Differences in Electroencephalography Power Levels between Poor and Good Performance in Attentional Tasks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Task
2.3. Experimental Protocol
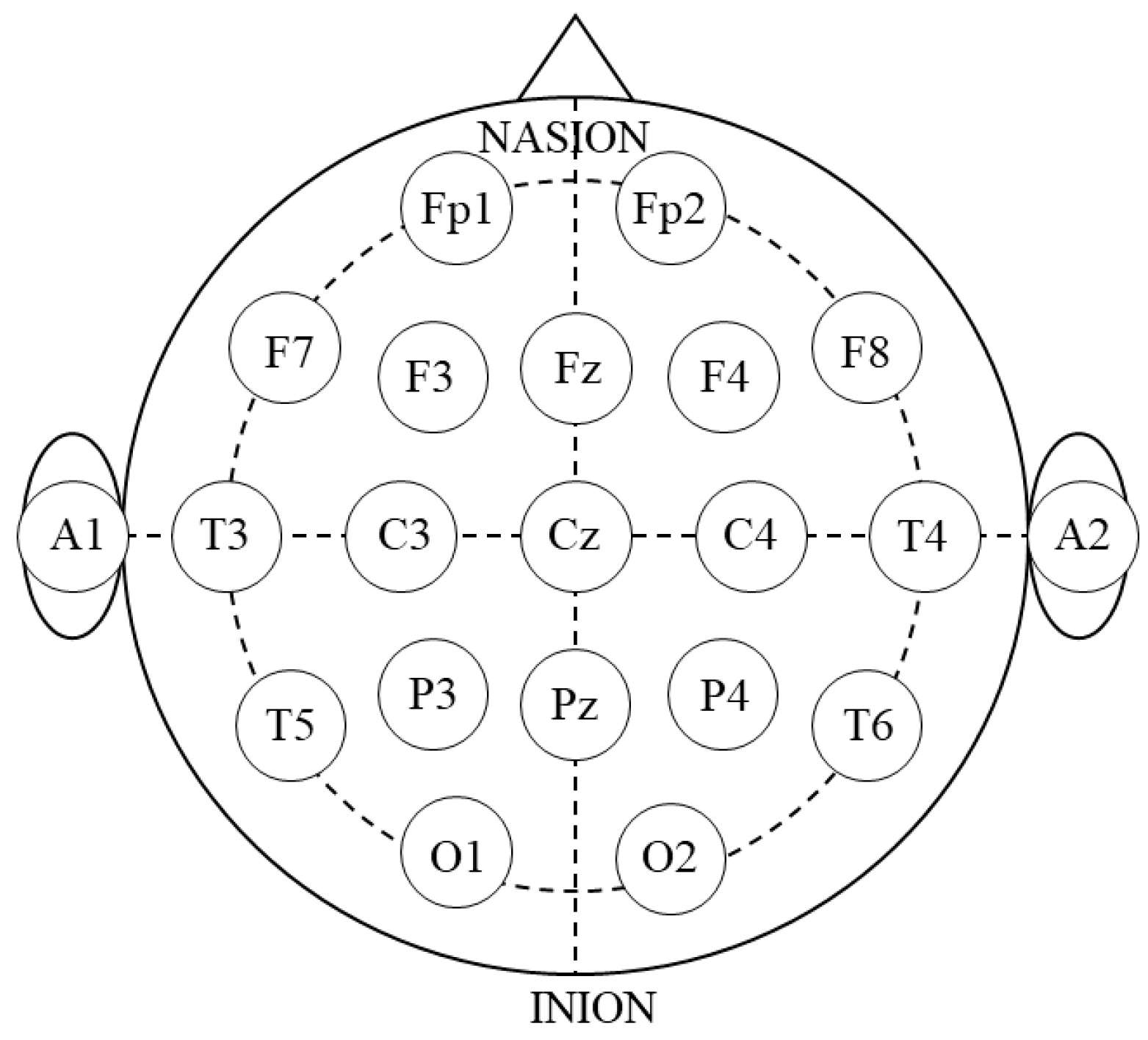
2.4. Data Analysis
2.5. Statistical Analysis
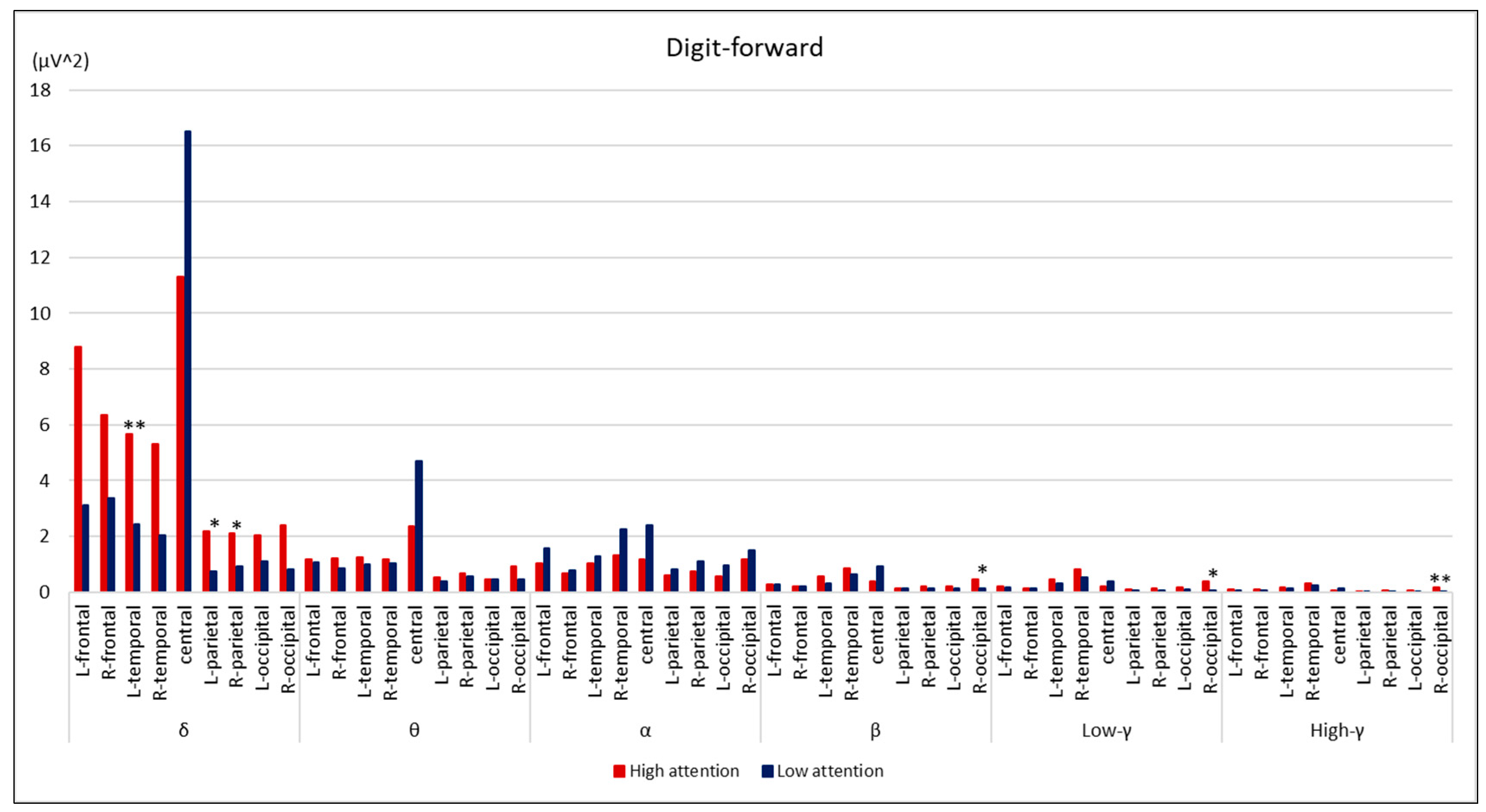
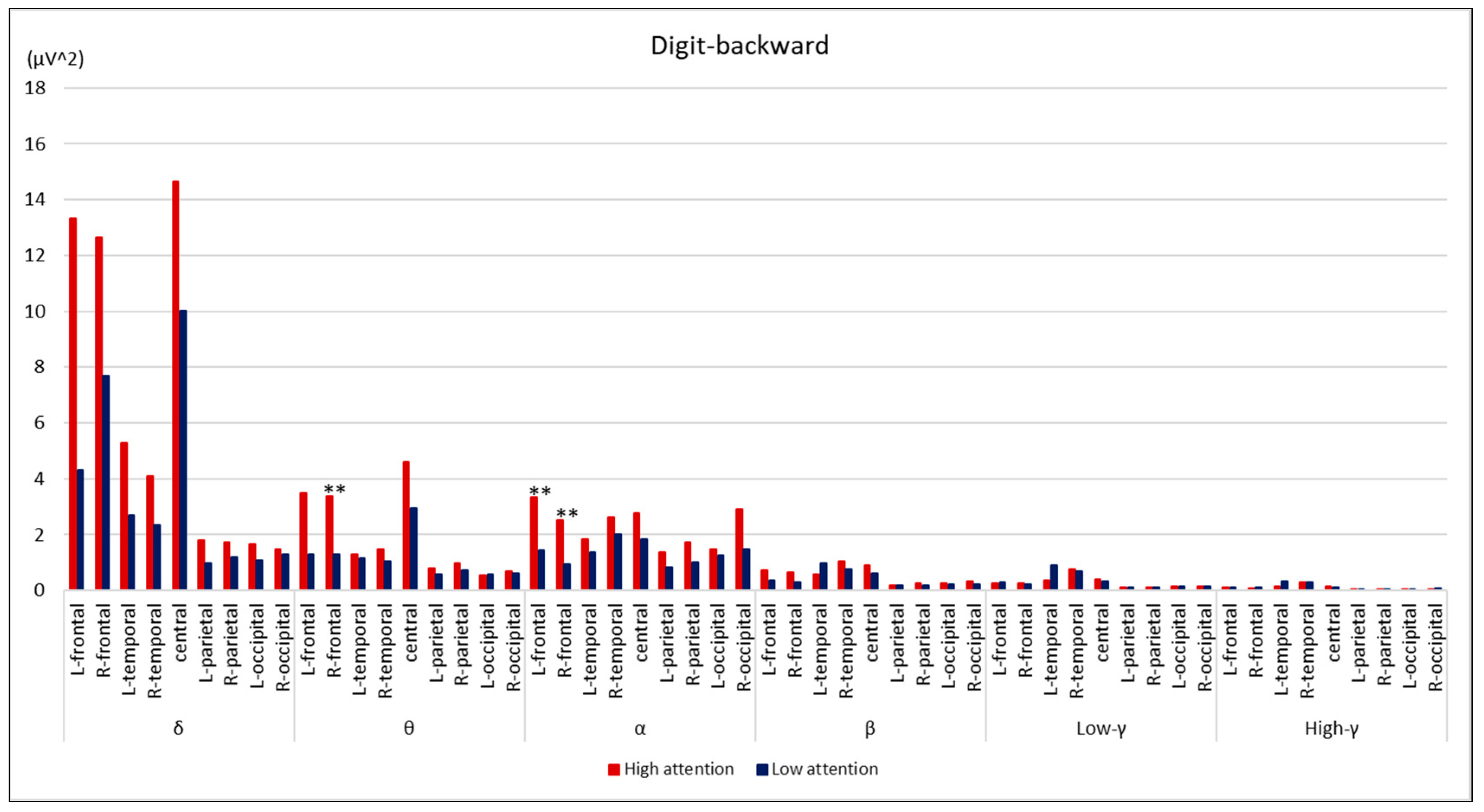
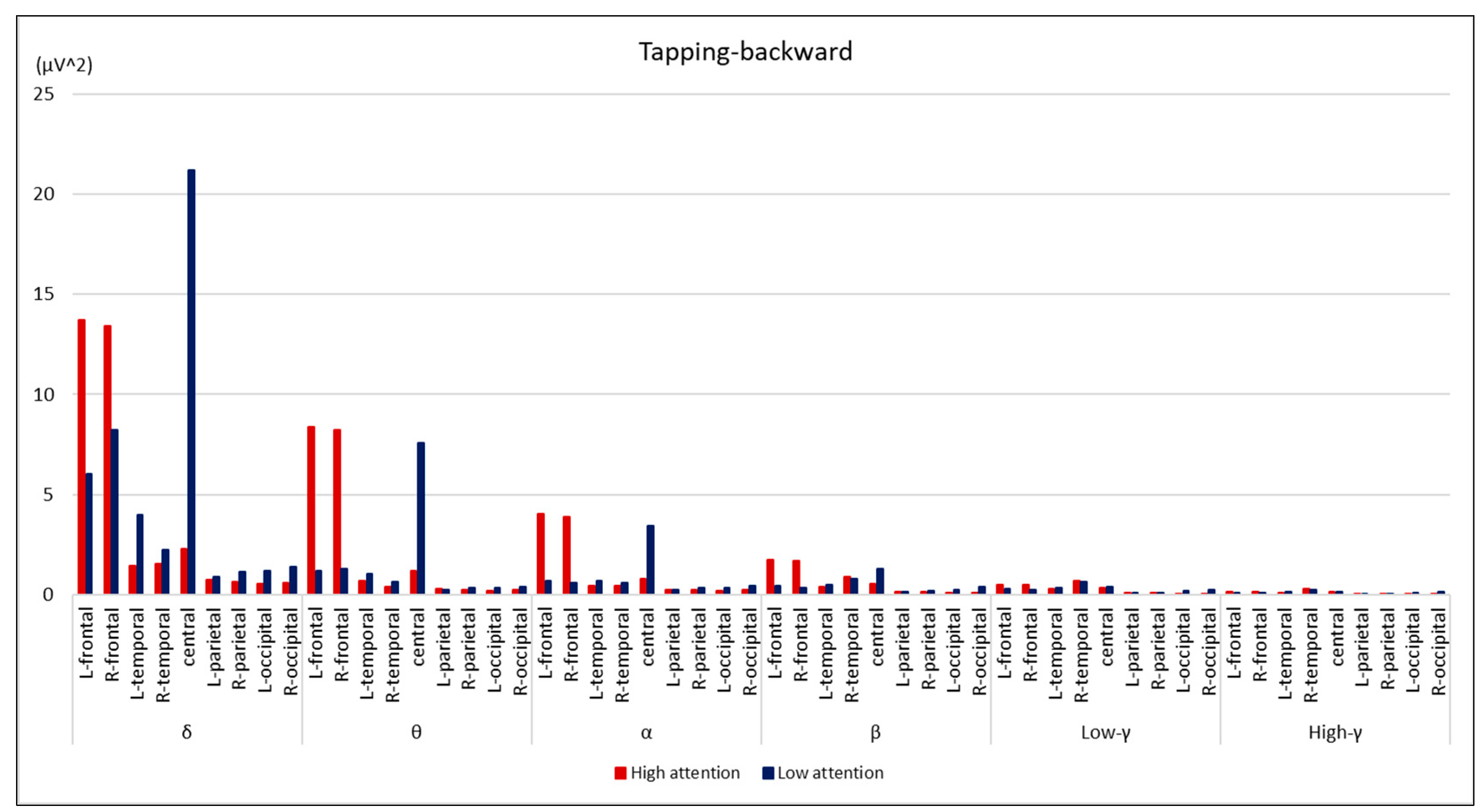
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Posner, M.I.; Petersen, S.E. The attention system of the human brain. Annu. Rev. Neurosci. 1990, 13, 25–42. [Google Scholar] [CrossRef]
- Crosson, B.; Ford, A.; McGregor, K.M.; Meinzer, M.; Cheshkov, S.; Li, X.; Walker-Batson, D.; Briggs, R.W. Functional imaging and related techniques: An introduction for rehabilitation researchers. J. Rehabil. Res. Dev. 2010, 47, vii–xxxiv. [Google Scholar] [CrossRef]
- Light, G.A.; Williams, L.E.; Minow, F.; Sprock, J.; Rissling, A.; Sharp, R.; Swerdlow, N.R.; Braff, D.L. Electroencephalography (EEG) and event-related potentials (ERPs) with human participants. Curr. Protoc. Neurosci. 2010, 25, 6–25. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.M. EEG in neurological conditions other than epilepsy: When does it help, what does it add? J. Neurol. Neurosurg. Psychiatry 2005, 76 (Suppl. S2), ii8–ii12. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.K.; Makeig, S. Clinical utility of EEG in attention-deficit/hyperactivity disorder: A research update. Neurotherapeutics 2012, 9, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Jurgiel, J.; Miyakoshi, M.; Dillon, A.; Piacentini, J.; Loo, S.K. Additive and interactive effects of attention-deficit/hyperactivity disorder and tic disorder on brain connectivity. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2023, 8, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Peksa, J.; Mamchur, D. State-of-the-art on brain-computer interface technology. Sensors 2023, 23, 6001. [Google Scholar] [CrossRef]
- Llorella, F.R.; Patow, G.; Azorín, J.M. Convolutional neural networks and genetic algorithm for visual imagery classification. Phys. Eng. Sci. Med. 2020, 43, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Acker, L.; Wong, M.K.; Wright, M.C.; Reese, M.; Giattino, C.M.; Roberts, K.C.; Au, S.; Colon-Emeric, C.; Lipsitz, L.A.; Devinney, M.J.; et al. Preoperative electroencephalographic alpha-power changes with eyes opening are associated with postoperative attention impairment and inattention-related delirium severity. Br. J. Anaesth. 2024, 132, 154–163. [Google Scholar] [CrossRef]
- Yang, K.; Hu, Y.; Zeng, Y.; Tong, L.; Gao, Y.; Pei, C.; Li, Z.; Yan, B. EEG Network Analysis of Depressive Emotion Interference Spatial Cognition Based on a Simulated Robotic Arm Docking Task. Brain Sci. 2023, 14, 44. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research in-volving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Gunawardena, R.; Sarrigiannis, P.G.; Blackburn, D.J.; He, F. Kernel-based nonlinear manifold learning for EEG-based functional connectivity analysis and channel selection with application to Alzheimer’s disease. Neuroscience 2023, 523, 140–156. [Google Scholar] [CrossRef]
- Matsuo, M.; Higuchi, T.; Miyabara, H.; Higashijima, M.; Oshikawa, T.; Nakamura, M.; Yamaguchi, Y.; Higashionna, T. Assessing attentional task-related electroencephalogram signal variations by using mobile electroencephalogram technology: An experimental study. Medicine 2023, 102, e35801. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Higuchi, T.; Ishibashi, T.; Egashira, A.; Abe, T.; Miyabara, H. Enhancement of visual attention by color revealed using electroencephalography. Open J. Ther. Rehabil. 2024, 12, 1–9. [Google Scholar] [CrossRef]
- Chao, H.; Cao, Y.; Liu, Y. Multi-channel EEG emotion recognition through residual graph attention neural network. Front. Neurosci. 2023, 17, 1135850. [Google Scholar] [CrossRef]
- Qiao, R.; Zhang, H.; Tian, Y. EEG cortical network reveals the temporo-spatial mechanism of visual search. Brain Res. Bull. 2023, 203, 110758. [Google Scholar] [CrossRef] [PubMed]
- Aydin, Ü.; Gyurkovics, M.; Ginestet, C.; Capp, S.; Greven, C.U.; Palmer, J.; McLoughlin, G. Genetic overlap between midfrontal theta signals and attention-deficit/hyperactivity disorder and autism spectrum disorder in a longitudinal twin cohort. Biol. Psychiatry 2023, 94, 823–832. [Google Scholar] [CrossRef]
- Tichelman, N.L.; Foerges, A.L.; Elmenhorst, E.M.; Lange, D.; Hennecke, E.; Baur, D.M.; Beer, S.; Kroll, T.; Neumaier, B.; Bauer, A.; et al. A genetic variation in the adenosine A2a receptor gene contributes to variability in oscillatory alpha power in wake and sleep EEG and A1 adenosine receptor availability in the human brain. Neuroimage 2023, 280, 120345. [Google Scholar] [CrossRef]
- Babel, S.; Baral, S.; Srivastava, A. Impact of listening to Indian classical music, or Rāgas, on the electroencephalogram: A meta-analysis. Cureus 2023, 15, e49592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, Y.; Deng, Y.; Kong, L.; Zhang, X.; Wei, X.; Mao, T.; Xu, Y.; Jiang, C.; Rao, H. Vigilant attention mediates the association between resting EEG alpha oscillations and word learning ability. Neuroimage 2023, 281, 120369. [Google Scholar] [CrossRef]
- Li, S.; Seger, C.A.; Zhang, J.; Liu, M.; Dong, W.; Liu, W.; Chen, Q. Alpha oscillations encode Bayesian belief updating underlying attentional allocation in dynamic environments. Neuroimage 2023, 284, 120464. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Huang, H.; Bao, X.; Pan, J.; Li, Y. An EEG-based attention recognition method: Fusion of time domain, frequency domain, and non-linear dynamics features. Front. Neurosci. 2023, 17, 1194554. [Google Scholar] [CrossRef] [PubMed]
- Radecke, J.O.; Fiene, M.; Misselhorn, J.; Herrmann, C.S.; Engel, A.K.; Wolters, C.H.; Schneider, T.R. Personalized α-tACS targeting left posterior parietal cortex modulates visuo-spatial attention and posterior evoked EEG activity. Brain Stimul. 2023, 16, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Silas, J.; Jones, A.; Yarrow, K.; Anderson, W. Spatial attention is not affected by α or β transcranial alternating current stimulation: A registered report. Cortex 2023, 164, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Perellón-Alfonso, R.; Oblak, A.; Kuclar, M.; Škrlj, B.; Pileckyte, I.; Škodlar, B.; Pregelj, P.; Abellaneda-Pérez, K.; Bartrés-Faz, D.; Repovš, G.; et al. Dense attention network identifies EEG abnormalities during working memory performance of patients with schizophrenia. Front. Psychiatry 2023, 14, 1205119. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, H.; Xing, Y.; Liu, J.; Gao, Y.; Gu, E.; Zhang, L.; Chen, L. Effect of raw electroencephalogram-guided anesthesia administration on postoperative outcomes in elderly patients undergoing abdominal major surgery: A randomized controlled trial. BMC Anesthesiol. 2023, 23, 337. [Google Scholar] [CrossRef]
- Janjua, M.S.; Spurling, B.C.; Arthur, M.E. Postoperative delirium. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Fuentes-Martinez, V.J.; Romero, S.; Lopez-Gordo, M.A.; Minguillon, J.; Rodríguez-Álvarez, M. Low-cost EEG multi-subject recording platform for the assessment of students’ attention and the estimation of academic performance in secondary school. Sensors 2023, 23, 9361. [Google Scholar] [CrossRef]
- Trenado, C.; Trauberg, P.; Elben, S.; Dimenshteyn, K.; Folkerts, A.K.; Witt, K.; Weiss, D.; Liepelt-Scarfone, I.; Kalbe, E.; Wojtecki, L. Resting state EEG as biomarker of cognitive training and physical activity’s joint effect in Parkinson’s patients with mild cognitive impairment. Neurol. Res. Pract. 2023, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Teicher, M.H.; Bolger, E.; Garcia, L.C.H.; Hafezi, P.; Weiser, L.P.; McGreenery, C.E.; Khan, A.; Ohashi, K. Bright light therapy and early morning attention, mathematical performance, electroencephalography and brain connectivity in adolescents with morning sleepiness. PLoS ONE. 2023, 18, e0273269. [Google Scholar] [CrossRef]
- Klooster, D.; Voetterl, H.; Baeken, C.; Arns, M. Evaluating robustness of brain stimulation biomarkers for depression: A systematic review of magnetic resonance imaging and electroencephalography studies. Biol. Psychiatry 2024, 95, 553–563. [Google Scholar] [CrossRef]
- Crétot-Richert, G.; De Vos, M.; Debener, S.; Bleichner, M.G.; Voix, J. Assessing focus through ear-EEG: A comparative study between conventional cap EEG and mobile in- and around-the-ear EEG systems. Front. Neurosci. 2023, 17, 895094. [Google Scholar] [CrossRef] [PubMed]
- Eyamu, J.; Kim, W.S.; Kim, K.; Lee, K.H.; Kim, J.U. Prefrontal event-related potential markers in association with mild cognitive impairment. Front. Aging Neurosci. 2023, 15, 1273008. [Google Scholar] [CrossRef] [PubMed]
- Kember, J.; Stepien, L.; Panda, E.; Tekok-Kilic, A. Resting-state EEG dynamics help explain differences in response control in ADHD: Insight into electrophysiological mechanisms and sex differences. PLoS ONE 2023, 18, e0277382. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuo, M.; Higuchi, T.; Ichibakase, T.; Suyama, H.; Takahara, R.; Nakamura, M. Differences in Electroencephalography Power Levels between Poor and Good Performance in Attentional Tasks. Brain Sci. 2024, 14, 527. https://doi.org/10.3390/brainsci14060527
Matsuo M, Higuchi T, Ichibakase T, Suyama H, Takahara R, Nakamura M. Differences in Electroencephalography Power Levels between Poor and Good Performance in Attentional Tasks. Brain Sciences. 2024; 14(6):527. https://doi.org/10.3390/brainsci14060527
Chicago/Turabian StyleMatsuo, Moemi, Takashi Higuchi, Taiyo Ichibakase, Hikaru Suyama, Runa Takahara, and Masatoshi Nakamura. 2024. "Differences in Electroencephalography Power Levels between Poor and Good Performance in Attentional Tasks" Brain Sciences 14, no. 6: 527. https://doi.org/10.3390/brainsci14060527
APA StyleMatsuo, M., Higuchi, T., Ichibakase, T., Suyama, H., Takahara, R., & Nakamura, M. (2024). Differences in Electroencephalography Power Levels between Poor and Good Performance in Attentional Tasks. Brain Sciences, 14(6), 527. https://doi.org/10.3390/brainsci14060527






