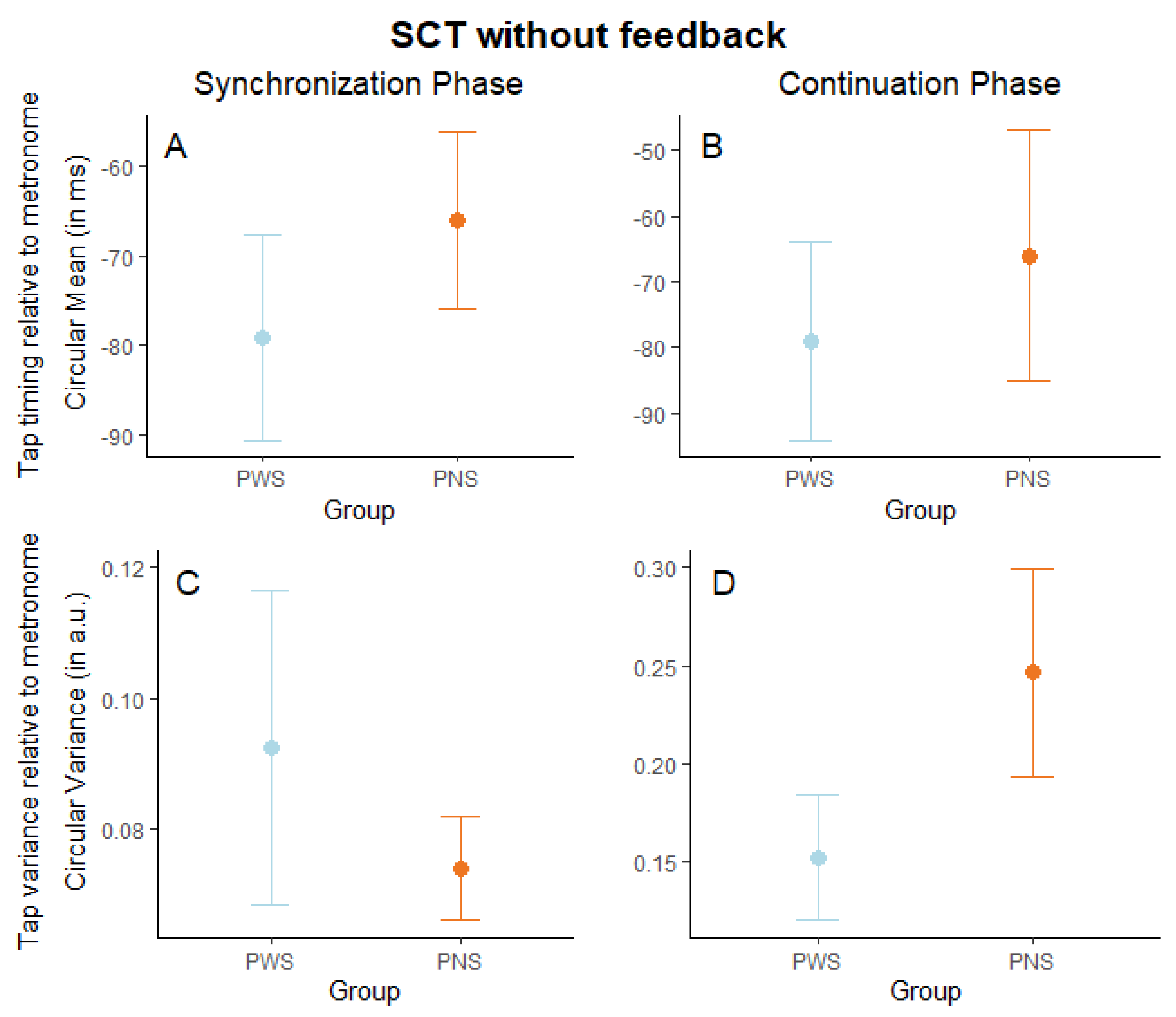
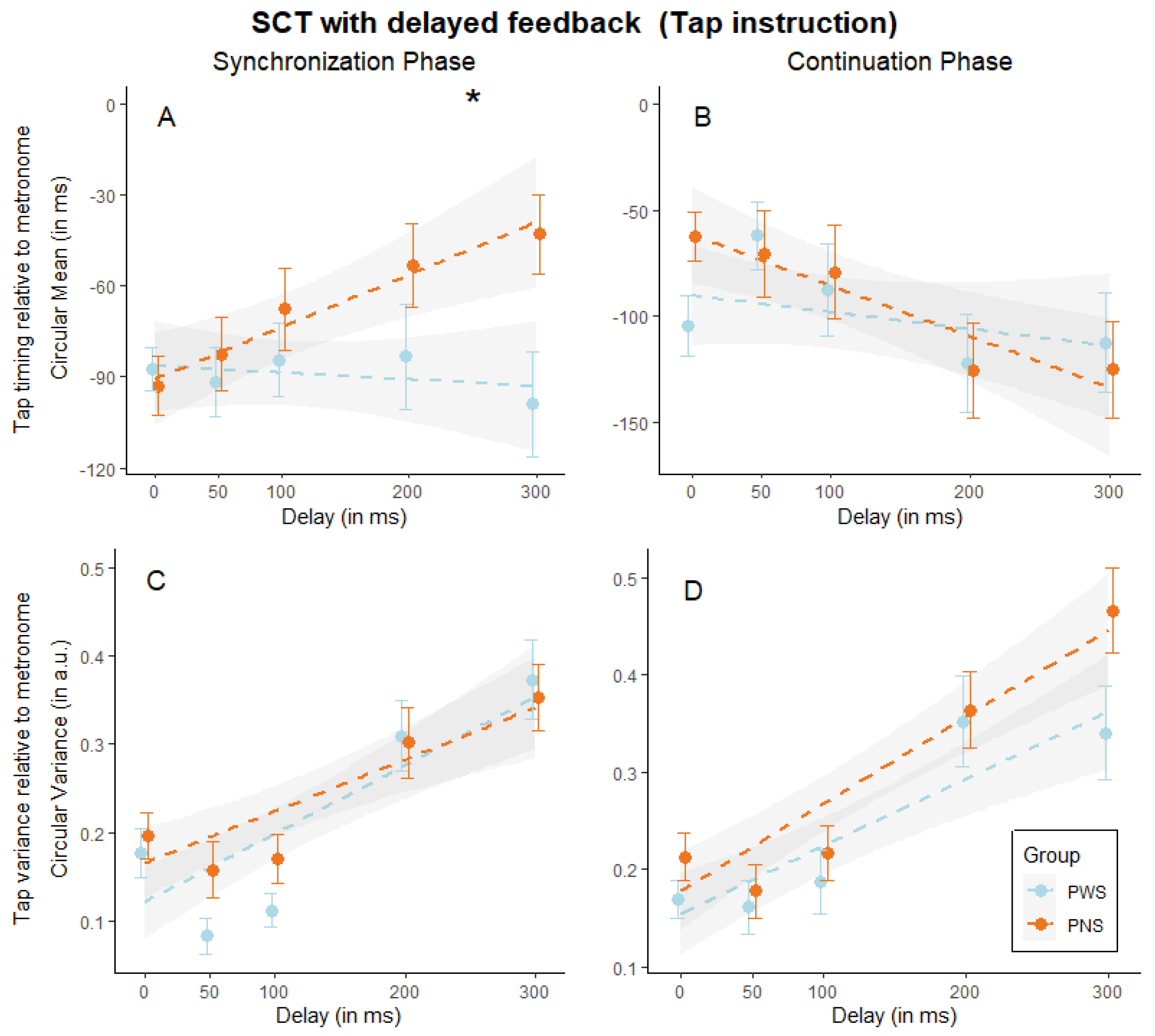
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition: DSM-5, 5th ed.; American Psychiatric Pub: Arlington, VA, USA, 2013. [Google Scholar]
- Lense, M.D.; Ladányi, E.; Rabinowitch, T.C.; Trainor, L.; Gordon, R. Rhythm and timing as vulnerabilities in neurodevelopmental disorders. Philos. Trans. R. Soc. B 2021, 376, 20200327. [Google Scholar] [CrossRef] [PubMed]
- Büchel, C.; Sommer, M. What causes stuttering? PLoS Biol. 2004, 2, e46. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.; MacKay, D.G. The Production of Speech; Linguistic Society of America: Washington, DC, USA, 1984. [Google Scholar]
- Borden, G.J. Initiation versus execution time during manual and oral counting by stutterers. J. Speech Lang. Hear. Res. 1983, 26, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Hulstijn, W.; Summers, J.J.; van Lieshout, P.H.; Peters, H.F. Timing in finger tapping and speech: A comparison between stutterers and fluent speakers. Hum. Mov. Sci. 1992, 11, 113–124. [Google Scholar] [CrossRef]
- Archibald, L.; De Nil, L.F. The relationship between stuttering severity and kinesthetic acuity for jaw movements in adults who stutter. J. Fluen. Disord. 1999, 24, 25–42. [Google Scholar] [CrossRef]
- Smits-Bandstra, S.; De Nil, L.; Rochon, E. The transition to increased automaticity during finger sequence learning in adult males who stutter. J. Fluen. Disord. 2006, 31, 22–42. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, N.G. Theoretical perspectives on the cause of stuttering. Contemp. Issues Commun. Sci. Disord. 2004, 31, 80–91. [Google Scholar] [CrossRef]
- Max, L.; Caruso, A.J.; Gracco, V.L. Kinematic analyses of speech, orofacial nonspeech, and finger movements in stuttering and nonstuttering adults. J. Speech Lang. Hear. Res. 2003, 46, 215–232. [Google Scholar] [CrossRef]
- Cai, S.; Beal, D.S.; Ghosh, S.S.; Tiede, M.K.; Guenther, F.H.; Perkell, J.S. Weak responses to auditory feedback perturbation during articulation in persons who stutter: Evidence for abnormal auditory-motor transformation. PLoS ONE 2012, 7, e41830. [Google Scholar] [CrossRef]
- Foundas, A.L.; Bollich, A.M.; Feldman, J.; Corey, D.M.; Hurley, M.; Lemen, L.C.; Heilman, K.M. Aberrant auditory processing and atypical planum temporale in developmental stuttering. Neurology 2004, 63, 1640–1646. [Google Scholar] [CrossRef]
- Kalinowski, J.; Armson, J.; Stuart, A.; Gracco, V.L. Effects of alterations in auditory feedback and speech rate on stuttering frequency. Lang. Speech 1993, 36, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bloodstein, O.; Bernstein Ratner, N. A Handbook on Stuttering, 6th ed.; Delmar Learning: Clifton Park, NY, USA, 2008. [Google Scholar]
- Braun, A.R.; Varga, M.; Stager, S.; Schulz, G.; Selbie, S.; Maisog, J.M.; Carson, R.E.; Ludlow, C.L. Altered patterns of cerebral activity during speech and language production in developmental stuttering. An H2 (15) O positron emission tomography study. Brain 1997, 120, 761–784. [Google Scholar] [CrossRef]
- Chang, S.E.; Horwitz, B.; Ostuni, J.; Reynolds, R.; Ludlow, C.L. Evidence of left inferior frontal–premotor structural and functional connectivity deficits in adults who stutter. Cereb. Cortex 2011, 21, 2507–2518. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.T.; Ingham, R.J.; Ingham, J.C.; Zamarripa, F.; Xiong, J.H.; Lancaster, J.L. Brain correlates of stuttering and syllable production: A PET performance-correlation analysis. Brain 2000, 123, 1985–2004. [Google Scholar] [CrossRef]
- Toyomura, A.; Fujii, T.; Kuriki, S. Effect of external auditory pacing on the neural activity of stuttering speakers. Neuroimage 2011, 57, 1507–1516. [Google Scholar] [CrossRef]
- Watkins, K.E.; Smith, S.M.; Davis, S.; Howell, P. Structural and functional abnormalities of the motor system in developmental stuttering. Brain 2008, 131, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Hickok, G.; Poeppel, D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition 2004, 92, 67–99. [Google Scholar] [CrossRef] [PubMed]
- Rauschecker, J.P. An expanded role for the dorsal auditory pathway in sensorimotor control and integration. Hear. Res. 2011, 271, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Penhune, V.B.; Zatorre, R.J. Listening to musical rhythms recruits motor regions of the brain. Cereb. Cortex 2008, 18, 2844–2854. [Google Scholar] [CrossRef]
- Lega, C.; Stephan, M.A.; Zatorre, R.J.; Penhune, V. Testing the role of dorsal premotor cortex in auditory-motor association learning using transcranical magnetic stimulation (TMS). PLoS ONE 2016, 11, e0163380. [Google Scholar] [CrossRef]
- Bengtsson, S.L.; Ullén, F.; Henrik Ehrsson, H.; Hashimoto, T.; Kito, T.; Naito, E.; Forssberg, H.; Sadato, N. Listening to rhythms activates motor and premotor cortices. Cortex 2009, 45, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Grahn, J.A. The role of the basal ganglia in beat perception: Neuroimaging and neuropsychological investigations. Ann. N. Y. Acad. Sci. 2009, 1169, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Grahn, J.A.; Brett, M. Rhythm and beat perception in motor areas of the brain. J. Cogn. Neurosci. 2007, 19, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Grahn, J.A.; Rowe, J.B. Finding and feeling the musical beat: Striatal dissociations between detection and prediction of regularity. Cereb. Cortex 2013, 23, 913–921. [Google Scholar] [CrossRef]
- Proksch, S.; Comstock, D.C.; Médé, B.; Pabst, A.; Balasubramaniam, R. Motor and predictive processes in auditory beat and rhythm perception. Front. Hum. Neurosci. 2020, 14, 578546. [Google Scholar] [CrossRef]
- Sares, A.G.; Deroche, M.L.; Shiller, D.M.; Gracco, V.L. Adults who stutter and metronome synchronization: Evidence for a nonspeech timing deficit. Ann. N. Y. Acad. Sci. 2019, 1449, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Falk, S.; Müller, T.; Dalla Bella, S. Non-verbal sensorimotor timing deficits in children and adolescents who stutter. Front. Psychol. 2015, 6, 847. [Google Scholar] [CrossRef] [PubMed]
- Slis, A.; Savariaux, C.; Perrier, P.; Gerber, S.; Garnier, M. Complexity of Rhythmic Tapping Task and Stuttering. In Proceedings of the ISSP 2020-12th International Seminar on Speech Production, Rhodes Island, RI, USA, 14–18 December 2020. [Google Scholar]
- Slis, A.; Savariaux, C.; Perrier, P.; Garnier, M. Rhythmic tapping difficulties in adults who stutter: A deficit in beat perception, motor execution, or sensorimotor integration? PLoS ONE 2023, 18, e0276691. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Zimmermann, G.N.; Linville, R.N.; Hegmann, J.P. Variations in self-paced behaviors in stutterers and nonstutterers. J. Speech Lang. Hear. Res. 1990, 33, 317–323. [Google Scholar] [CrossRef]
- Cooper, M.H.; Allen, G.D. Timing control accuracy in normal speakers and stutterers. J. Speech Hear. Res. 1977, 20, 55–71. [Google Scholar] [CrossRef]
- Zelaznik, H.N.; Smith, A.; Franz, E.A.; Ho, M. Differences in bimanual coordination associated with stuttering. Acta Psychol. 1997, 96, 229–243. [Google Scholar] [CrossRef]
- Zelaznik, H.N.; Smith, A.; Franz, E.A. Motor performance of stutterers and nonstutterers on timing and force control tasks. J. Mot. Behav. 1994, 26, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Max, L.; Yudman, E.M. Accuracy and variability of isochronous rhythmic timing across motor systems in stuttering versus nonstuttering individuals. J. Speech Lang. Hear. Res. 2003, 46, 146–163. [Google Scholar] [CrossRef] [PubMed]
- Aschersleben, G. Temporal control of movements in sensorimotor synchronization. Brain Cogn. 2002, 48, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Yairi, E. Identification of traits associated with stuttering. J. Commun. Disord. 2006, 39, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Webster, W.G. Neuropsychological models of stuttering—I. Representation of sequential response mechanisms. Neuropsychologia 1985, 23, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Olander, L.; Smith, A.; Zelaznik, H.N. Evidence that a motor timing deficit is a factor in the development of stuttering. J. Speech Lang. Hear. Res. 2010, 53, 876–886. [Google Scholar] [CrossRef] [PubMed]
- van de Vorst, R.; Gracco, V.L. Atypical non-verbal sensorimotor synchronization in adults who stutter may be modulated by auditory feedback. J. Fluen. Disord. 2017, 53, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Stuart, A.; Kalinowski, J.; Rastatter, M.P.; Lynch, K. Effect of delayed auditory feedback on normal speakers at two speech rates. J. Acoust. Soc. Am. 2002, 111 Pt 1, 2237–2241. [Google Scholar] [CrossRef]
- Swink, S.; Stuart, A. The effect of gender on the N1–P2 auditory complex while listening and speaking with altered auditory feedback. Brain Lang. 2012, 122, 25–33. [Google Scholar] [CrossRef]
- Black, J.W. The effect of delayed sidetone upon vocal rate and intensity. J. Speech Hear. Disord. 1951, 16, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Lee, B. Artificial stutter. J. Speech Hear. Disord. 1951, 16, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Havlicek, L.L. Effect of delayed auditory feedback on musical performance. J. Res. Music Educ. 1968, 16, 308–318. [Google Scholar] [CrossRef]
- Gates, A.; Bradshaw, J.L.; Nettleton, N.C. Effect of different delayed auditory feedback intervals on a music performance task. Percept. Psychophys. 1974, 15, 21–25. [Google Scholar] [CrossRef]
- Pfordresher, P.Q.; Benitez, B. Temporal coordination between actions and sound during sequence production. Hum. Mov. Sci. 2007, 26, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Pfordresher, P.Q.; Dalla Bella, S. Delayed auditory feedback and movement. J. Exp. Psychol. Hum. Percept. Perform. 2011, 37, 566. [Google Scholar] [CrossRef] [PubMed]
- Finney, S.A.; Warren, W.H. Delayed auditory feedback and rhythmic tapping: Evidence for a critical interval shift. Percept. Psychophys. 2002, 64, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.A.; Galloway, F.T. Performances of normal-hearing and hard-of-hearing persons on the delayed feedback task. J. Speech Hear. Res. 1959, 2, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Fairbanks, G. Selective vocal effects of delayed auditory feedback. J. Speech Hear. Disord. 1955, 20, 333–345. [Google Scholar] [CrossRef]
- MacKay, D.G. Metamorphosis of a critical interval: Age-linked changes in the delay in auditory feedback that produces maximal disruption of speech. J. Acoust. Soc. Am. 1968, 43, 811–821. [Google Scholar] [CrossRef]
- Cai, S.; Ghosh, S.S.; Guenther, F.H.; Perkell, J.S. Focal manipulations of formant trajectories reveal a role of auditory feedback in the online control of both within-syllable and between-syllable speech timing. J. Neurosci. 2011, 31, 16483–16490. [Google Scholar] [CrossRef] [PubMed]
- Floegel, M.; Fuchs, S.; Kell, C.A. Differential contributions of the two cerebral hemispheres to temporal and spectral speech feedback control. Nat. Commun. 2020, 11, 2839. [Google Scholar] [CrossRef]
- Mitsuya, T.; Munhall, K.G.; Purcell, D.W. Modulation of auditory-motor learning in response to formant perturbation as a function of delayed auditory feedback. J. Acoust. Soc. Am. 2017, 141, 2758–2767. [Google Scholar] [CrossRef] [PubMed]
- Ogane, R.; Honda, M. Speech compensation for time-scale-modified auditory feedback. J. Speech Lang. Hear. Res. 2014, 57, S616–S625. [Google Scholar] [CrossRef] [PubMed]
- Oschkinat, M.; Hoole, P. Compensation to real-time temporal auditory feedback perturbation depends on syllable position. J. Acoust. Soc. Am. 2020, 148, 1478–1495. [Google Scholar] [CrossRef]
- Daliri, A.; Max, L. Stuttering adults’ lack of pre-speech auditory modulation normalizes when speaking with delayed auditory feedback. Cortex 2018, 99, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Tourville, J.A.; Beal, D.S.; Perkell, J.S.; Guenther, F.H.; Ghosh, S.S. Diffusion imaging of cerebral white matter in persons who stutter: Evidence for network-level anomalies. Front. Hum. Neurosci. 2014, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Frankford, S.A.; Cai, S.; Nieto-Castañón, A.; Guenther, F.H. Auditory feedback control in adults who stutter during metronome-paced speech I. Timing Perturbation. J. Fluen. Disord. 2023, 75, 105943. [Google Scholar] [CrossRef] [PubMed]
- Howell, P.; El-Yaniv, N.; Powell, D.J. Factors affecting fluency in stutterers when speaking under altered auditory feedback. In Speech Motor Dynamics in Stuttering; Springer: Vienna, Austria, 1987; pp. 361–369. [Google Scholar]
- Kalinowski, J.; Stuart, A.; Sark, S.; Armson, J. Stuttering amelioration at various auditory feedback delays and speech rates. Int. J. Lang. Commun. Disord. 1996, 31, 259–269. [Google Scholar] [CrossRef]
- Lincoln, M.; Packman, A.; Onslow, M. Altered auditory feedback and the treatment of stuttering: A review. J. Fluen. Disord. 2006, 31, 71–89. [Google Scholar] [CrossRef]
- Drewing, K. Delayed auditory feedback in repetitive tapping: A role for the sensory goal. Q. J. Exp. Psychol. 2013, 66, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Kulpa, J.D.; Pfordresher, P.Q. Effects of delayed auditory and visual feedback on sequence production. Exp. Brain Res. 2013, 224, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Pfordresher, P.Q. Auditory feedback in music performance: Evidence for a dissociation of sequencing and timing. J. Exp. Psychol. Hum. Percept. Perform. 2003, 29, 949. [Google Scholar] [CrossRef] [PubMed]
- Pfordresher, P.; Palmer, C. Effects of delayed auditory feedback on timing of music performance. Psychol. Res. 2002, 66, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Aschersleben, G.; Prinz, W. Delayed auditory feedback in synchronization. J. Mot. Behav. 1997, 29, 35–46. [Google Scholar] [CrossRef]
- Repp, B.H.; Su, Y.-H. Sensorimotor synchronization: A review of recent research (2006–2012). Psychon. Bull. Rev. 2013, 20, 403–452. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, F.T.; Tillmann, B. Thresholds of auditory-motor coupling measured with a simple task in musicians and non-musicians: Was the sound simultaneous to the key press? PLoS ONE 2014, 9, e87176. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Riley, G. The Stuttering Severity Instrument for Adults and Children (SSI-4), 4th ed.; PRO-ED: Austin, TX, USA, 2009. [Google Scholar]
- Lagrois, M.É.; Palmer, C.; Peretz, I. Poor synchronization to musical beat generalizes to speech. Brain Sci. 2019, 9, 157. [Google Scholar] [CrossRef]
- Namasivayam, A.K.; Van Lieshout, P.; McIlroy, W.E.; De Nil, L. Sensory feedback dependence hypothesis in persons who stutter. Hum. Mov. Sci. 2009, 28, 688–707. [Google Scholar] [CrossRef]
- Falk, S.; Müller, T.; Dalla Bella, S. Sensorimotor synchronization in stuttering children and adolescents. Procedia-Soc. Behav. Sci. 2014, 126, 206–207. [Google Scholar] [CrossRef]
- Schultz, B.G.; van Vugt, F.T. Tap Arduino: An Arduino microcontroller for low-latency auditory feedback in sensorimotor synchronization experiments. Behav. Res. Methods 2016, 48, 1591–1607. [Google Scholar] [CrossRef]
- van Vugt, F.T. The TeensyTap Framework for Sensorimotor Synchronization Experiments. Adv. Cogn. Psychol. 2020, 16, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Fisher, N.I.; Lewis, T.; Embleton, B.J. Statistical Analysis of Spherical Data; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Pecenka, N.; Keller, P.E. The role of temporal prediction abilities in interpersonal sensorimotor synchronization. Exp. Brain Res. 2011, 211, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Cremers, J.; Klugkist, I. One direction? A tutorial for circular data analysis using R with examples in cognitive psychology. Front. Psychol. 2018, 9, 396689. [Google Scholar] [CrossRef]
- Gelman, A.; Hill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 2 May 2024).
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Loucks, T.; Chon, H.; Han, W. Audiovocal integration in adults who stutter. Int. J. Lang. Commun. Disord. 2012, 47, 451–456. [Google Scholar] [CrossRef]
- Nudelman, H.B.; Herbrich, K.E.; Hess, K.R.; Hoyt, B.D.; Rosenfield, D.B. A model of the phonatory response time of stutterers and fluent speakers to frequency-modulated tones. J. Acoust. Soc. Am. 1992, 92, 1882–1888. [Google Scholar] [CrossRef]
- Fukawa, T.; Yoshioka, H.; Ozawa, E.; Yoshida, S. Difference of susceptibility to delayed auditory feedback between stutterers and nonstutterers. J. Speech Lang. Hear. Res. 1988, 31, 475–479. [Google Scholar] [CrossRef]
- Chon, H.; Jackson, E.S.; Kraft, S.J.; Ambrose, N.G.; Loucks, T.M. Deficit or difference? Effects of altered auditory feedback on speech fluency and kinematic variability in adults who stutter. J. Speech Lang. Hear. Res. 2021, 64, 2539–2556. [Google Scholar] [CrossRef]
- Etchell, A.C.; Johnson, B.W.; Sowman, P.F. Behavioral and multimodal neuroimaging evidence for a deficit in brain timing networks in stuttering: A hypothesis and theory. Front. Hum. Neurosci. 2014, 8, 467. [Google Scholar] [CrossRef]
- Max, L.; Guenther, F.H.; Gracco, V.L.; Ghosh, S.S.; Wallace, M.E. Unstable or insufficiently activated internal models and feedback-biased motor control as sources of dysfluency: A theoretical model of stuttering. Contemp. Issues Commun. Sci. Disord. 2004, 31, 105–122. [Google Scholar] [CrossRef]
- Wieland, E.A.; McAuley, J.D.; Dilley, L.C.; Chang, S.E. Evidence for a rhythm perception deficit in children who stutter. Brain Lang. 2015, 144, 26–34. [Google Scholar] [CrossRef]
- Rimmele, J.M.; Morillon, B.; Poeppel, D.; Arnal, L.H. Proactive sensing of periodic and aperiodic auditory patterns. Trends Cogn. Sci. 2018, 22, 870–882. [Google Scholar] [CrossRef]
- Patel, A.D.; Iversen, J.R. The evolutionary neuroscience of musical beat perception: The Action Simulation for Auditory Prediction (ASAP) hypothesis. Front. Syst. Neurosci. 2014, 8, 57. [Google Scholar] [CrossRef]
- Cannon, J.J.; Patel, A.D. How beat perception co-opts motor neurophysiology. Trends Cogn. Sci. 2021, 25, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Wieneke, G.; Janssen, P.; Brutten, G.J. Variance of central timing of voiced and voiceless periods among stutterers and nonstutterers. J. Fluen. Disord. 1995, 20, 171–189. [Google Scholar] [CrossRef]

| Phenomenon | Studies Finding Differences in PWS vs. PNS | Studies Finding No Differences in PWS vs. PNS |
|---|---|---|
| Slower spontaneous motor performance in PWS vs. PNS | Brown, 1990 [33]; Subramanian and Yairi, 2006 [39] | Webster, 1985 [40] |
| Higher tapping variability in PWS vs. PNS | Cooper and Allen, 1977 [34]; Falk et al., 2015 [30]; Olander et al., 2010 [41]; Sares et al., 2019 [29]; Slis et al., 2022 [31]; 2023 [32]; Smits-Bandstra et al., 2006 [8]; van de Vorst and Gracco, 2017 [42]; Zelaznik et al., 1997 [35] | Hulstijn et al., 1992 [6]; Max and Yudman, 2003 [37]; Zelaznik et al., 1994 [36] |
| Greater number of missed taps in PWS vs. PNS | Slis et al., 2022 [31] | Zelaznik et al., 1994 [36] |
| Greater negative mean asynchrony (NMA) in PWS vs. PNS | Falk et al., 2015 [30]; Sares et al., 2019 [29]; Slis et al., 2023 [32] | Hulstijn et al., 1992 [6]; Max and Yudman, 2003 [37]; van de Vorst and Gracco, 2017 [42]; Zelaznik et al., 1994 [36] |
| Tempo drift when metronome is discontinued | None | Hulstijn et al., 1992 [6]; Max and Yudman, 2003 [37]; Sares, et al., 2019 [29]; Slis et al., 2023 [32] |
| Participant ID | Gender | Age (Years) | SSI Score—Severity | Stuttering Frequency %SS |
|---|---|---|---|---|
| 1 | Female | 24 | 17 (very mild) | 2.98 |
| 2 | Female | 24 | 6 (very mild) | 0.75 |
| 3 | Male | 27 | 16 (very mild) | 3.79 |
| 4 | Female | 30 | 35 (severe) | 34.29 |
| 5 | Male | 23 | 14 (very mild) | 2.50 |
| 6 | Female | 24 | 22 (mild) | 16.69 |
| 7 | Female | 28 | 4 (very mild) | 0.37 |
| 8 | Male | 32 | 21 (mild) | 7.48 |
| 9 * | Female | 30 | 0 | 0 |
| 10 | Female | 26 | 20 (mild) | 5.78 |
| 11 | Male | 35 | 10 (very mild) | 0.95 |
| Circular Mean | Circular Variance | |||
|---|---|---|---|---|
| Synchronization | Continuation | Synchronization | Continuation | |
| Delay | ꭓ2 (1) = 7.99, p = 0.005 | ꭓ2 (1) = 10.39, p = 0.001 | ꭓ2 (1) = 68.23, p < 0.001 | ꭓ2 (1) = 107.90, p < 0.001 |
| Group | ꭓ2 (1) = 0.56, p = 0.455 | ꭓ2 (1) = 0.27, p = 0.605 | ꭓ2 (1) = 0.17, p = 0.679 | ꭓ2 (1) = 0.51, p = 0.476 |
| Delay × Group | ꭓ2 (1) = 10.86, p < 0.001 | ꭓ2 (1) = 2.38, p = 0.123 | ꭓ2 (1) = 1.31, p = 0.252 | ꭓ2 (1) = 1.72, p = 0.190 |
| Goodness-of-fit (R2) | Marginal R2 = 0.030, Conditional R2 = 0.338 | Marginal R2 = 0.021, Conditional R2 = 0.119 | Marginal R2 = 0.082, Conditional R2 = 0.338 | Marginal R2 = 0.116, Conditional R2 = 0.426 |
| Circular Mean | Circular Variance | |||
|---|---|---|---|---|
| Synchronization | Continuation | Synchronization | Continuation | |
| Delay | ꭓ2 (1) = 0.28, p = 0.595 | ꭓ2 (1) = 17.67, p < 0.001 | ꭓ2 (1) = 147.32, p < 0.001 | ꭓ2 (1) = 47.13, p < 0.001 |
| Group | ꭓ2 (1) = 0.03, p = 0.859 | ꭓ2 (1) = 0.05, p = 0.829 | ꭓ2 (1) = 1.09, p = 0.296 | ꭓ2 (1) = 2.73, p = 0.098 |
| Delay × Group | ꭓ2 (1) = 0.47, p = 0.492 | ꭓ2 (1) = 0.99, p = 0.320 | ꭓ2 (1) = 0.80, p = 0.370 | ꭓ2 (1) = 2.93, p = 0.087 |
| Goodness-of-fit (R2) | Marginal R2 = 0.001, Conditional R2 = 0.267 | Marginal R2 = 0.030, Conditional R2 = 0.090 | Marginal R2 = 0.164, Conditional R2 = 0.409 | Marginal R2 = 0.088, Conditional R2 = 0.308 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzari, G.; van de Vorst, R.; van Vugt, F.T.; Lega, C. Subtle Patterns of Altered Responsiveness to Delayed Auditory Feedback during Finger Tapping in People Who Stutter. Brain Sci. 2024, 14, 472. https://doi.org/10.3390/brainsci14050472
Lazzari G, van de Vorst R, van Vugt FT, Lega C. Subtle Patterns of Altered Responsiveness to Delayed Auditory Feedback during Finger Tapping in People Who Stutter. Brain Sciences. 2024; 14(5):472. https://doi.org/10.3390/brainsci14050472
Chicago/Turabian StyleLazzari, Giorgio, Robert van de Vorst, Floris T. van Vugt, and Carlotta Lega. 2024. "Subtle Patterns of Altered Responsiveness to Delayed Auditory Feedback during Finger Tapping in People Who Stutter" Brain Sciences 14, no. 5: 472. https://doi.org/10.3390/brainsci14050472






