Abstract
Depression is the most common mental disorder worldwide. Both antidepressants and psychotherapy are effective in treating depression, but the response to these treatments is often incomplete. Yoga-based interventions (YBIs) have been advocated by some researchers as a promising form of alternative treatment for depression. Recent research has attempted to identify the biological mechanisms associated with the antidepressant actions of YBIs. In this scoping review, conducted according to the PRISMA-ScR guidelines, the PubMed and Scopus databases were searched to retrieve research on biomarkers of response to YBIs in patients with depression. These studies were also critically reviewed to evaluate their methodological quality and any sources of bias. Nineteen studies were included in the review. Based on these studies, there is preliminary evidence that YBIs may be associated with increased serum brain-derived neurotrophic factor (BDNF) and reduced serum cortisol and interleukin-6 (IL-6) in patients with depression. However, many of these changes were also observed in the control arms, and the overall quality of the research was low. At present, it cannot be concluded that there are reliable biomarkers of response to YBIs in depression, though there are some potential biological correlates. Further advances in this field will depend critically on improvements in study design, particularly the minimization of sources of bias and the selection of more specific and sensitive biomarkers based on existing evidence from other treatment modalities.
1. Introduction
Depressive disorders are one of the leading causes of morbidity and disability worldwide [1]. This group of disorders is characterized by persistent low mood with associated changes in cognition, behavior, and biological functions. It includes major depressive disorder (MDD), characterized by discrete episodes of a greater severity, and dysthymia, characterized by chronic, “low-grade” depressive symptoms [2]. It is estimated that at least 300 million people suffer from depressive disorders globally; in the year 2017 alone, it was estimated that there were around 250 million new cases or episodes of depression [3]. Depression arises from a complex interplay of biological, psychological, and social factors, which results in significant cross-national and cross-regional variations in its occurrence. It is estimated that the prevalence of depression is 4.1–5.8% in European countries, 5.9% in the United States, 3.4–4.8% in Africa, and 3–4.5% in Asian countries. In India, the prevalence of depression is estimated at 4.5% [3].
The treatment of depressive disorders usually involves pharmacological treatment with antidepressant medications and psychotherapies such as interpersonal therapy (IPT) and cognitive behavioral therapy (CBT) [4]. Though these treatments are effective, there is a significant number of patients who do not respond to them. It is estimated that at least 30–40% of patients will not respond to initial treatment with antidepressants, and the likelihood of response to subsequent treatments is of a similar order [5,6,7,8]. Moreover, some patients who respond initially to medication may relapse despite continued treatment [9]. Psychotherapies have advantages over medication in terms of effectiveness, safety, and sustained response [10], but may not be readily available in low-resource settings [11]. There is significant heterogeneity within the group of depressive disorders, and patients with different symptom profiles may respond better to specific types of treatment or combinations of treatments [12].
The limitations of conventional monotherapies for depression were highlighted by a recent meta-analysis, which showed that combined therapies were superior both in terms of efficacy and of sustained improvement in symptoms of depression [13]. However, providing combined pharmacotherapy and psychotherapy poses its own challenges in terms of requirements in time, personnel, and infrastructure.
The increasing awareness of the limits of “conventional” treatment for depression has led to a renewed interest in complementary and alternative therapies. This term “complementary and alternative medicine” (CAM) or “integrative medicine” encompasses a wide range of interventions that fall outside the umbrella of “allopathic” medicine [14]. A wide range of CAM-based treatments have been tested in depression, but only a few have shown replicated evidence of efficacy [15,16]. Most of these treatments have been used as adjuncts to standard treatments, though some have been tried as monotherapies for milder forms of depression [17].
1.1. Yoga as a Treatment for Depression
Yoga is an ancient spiritual discipline originating from India. The word yoga is derived from a Sanskrit root meaning “union”, which refers to the aim of this discipline—to achieve union between individual and universal (divine) consciousness, and between mind and body [18]. The disciplines that constitute yoga can be broadly divided into four components: physical movements (asana), relaxation (savasana), breathing techniques (pranayama), and meditative techniques (dhyana) [19]. Yoga is sometimes classed together with CAM or integrative therapies. Experts in the field emphasize that it is not primarily a “treatment” or an “intervention” for physical or mental illnesses; rather, it is a set of practices aimed at mind–body “oneness” or “integration” [20,21]. Nevertheless, some researchers have investigated the potential of yoga as a treatment for mental disorders, including depressive disorders. The first clinical trials in this field, conducted over two decades ago, found that yoga was effective in the treatment of both major depression and dysthymia, with an efficacy comparable to that of antidepressant monotherapy. However, these reports came from a single research center in India and required replication [22,23].
Subsequent studies have tended to confirm this result, with some caveats. A systematic review of yoga for depression, published in 2017, concluded that it did have positive effects that exceeded those of placebo. This review also identified significant methodological concerns in existing research, pertaining to study power, differences between monotherapy and combined therapy trials, and a lack of medium- or long-term results. These limitations reduced the confidence that could be placed in “positive” research reports [24]. A more recent meta-analysis, published in 2023, found that yoga had a moderate effect in reducing symptoms of depression (Cohen’s d = −0.60 to −0.64) and a small effect on associated symptoms of anxiety (Cohen’s d = −0.26). The authors of this review cautioned that many of the included studies had a significant risk of bias. They concluded that overall quality of evidence for yoga in depression was “low to moderate” in quality as per the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines. Another limitation of this review is that it included some studies in which depression was only a secondary outcome, and in which patients did not fulfill diagnostic criteria for MDD [25].
Results from the most recent published reports suggest that yoga, when used as an adjunct or “add-on” to treatment as usual (TAU), may be effective and cost-effective in the management of depressive episodes. Such results have been obtained both from India [26,27] and from high-income European countries [28,29]. However, it is unclear if consideration of these subsequent results would substantially alter the findings of the most recent meta-analysis.
As a result, expert recommendations for the treatment of depression have taken varying positions on the value of yoga, with no clear consensus [30,31]. The conclusion reached by the Indian Psychiatric Society (IPS) is particularly relevant in this context, as India is the country in which most clinical trials of yoga for MDD have been conducted. The IPS guideline concludes, “Studies related to role of the traditional therapies like meditation, yoga and related techniques have been mostly published in documents of various organizations propagating that particular technique. Well-designed scientific studies to authenticate these claims need to be conducted” [32].
1.2. Can Biomarkers Be Used to Improve the Quality of Yoga Research in Depression?
1.2.1. Limitations of Clinical Research Pertaining to Yoga in Depression
Based on the current evidence, it can be tentatively concluded that YBIs are effective in reducing depressive symptoms in some patients, but that these results cannot be readily generalized due to methodological limitations, including the possibility of bias. In a review addressing this issue, Nauphal et al. critically evaluated the limitations and outlined an agenda for future research [33]. They identified five questions that needed to be addressed by researchers before firm recommendations regarding yoga could be incorporated into standard practice. These questions can be briefly summarized las follows:
- Are specific types of YBIs better than others in the treatment of depression? This is important because different authors have used different yoga-based techniques or modules, with different admixtures of some or all of the domains of yoga [19]. Variations in the exact type of intervention can lead to significant variability in observed outcomes [30].
- How often/for how long should YBIs be used to achieve a meaningful response? Most available research has only assessed short-term response over a period of 8 weeks or less, but depression is a chronic and recurrent illness, and it is not clear if brief YBIs have a sustained effect [34].
- Are there any adverse effects associated with the use of YBIs in depression? One of the “selling points” of YBIs is that they are safer than pharmacological treatments. However, there have been reports of adverse events, such as paradoxical increases in anxiety or depression, particularly with the use of meditation-based techniques [35,36].
- How do YBIs synergize with “standard” treatments for depression? Several clinical trials in this field have involved the comparison of adjunctive YBIs to “treatment as usual” in patients receiving recommended doses of antidepressants. However, it is unknown if the observed effects of YBIs in this context are simply additive, or if they enhance the effects of prior treatment. It is also unknown if YBIs are superior to other forms of adjunctive treatment, such as pharmacological augmentation or combined pharmacotherapy and psychotherapy [37].
- What are the barriers and challenges in accessing YBIs for patients in diverse settings? Several factors could potentially reduce the acceptability of YBIs, though they have not been specifically studied in depression. These include physical pain or disability, varying cultural attitudes towards the acceptability of yoga, a lack of qualified trainers or therapists, and financial constraints [38].
To some extent, these questions are being answered by recent research. For example, there is now more evidence on the combination of YBIs with standard therapy than there was in 2019 [26,27,28,29,39,40]. We know that a wide range of YBIs show comparable efficacy in depression, including hatha yoga, Sudharshan Kriya Yoga (SKY), body-oriented yoga, mindful yoga, and even yoga packages developed specifically for depression [39,40,41]. However, there have been no head-to-head comparisons of specific YBI techniques or modules, or attempts to identify relationships between specific techniques and improvements in specific symptoms of depression.
It is possible that controlled clinical trials with a better design and a reduced risk of bias could provide more definitive evidence on the role of YBIs in depression. However, even if evidence of clinical improvement is demonstrated, the mechanisms through which such improvement occurs are unknown. There is an unanswered, sixth question that remains to be addressed: What are the molecular and cellular processes associated with the response to yoga in depression? Depression is a complex syndrome associated with alterations in central nervous, autonomic, neuroendocrine, immune-inflammatory, and gut–brain axis functioning [42,43]. Distinct yoga techniques can potentially exert beneficial effects on pathways implicated in the pathogenesis of depression. For example, relaxation and breathing techniques may ameliorate the neuroendocrine dysregulation seen in depression [44], while meditation may have beneficial effects on the functioning of specific regions of the cerebral cortex [45]. There is a need to understand and characterize these mechanisms, so that improvements in symptoms can be correlated with underlying physiological and biochemical changes [46]. Understanding these changes at the molecular, cellular, and organ level would have three key benefits [47]:
- A better understanding of the biological correlates of symptom improvement in depression.
- A more evidence-based approach to YBI, facilitating a rapprochement between yoga practitioners and practitioners of modern medicine, thereby avoiding the extremes of skepticism and unrealistic claims [32].
- A personalized approach to the use of YBI in depression, through the identification of those patients who would respond best to them.
1.2.2. Biomarkers and Their Subtypes
In other words, what is required is a careful study of biological correlates, and if possible of biomarkers, associated with response to yoga-based interventions in depression. A biomarker has been defined by the United States Food and Drug Administration (FDA) and National Institutes of Health (NIH) Biomarker Working Group as “A defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes or responses to an exposure or intervention” [48]. Biomarkers are measures based on “molecular, histologic, radiographic or physiologic characteristics”, and are distinct from measures of an individual’s emotions, behavior, or responses to a questionnaire [49]. They are objective measures of cellular, organ, or system function that can be correlated with changes in self- or clinician-rated (subjective) status. Characterization of a biomarker requires a description of its identity, biological plausibility, and measurement method [48].
Biomarkers have been subtyped into five broad categories: diagnostic, monitoring, response, predictive, and prognostic [48,49]. A brief description of these subtypes is provided in Table 1.

Table 1.
Types of biomarkers in medical research, based on FDA-NIH guidelines.
1.2.3. Biomarkers Related to Depression and Its Treatment
There is as yet no biomarker that can reliably diagnose depression [43]. However, several potential biomarkers have been evaluated for their utility in assessing disease activity, treatment response, and illness course in depression [50,51]. Response biomarkers for antidepressant therapies fall broadly into five categories: imaging, electrophysiology, cognition, biochemical, and genetic [52,53]. More recently, novel biomarkers such as levels of specific microRNAs or measures of DNA methylation have also been studied in relation to treatment response in depression [54]. In terms of the strength of evidence for each of these groups of biomarkers, large effect sizes (combined Hedge’s d > 1) have been noted for imaging, electrophysiology, cognitive and protein/nucleotide markers, and small to moderate effective sizes (combined Hedge’s d = 0.5) for genetic markers. However, marked inter-study variability has been observed in studies of electrophysiology, cognitive, and genetic biomarkers [52]. More details of these categories of biomarkers in depression research are provided in Table 2.

Table 2.
Biomarkers used to predict treatment response to antidepressants in patients with depression.
Most studies of treatment response in depression have evaluated biomarkers in relation to antidepressant drug therapy. However, these markers may also be useful in evaluating the response to other forms of treatment, such as electroconvulsive therapy [55], repetitive transcranial magnetic stimulation [56], and psychotherapies such as cognitive behavioral therapy (CBT) [57].
1.2.4. The Difference between “Biological Correlates” and “Biomarkers”
In this context, it is important to note that several studies have examined changes in the levels of other molecules implicated in the pathogenesis of depression, such as pro-inflammatory cytokines and other inflammatory mediators, in relation to antidepressant response. Though some experts consider these valuable predictors of treatment response [58], others differ with this opinion and prefer to label them “biological correlates” instead of “predictors” or “biomarkers” [52]. This distinction is important, because not all biological changes seen in the course of treatment for depression correlate directly with the response. Moreover, levels of some of these proteins or hormones may remain elevated even after successful treatment. Such biochemical parameters cannot be truly called “biomarkers” [48]. The controversy over whether changes in cytokines are “response” or “predictive” biomarkers in relation to treatment in depression is unresolved; as of now, the consensus is that these molecules are biological correlates that may prove to be biomarkers pending future research [50,59]. This distinction will prove to be important when evaluating claimed “biomarkers” of response to YBIs.
1.2.5. Possible Biological Correlates of Yoga and Their Relevance to Depression
Experts in the field of yoga have claimed that its regular practice has beneficial effects on a number of biological processes. However, as with yoga research in general, research on the “biology” or “neurobiology” of yoga has yielded inconsistent results, and is subject to significant methodological limitations [60]. With these caveats, it may be noted that recent meta-analyses suggest that yoga may increase gray matter volumes in the insular and hippocampal cortices, improve functional connectivity in the prefrontal cortex and default mode networks, increase heart rate variability, and reduce levels of certain pro-inflammatory markers [61,62,63]. Theoretically, these changes could be beneficial for patients with depression [64]. This speculation could not be verified due to a lack of controlled clinical research on biological changes in patients with depression receiving YBIs. With the recent growth of research in this area, it is important to critically review the evidence that has now accumulated, and to see if it can bring a greater degree of objectivity to the use of YBIs in clinical practice.
The aims of this review are fourfold:
- to summarize the current state of research on possible biological correlates associated with yoga-based interventions in patients with depression;
- to assess whether any of these correlates qualify as biomarkers, using the rigorous definitions provided by the FDA-NIH guidelines and earlier researchers;
- to critically evaluate the quality of this research, with a particular focus on possible sources of bias;
- to situate these findings in the broader context of biomarker research related to treatment response in depression in general.
2. Review Process
The objective of the current paper is to review existing studies of potential biomarkers associated with response to YBI in patients with depression. Due to the heterogeneity of the available research, a scoping review methodology was adopted based on the PRISMA-ScR guidelines [65]. The completed PRISMA-ScR checklist for this review is available as Supplementary Material Table S1.
For this review, the PubMed and Scopus databases were searched for relevant papers from a scoping perspective. All relevant papers published up to March 2024 were included. The search strategy was constructed based on both the PRISMA-ScR guidelines and prior scoping reviews of biomarker research in psychiatry [65,66,67]. In the first step of this review, a broad search strategy was used to retrieve citations related to the broad area of yoga-based interventions for depression. The following terms were used: (“major depression” OR “depressive episode” OR “major depressive episode” OR “depressive disorder” OR “major depressive disorder” OR “recurrent depressive disorder” OR “dysthymia”) AND (“yoga” OR “hatha yoga” OR “yoga-based intervention” OR “yoga-based therapy” OR “yoga therapy”), included in either the title, abstract, or full text.
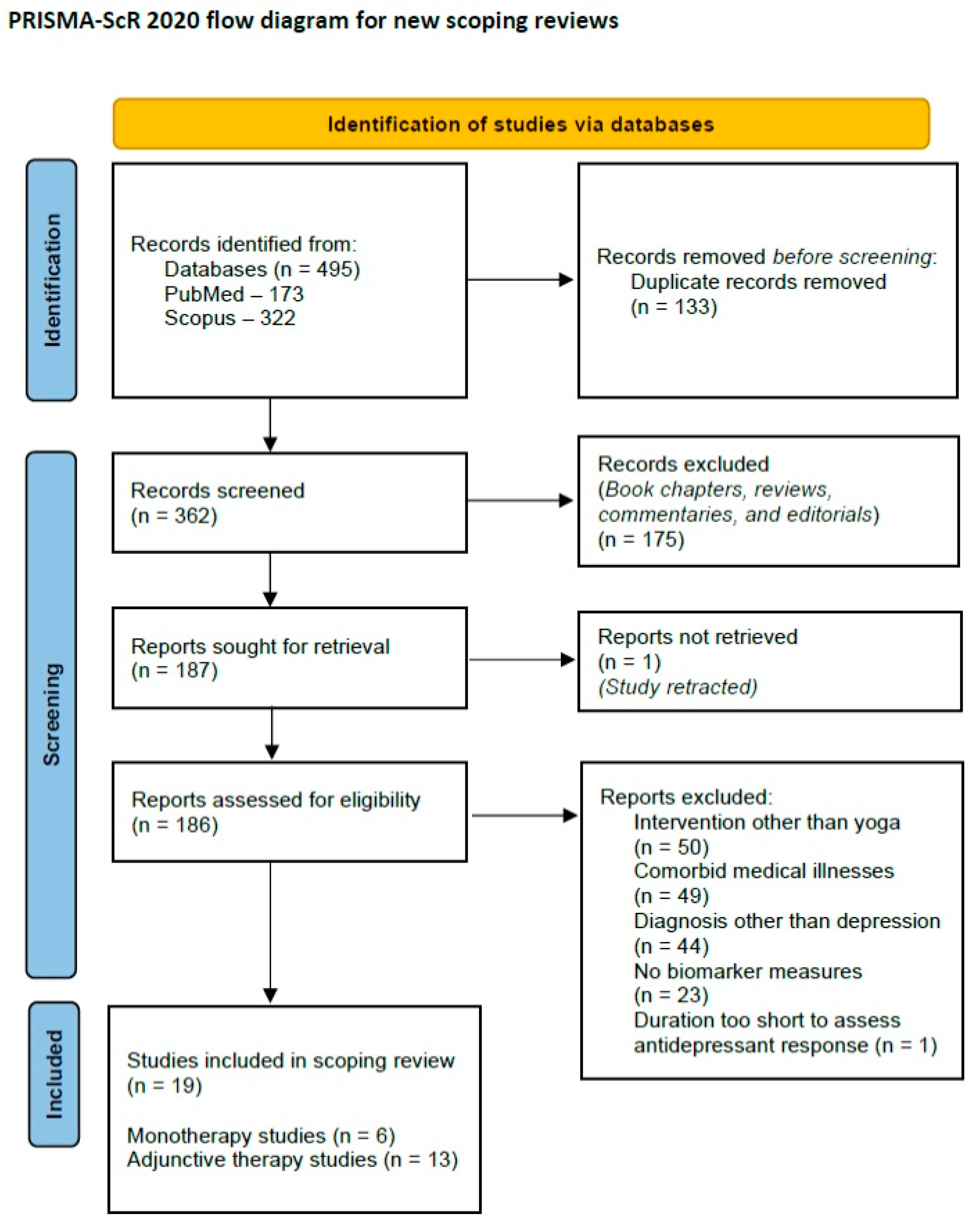
A total of 495 citations were retrieved at this step. After removal of duplicate citations and screening out of unrelated papers based on the title and abstract, 187 full-text articles were screened for inclusion of the scoping review. As one of these articles had been retracted due to errors in reporting the results, 186 full texts were reviewed. In the second step, full-text articles were screened for suitability for the review. Studies were selected if they fulfilled the following criteria:
- Clinical trials in patients with depression, defined as major depressive disorder or dysthymia, diagnosed using the standard diagnostic criteria of the American Psychiatric Association or World Health Organization.
- No primary medical comorbidity, such as diabetes mellitus, cancer, or cardiovascular disease.
- Use of a yoga-based intervention, either as a monotherapy or as an adjunct to standard treatment or “treatment as usual” (TAU).
- Assessment of response to treatment in terms of improvements in depressive symptoms using a standardized and validated rating scale, such as the Hamilton Depression Rating Scale (HDRS) or Montgomery–Asberg Depression Rating Scale (MADRS).
- Measurement of one or more potential biomarkers at baseline and/or during treatment. Biomarkers were selected only if they fulfilled the criteria of conceptual clarity and biological plausibility. To establish conceptual clarity, the definitions of “response” or “predictive” biomarkers were used as per the FDA-NIH criteria [48]. To establish biological plausibility, only those molecules that had been identified as markers or predictors of treatment response in depression in previous systematic reviews and meta-analyses were included [50,51,52,53,54].
- Papers published in English.
The following types of studies were excluded:
- Trials of patients with other medical conditions (e.g., fibromyalgia, pre-menstrual symptoms) in which depressive symptoms were measured as a secondary outcome.
- Trials in which information on reductions in depressive symptoms was not reported.
- Studies whose duration was too brief to meaningfully assess treatment response in depression. Most trials of any intervention of depression require a period of 4 to 8 weeks to observe a response; therefore, trials with a duration <4 weeks were not included in the review.
Based on these criteria, a total of nineteen papers were selected for inclusion in the review. A PRISMA-ScR flow diagram depicting the review process is provided below (Figure 1).
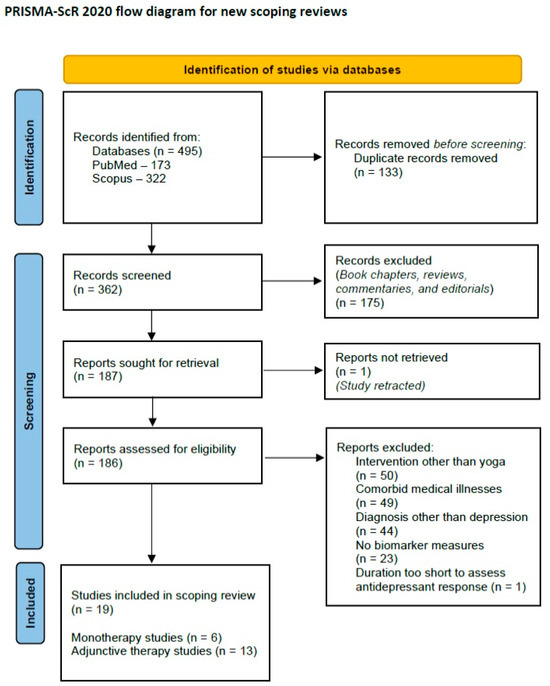
Figure 1.
PRISMA-ScR 2020 flow diagram for the current review.
At the third step, data from each paper were extracted and tabulated under the following headings:
- Study sample characteristics: country of origin, setting, sample size;
- Clinical diagnosis, including any comorbidities if documented;
- Treatment(s) received in addition to YBI, if specified;
- YBI characteristics: type of YBI, number of sessions;
- Biomarker types and measures;
- Study results, both positive and negative, in terms of changes in biomarkers and/or associations between biomarkers and treatment response;
- Whether the given biological correlate could be considered a possible biomarker, based on the FDA-NIH criteria.
The fourth step involved assessment of study quality. In view of the concerns raised regarding the methodological quality of research on YBIs for depression, two aspects of study quality were assessed for each paper. First, each study was assessed using the BIOCROSS tool. This tool was specifically designed to evaluate the quality of biomarker studies in medical research [68]. It assigns a total score of 0 to 20 for a given paper, with higher scores indicative of better methodological quality. Second, the risk of bias in each of the biomarker-based clinical trials was assessed using the Cochrane Risk of Bias tool, version 2 (RoB 2) [69]. This is important because a high level of bias in the original trials could lead to false-positive findings regarding biomarkers of response to YBIs for depression. If the information in the retrieved reports was not sufficient for a complete RoB rating, the original published reports of the clinical trial were retrieved and data were extracted from them. This was necessary for two of the included studies [70,71].
3. Review Findings
3.1. Characteristics of the Included Studies
Overall, a total of six papers on YBI monotherapy [72,73,74,75,76,77] and thirteen papers on adjunctive YBI in patients with depression [78,79,80,81,82,83,84,85,86,87,88,89,90] were included in this review. After correcting for duplication of samples across some studies, data were available for 559 patients across 19 reports. The complete details of all included publications are provided in Table 3.

Table 3.
Characteristics of studies examining biomarkers of response to yoga-based interventions in patients with depression.
Among these papers, sixteen included patients with MDD, one included patients with either MDD or dysthymia, one included patients with dysthymia alone, and one included patients with either MDD or anxiety disorders with significant depressive symptoms. In all but one of the studies of MDD, patients were diagnosed based on standard diagnostic criteria, namely the American Psychiatric Association’s Diagnostic and Statistical Manual (DSM-IV/DSM-5) or the World Health Organization’s International Classification of Diseases and Disorders (ICD-10).
The majority of published reports (14 of 19) were from India [72,73,75,77,78,79,80,81,82,84,86,87,88,90]. Nine of these fourteen reports were from a single Indian center [72,73,77,81,82,84,87,88,90]. There were three publications from the United States [74,76], one from Germany [83], and one from Italy [85].
3.2. Study Quality
Study quality was assessed using the BIOCROSS and RoB 2 instruments. The mean BIOCROSS score for the papers included in this study was 13.4 ± 3.3, indicating a generally acceptable methodological quality. The lowest score obtained was 8 (out of a maximum of 20) [72,77] and the highest was 19 [83]. Studies obtained high mean scores on assessments of study hypothesis (1.6 of a maximum of 2) and description of the biomarkers measured (1.6). The lowest scores were obtained for limitations (1.0), statistical modeling of biomarker changes in relation to clinical response (1.1), and statistical analysis in general (1.2).
In other words, most studies described their rationale in clear and logical terms, and used valid and replicable laboratory methods to estimate potential biomarkers. However, they had deficiencies in terms of statistical analysis, modelling biomarker changes in relation to therapeutic response, and of emphasizing study strengths and positive findings without fully acknowledging limitations.
When assessed for risk of bias, sixteen of the nineteen included studies were found to be at high risk of bias [72,73,74,76,77,78,79,80,81,82,83,84,85,86,87,90], and three had some concerns pertaining to bias [75,88,89]. None of the studies fell into the low-risk category. On average, studies were found to be at high risk of bias in a median of two domains. When considering the individual domains of the RoB 2, only one study showed evidence of bias arising from a possible deviation in protocol [72]. However, five studies had concerns related to missing outcomes, eight studies had concerns related to the allocation of subjects to groups, eight had concerns related to the selection of the result, and nine had potential biases related to outcome measurement.
Overall, bias was a significant issue of concern in the existing literature. There was no significant difference in the proportion of studies rated at high risk of bias between monotherapy and adjunctive studies (5/6 vs. 11/13; p = 1.000, Fisher’s exact test) and between studies from India and those from other countries (12/14 vs. 4/5; p = 1.000, Fisher’s exact test).
In addition to these findings, study sample sizes were low (mean 39.1 ± 24.7 participants, range 15–84), and none of the reports included a formal sample size estimation. This suggests that this research may have been statistically underpowered in general.
3.3. Biological Correlates, Symptom Severity, and Treatment Characteristics
A wide range of potential biomarkers were studied in relation to adjunctive yoga in the existing literature. Based on the taxonomy suggested by Voegeli et al. for biomarker studies of treatment response in depression, these could be classified as follows: electrophysiology (n = 8), biochemical (n = 7), cognitive (n = 2), neuroimaging (n = 1), and genetic (n = 1). Electrophysiological and biochemical markers were the most frequently assessed, while neuroimaging and genetic markers were rarely evaluated [52]. In two studies, biomarkers were estimated only at baseline [73,74]; in the remainder, they were measured both at baseline and after the completion of treatment.
In most studies (17 out of 19), symptom severity was measured using a standardized rating scale for depressive symptoms. The most commonly used instrument was the Hamilton Rating Scale for Depression (HAM-D), followed by the Beck Depression Inventory (BDI). A single study used the Quick Inventory of Depressive Symptomatology (QIDS). Two studies did not report symptom severity at baseline [77,87]. Among the seventeen studies using a rating scale, two used a HAM-D cutoff of 18, indicating moderate depression, to select patients, but did not provide mean or median symptom scores. In the remaining studies, mean symptom scores for MDD ranged from 17.6 to 24.6 on the HAM-D and 23.2 to 30.8 on the BDI, indicating moderate to severe depression.
Among studies of electrophysiological markers, four studies examined changes in brain electrical activity using electroencephalography (EEG) [72,73,77] or transcranial magnetic stimulation (TMS) [83]; the remaining four evaluated cardiac autonomic functioning [74,80,85,88]. Biochemical studies showed marked heterogeneity, including assessments of neuroendocrine functioning [83,84,86], immune-inflammatory markers [86,89,90], brain-derived neurotrophic factor (BDNF) [82,86], and oxidative stress [86].
The type and duration of yoga-based interventions varied significantly across studies. Techniques used included Iyengar yoga [74,76], Sahaj yoga meditation [78,79,80], Hatha yoga [83,89], a depression-specific yoga module developed by experts [91], Sudarshan Kriya yoga [72,73,85], and a yoga- and meditation-based lifestyle intervention [75,86]. The duration of treatment ranged from 2 to 12 weeks, though most studies provided yoga-based interventions for 8–12 weeks. Details of these types of YBI, and which of the four components of yoga were included in each of them, are provided in Table 4. All interventions included at least two of the components of yoga, and three of them included all four components—movement, breathing, relaxation, and meditation. The most frequently used YBI was the yoga therapy module for depression, which was used in seven studies, all from the same center [91].

Table 4.
Description of the different types of YBI used in the included studies.
In studies of adjunctive YBIs, concurrent treatments are an important confounding factor. Twelve of the thirteen studies of adjunctive therapy included patients with antidepressants “as usual”, meaning that they were allowed to continue stable doses of antidepressant treatment in a naturalistic manner. One study attempted to standardize pharmacological treatment by randomizing participants to receive either the antidepressant escitalopram (10 mg/day) or the atypical antipsychotic quetiapine (300 mg/day) [83]. In twelve of the studies, adjunctive yoga was compared to pharmacotherapy alone, which served as the control group. In a single report from the United States, the control group received an educational intervention on lifestyle modification in addition to medication [89].
3.4. Biological Correlates vs. Biomarkers of Yoga-Based Intervention
The body of research included in this review yielded mixed results in terms of changes in possible biomarkers related to yoga-based interventions. Twelve studies reported findings that could be interpreted as positive, six reported no significant effects of YBI on the specified biomarker, and one reported a trend towards a possible effect of yoga. The interpretation of these findings is complicated by the fact that very few studies directly modelled the statistical relationship between changes in biomarkers and changes in depressive symptoms. Four studies tested for such a relationship [82,83,85,86] but only two established a possible link with the YBI of interest [82,86]. Two studies reported a temporal association between biomarker changes and symptom improvement, but did not explore this further [72,77]. This reflects the deficiencies in statistical analysis identified by the BIOCROSS ratings, as mentioned in Section 3.2.
When applying the criteria for biomarkers of response based on the FDA-NIH definition, more significant concerns were identified. Of the nineteen papers reviewed, twelve (63%) did not satisfy the definition for a response or predictive biomarker. In other words, any findings reported in these papers can only be considered “biological correlates” of YBIs in depression and not biomarkers. Four papers [75,83,86,90] reported results that could be considered response biomarkers, and two [74,81] reported findings that were suggestive but not conclusive. Overall, only four of fourteen Indian studies (28.6%) and two of five studies from other countries (40%) attempted to check for genuine “biomarker” effects. This difference was numerically in favor of the studies outside India, but was not statistically significant (p > 0.5, Fisher’s exact test). The existing research could be best described as identifying biological correlates and not biomarkers of response to YBIs in patients with depression. Detailed study results are given in full in Table 3, with key findings discussed below.
3.4.1. Electrophysiological Studies
Among electrophysiological studies that involved measures of brain activity, a single study found that an adjunctive YBI module increased the cortical silent period. An association with symptom change was not reported [87]. Studies of electroencephalographic (EEG) parameters such as the P300 auditory event-related potential and alpha activity yielded inconsistent results [73,74,79].
Among studies of cardiac autonomic activity, a baseline component of heart rate variability (HRV) was associated with a better response to Iyengar yoga monotherapy [74], but studies of changes in HRV-related autonomic parameters following treatment yielded equivocal results [80,85,88].
All electrophysiological studies were assessed as being at a high risk of bias affecting study outcomes, except for a single study of heart rate variability [88].
3.4.2. Biochemical Studies
a. Neurotrophic factors: Three studies, one of monotherapy and two of adjunctive therapy, examined changes in BDNF in relation to YBIs for depression [77,81,86]. All three reported increases in serum BDNF after YBI. In one study, this change was also observed in patients receiving TAU [81]. Two of these studies reported a correlation between increased BDNF and improvement in depressive symptoms [81,86].
b. Immune-inflammatory mediators: Two studies reported a reduction in peripheral levels of pro-inflammatory cytokine IL-6 after adjunctive YBI [86,89], and one reported a reduction in the complement components C1q, Factor H, and properdin [90]. These changes were not significantly correlated with improvements in depression.
c. Neuroendocrine correlates: Two studies of adjunctive YBIs found a reduction in serum cortisol after treatment with yoga, but not TAU. The relationship of these changes to the response was unclear [84,86]. In contrast, a study of Hatha yoga found no effect of yoga on cortisol responses to dexamethasone or CRH stimulation. In the latter study, normalization of the cortisol response was associated with improvement in depression in both the yoga and TAU groups [83].
d. Others: A single study found that adjunctive YBI, but not TAU, was associated with reductions in markers of oxidative stress and cellular aging. These changes were not significantly associated with treatment response [86].
All these studies, except one study of pro-inflammatory cytokines [89], were rated as being at high risk of bias.
3.4.3. Neurocognitive, Genetic, and Imaging Studies
a. Neurocognition: A study of Sahaj yoga meditation found significant improvement in two tests of sustained attention in patients receiving yoga compared to TAU [78]. A study of an adjunctive YBI module found improvements in tests of concentration, processing speed, and verbal memory in both the yoga and TAU groups [82]. The relationship between these changes and treatment response was not studied. Both these studies were rated as having a high risk of bias.
b. Genetics: A single study examined the effect of serotonin transporter (SLC6A4) and methylene tetrahydrofolate reductase (MTHFR) functional polymorphisms on response to yoga-based monotherapy. No association was found between either variant and response to yoga, though SLC6A4 genotype was associated with antidepressant response [75]. This study was rated as having some concerns with regards to bias.
c. Neuroimaging: A single study found evidence of increased thalamic GABA following Iyengar yoga monotherapy over 12 weeks on magnetic resonance spectroscopy; this was not clearly related to treatment response [76]. This study was rated as having a high risk of bias.
4. Critical Analysis of the Review Findings
Yoga-based interventions (YBIs) have been advocated for by some researchers and experts as an emerging option for the treatment of depression. There has been a significant growth of clinical research in this field. Results of YBI monotherapy have been mixed, though there is substantial evidence that adjunctive YBI outperforms patients receiving treatment as usual, and is well-tolerated. The mechanisms underlying this possible therapeutic effect are largely unknown, and have been investigated only recently. It is possible that some of the biological pathways influenced by YBIs are similar to those implicated in the action of antidepressants [64]. However, as will be seen below, such a conclusion cannot yet be drawn confidently due to certain conceptual and methodological concerns.
4.1. Replicated Findings Pertaining to Biological Correlates of YBI Response in Depression
A wide range of putative biomarkers have been investigated in relation to YBIs for patients with depression. As seen in Section 3.3, the majority of these do not satisfy strict criteria for biomarkers of treatment response. Moreover, replication of findings is an important concern in biomarker research in depression, and findings from single studies must be interpreted with prudence [52]. If we confine ourselves to results that have been replicated, or demonstrated by at least two independent groups of researchers, then only three findings emerge as being of potential relevance: increased serum BDNF [77,81,86], reduced serum cortisol [84,86], and reduced serum IL-6 [86,89].
These findings are potentially relevant, because alterations in all these three biochemical markers have been implicated in the pathophysiology of depression and in the response to conventional antidepressant treatments. Depressive episodes are associated with reduced peripheral BDNF [92], increased peripheral IL-6 [93,94], and dysregulated functioning of the HPA axis [95,96]. To some extent, these alterations have been found to normalize after successful treatment with medications, psychotherapy, or electroconvulsive therapy [97,98,99], though it should be noted that none of these have been consistently identified as biomarkers of treatment response in depression [52].
In addition, the biological processes indexed by these molecules are intimately linked to each other in the genesis of depressive disorders. Early childhood adversity, chronic stress, or depression itself can lead to HPA axis dysregulation, associated with increased cortisol levels and dexamethasone non-suppression [100,101]. These changes in HPA axis responsiveness lead to an increase in peripheral inflammation, as evidenced by increased peripheral levels of cytokines such as IL-6 [102]. Increases in peripheral inflammation can trigger microglial activation and neuroinflammation, leading to reduced neural plasticity, which is correlated with reduced levels of BDNF and with depressive symptomatology [103,104].
Additional support for a possible beneficial effect of YBIs on these molecular pathways comes from studies of yoga in healthy controls and in patients with disorders other than depression. In these patients, the practice of yoga was also associated with apparently beneficial reductions in peripheral pro-inflammatory cytokine and cortisol levels [105,106,107]. However, as noted in a recent meta-analysis, changes in these markers are not always statistically significant, and their clinical relevance is open to question [63].
The convergence of these three lines of evidence suggests that the effects of YBIs on HPA axis functioning, reduced peripheral inflammation, and increased neurotrophic factor production may be related to their benefits in patients with depression. However, even this conclusion is only provisional, as most of these studies did not directly model the relationship between changes in the potential biomarkers and reductions in depressive symptoms. In one study, there appeared to be a significant association between increased BDNF and treatment response [86], but this was not consistent across studies [81].
Findings related to other biological correlates, such as measures of oxidative stress, cerebral cortical inhibition, heart rate variability and brain GABA levels, cannot be definitively interpreted due to the limited quantity and quality of evidence available. There is evidence that these processes are dysregulated in patients with depression, lending some support to these findings [108,109,110,111]. Among these results, perhaps the most plausible are those related to HRV. This parameter, which is a measure of the balance between cardiac parasympathetic and sympathetic activity, is reduced in depression and may predict response to antidepressants [110]. Moreover, the practice of yoga may increase HRV in healthy individuals [112]. Though it is possible that HRV may prove to be a useful marker response to YBIs in depression, the inconsistency and low quality of the available evidence precludes such a conclusion at the moment [74,85,88].
4.2. Correlation or Causation?
The key question to be considered when appraising the results of biomarker studies is whether changes in specific biological processes are meaningfully associated with changes in the patient’s symptomatic status. Stated in simple terms, it is necessary not just to show that yoga changes levels of certain well-defined markers, but that these changes correlate with reductions in depressive symptoms. If this cannot be established, then one cannot speak of “biomarkers” in the most accurate sense, as explained in Section 1.2.5 and Section 3.3. In fact, accurate modeling of the relationships between biomarker levels and clinical status is one of the criteria for assessing the quality of biomarker research [48,64]. If biological parameters are altered in patients with depression, but do not correlate meaningfully with symptoms or their improvement, they can only be considered “disease markers” but not response biomarkers as per the FDA-NIH guidelines [48,52]. As noted in Section 3.3, only four of the studies attempted to model a possible causal relationship between changes in biomarkers and symptomatic improvement, and this was tentatively confirmed in two of these studies, both involving BDNF [82,86]. Two further studies reported a temporal association between biomarker changes and clinical response to YBIs. While temporal association is necessary to establish that a given marker is a response biomarker, it is not sufficient, as certain parameters may change contemporaneously with clinical improvement without being meaningfully linked to it. For example, treatment with antipsychotics can lead to objective, measurable increases in body weight and metabolic parameters in patients that are concurrent with clinical response. However, these increases occur regardless of patient diagnosis, and are not meaningfully connected with contemporary models of the pathogenesis of psychosis. Therefore, they are not biologically plausible biomarkers of antipsychotic response [113].
In conclusion, the existing research cannot confirm a causal relationship between any particular biomarker—with the possible exception of serum BDNF—and response to YBIs in depression. This limitation, which arises from shortcomings in study design and data analysis (Section 3.2), needs to be addressed in future research in this field.
4.3. Limitations of the Existing Research
Apart from concerns related to replication and causality, there are certain important limitations of the existing research. These should be considered when interpreting the results of individual studies, and are enumerated below.
- Biomarker selection: Recent reviews of biomarker research in depression have highlighted the limitations of certain biological correlates—particularly electrophysiological and biochemical measures—as genuine markers of treatment response. Experts in the field believe that there is a need to move beyond these biomarkers and to investigate novel predictors of treatment response in depression, such as measures of DNA methylation or protein expression, in combination with digital methods of phenotyping and machine learning [114,115,116,117]. In contrast, most studies of YBI in depression have used biological correlates that have already been extensively investigated and found to be of limited specificity and predictive power [52,56,92]. Similarly, though neuroimaging and cognitive findings have yielded the best effect sizes for prediction of antidepressant response [52], only three of the studies reviewed here made use of these markers [76,80,82]. In addition, some studies have used markers that are not of proven value in predicting treatment response in depression, such as heart rate or EEG alpha activity [79,80]. Results from such research, even if positive, are of limited value.
- Secondary analyses and multiple comparisons: Some of the studies presented in this paper are based on re-analyses or secondary analyses of original trial data [78,79,80,81,82,84,87,88]. Such secondary analyses introduce a significant risk of false positive results, as they are not based on analysis of a pre-planned primary biomarker outcome [118]. Likewise, some of the included studies have analyzed a large number of previously untested biomarkers. Though this approach may be useful in generating novel hypotheses [119], it increases the possibility of false positive findings, especially if appropriate statistical corrections are not made [120]. For example, one of the included studies examined multiple markers of neuroplasticity, oxidative stress, cellular aging, and neural plasticity [86]; if twenty such markers are evaluated, at least one false positive can be expected at p < 0.05 [121]. As a result, even the positive findings identified in this review should be viewed with caution.
- Participant-related biases: The majority of included studies are from India, where yoga has its historical and spiritual origin. The practice of yoga in Indian patients is imbued with cultural meanings that extend beyond its use as an “antidepressant” treatment [122]. Such factors may affect patients’ preferences for YBIs, leading to biases in sample selection even when patients’ depression severity scores appear comparable. Moreover, when these patients practice a YBI for depression, they may experience an accentuated “meaning response”, similar to that observed when receiving placebo treatments in a drug trial. This can produce biochemical and physiological changes that are not specifically related to yoga [123].
- Confounding effects of variations in yoga technique: As illustrated in Table 4, the YBIs used by different groups of researchers vary widely. Though they may have certain key components or principles in common, the intensity at which these components are administered, and their relative emphasis in each treatment “module” or “package”, vary widely. Moreover, there are variations in therapist skill and experience and patient adherence to home-based practice that are difficult to quantify. As a result of this, a positive finding obtained in one study cannot be generalized to clinical practice if the YBI differs substantially. Thus, for example, some studies of the “yoga therapy module for depression” found evidence of an increase in BDNF following treatment [77,82]; however, it cannot be concluded that the same effect will be observed in patients receiving Hatha yoga or Sudarshan Kriya Yoga. This is an important limitation of yoga research in depression in general [33,124].
- Effects of other confounders on biological correlates: As noted in (a) above, most of the putative biomarkers studied in relation to YBIs for depression are non-specific in nature. This means, inter alia, that their levels can be altered by a number of factors not directly related either to depression or to yoga. Such confounding factors include age, gender, levels of physical activity, dietary practices, obesity, exposure to psychosocial adversity during childhood, ongoing stressors, and comorbid medical illnesses [94,125,126]. The studies reviewed in this paper have made little or no attempt to model for the possible effect of these confounders; as a result, it is difficult to conclude that either the levels of these biomolecules, or changes in them, are specifically linked to the presumed benefits of YBIs.
- Low methodological quality: As can be seen from the BIOCROSS and RoB 2 assessments of individual studies, particularly the latter (Section 3.2), the majority of included studies were at a high risk of bias, particularly with regards to outcome measures and their analysis. Moreover, statistical modeling of biomarker outcomes was often simplistic and could not establish a clear causal relationship between treatment and changes in biochemical or physiological parameters. Such evidence would receive an overall quality of “low” according to the GRADE guidelines [127], and cannot be reliably used to guide clinical practice.
- Lack of specificity of the observed changes to yoga: Even if the findings summarized in Table 3 can be established as biomarkers through further research, there is a key issue that remains unresolved—namely, the lack of specificity of such biological changes to yoga or YBIs per se. Similar changes in immune, autonomic, and endocrine parameters have been documented for other “body-oriented” or “mind-body” interventions, including exercise [128], meditation [129], relaxation techniques such as deep breathing or progressive muscle relaxation [130], and traditional practices from other countries [131]. Therefore, it is possible that the changes observed in YBI research are non-specific concomitants of participation in several meditation- or movement-based therapies. As there are no head-to-head trials comparing changes in biological parameters in patients receiving YBI and those receiving these treatments, this question remains unresolved.
- Country of origin effects: It has been known for at least the past two decades that the country of origin is an important confounder when evaluating the “positivity” of study outcomes. For example, studies of acupuncture from China or Japan are more likely to yield positive outcomes than those from other countries. This may reflect both participant biases, as mentioned in (c) above, and implicit biases on the part of researchers and regional journals [132]. In the case of yoga, it has been documented that studies from India are up to 25 times more likely to be positive than those from other countries [133]. A subsequent systematic review involving a larger number of studies found that this was probably due to selection bias [134]. While the measurement of biological parameters might appear to reduce this risk, it cannot negate it entirely, and it is possible that subtle biases may enter both during the study and in subsequent decisions to publish specific results [68,132,134]. Such methodological concerns have even been flagged by Indian researchers working in the field of complementary and alternative medicine [135]. This is a particular cause for concern in the current review, which is largely based on studies for India.
In conclusion, it can be stated that the overall quality of the evidence reviewed in this is low, and is subject to bias from multiple sources. Such evidence cannot be reliably used to predict treatment response in clinical practice. At best, it can be tentatively stated that YBIs for depression are accompanied by changes in certain biological processes, but that the significance of these changes remains unclear. There is an urgent need for better-designed studies with more rigorous statistical analyses and attempts to address potential sources of bias.
4.4. Synthesis and Future Directions
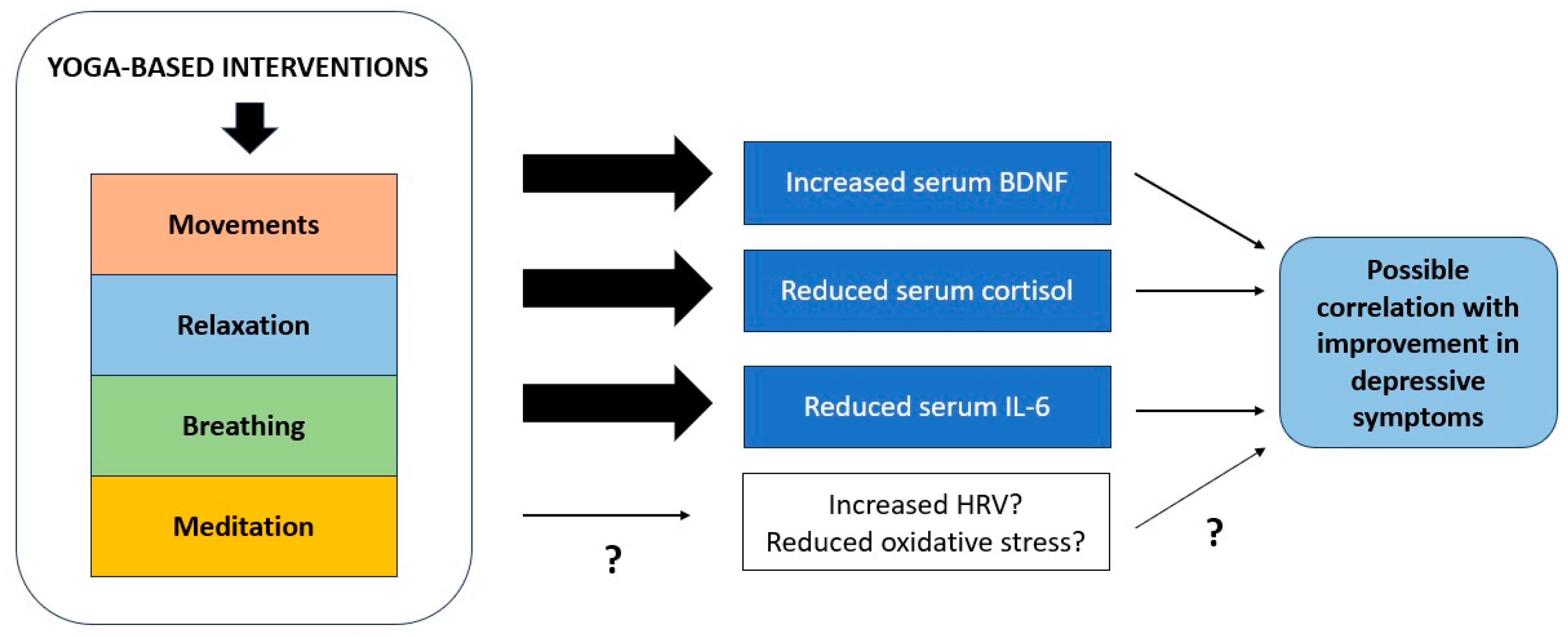
The existing research has uncovered a limited number of potential biological correlates of response to YBI. Though there are significant flaws in the underlying research; these changes can all be tentatively linked to a biological pathway that connects stress responses, peripheral inflammation, neuroinflammation, and neural plasticity. These mechanisms are illustrated graphically in Figure 2 below. This pathway has been implicated in the pathogenesis of depressive states by an extensive body of research, both in translational models and in patients with the disorder. It is possible that YBIs may have beneficial effects on depression through other pathways, including modulation of parasympathetic function and cortical inhibition, but these findings require replication. Moreover, none of these changes qualify as “biomarkers” using contemporary definitions, for the reasons summarized in Section 4.3.
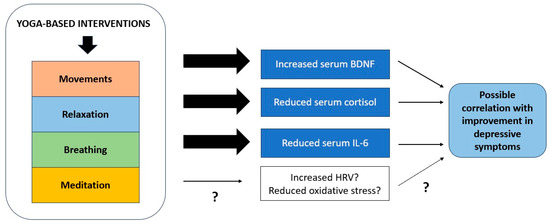
Figure 2.
Mechanisms through which yoga-based interventions may influence biological processes involved in recovery from depression. “?” denotes that there is inconsistent or inadequate evidence to confirm the relevance of a particular process.
The overall level of confidence that one can place in this evidence is low, due to various methodological limitations and sources of bias [136,137]. Therefore, the order of the day in this field would be to attempt to replicate these results in trials with a better design. This would include:
- Selection of study participants in such a way as to minimize bias, including multicentric trials with more sophisticated randomization methods.
- Comparative studies involving a YBI arm and a control arm receiving a different form of movement-related or mind–body therapy, such as exercise or meditation.
- Standardization of YBI modules and packages, to ensure comparability across studies, and to identify how specific yoga techniques affect discrete biological pathways.
- Selection of biochemical and physiological markers based on the most recent and consistent evidence from other studies of treatment response in depression. This would imply more research on neuroimaging and neurocognitive markers, as well as studies of novel biomarkers derived from epigenetic and proteomic research.
- Better handling of drop-outs, to avoid over-estimation of possible changes in biomarkers in patients receiving YBIs.
- Improved statistical analyses to minimize confounding effects and to reduce the likelihood of false positive findings.
Such improvements could be achieved through the development of standardized guidelines for conducting and reporting research on YBIs in depression, with reference to both biological correlates and clinical outcomes [138,139].
4.5. Strengths and Limitations of the Current Review
The chief strengths of this review are two-fold. First, it represents the first attempt to synthesize the existing evidence on the biological correlates of response to YBIs for depression. Second, it presents a critical analysis of several factors that could affect the quality of this evidence, and highlights areas where improvements or advances are crucially needed in future. For these reasons, it is of use both to clinicians with an interest in this form of therapy, and to researchers who wish to improve on existing research.
The limitations of this review should also be acknowledged. First, due to the heterogeneity of methods and outcomes, a formal systematic review or meta-analysis could not be carried out. Second, as only papers written in English were included, it is possible that relevant trials of YBIs written in other languages may have been omitted. Third, the study relied on citations retrieved from databases of biomedical research, in which studies of yoga may have been under-represented. Finally, it is possible that YBIs may improve depression through as yet unidentified biological mechanisms that were not considered in this review.
5. Conclusions
Based on the existing evidence, it is possible that biological mechanisms related to the stress response, immune-inflammatory activity, and neural plasticity may be involved in the beneficial effects of yoga-based interventions for patients with depression. Though these findings are plausible on the surface, they should be interpreted with caution due to several methodological shortcomings, and the overall quality of the available evidence is low. This review is a preliminary attempt at delineating both the biological processes that may underpin response to YBIs in depression, and the study-related errors and limitations that need to be addressed by future researchers in this field. The findings of this review are of potential interest both to clinicians involved in the evidence-based use of YBIs, and to researchers seeking a better understanding of biological changes associated with specific yoga techniques in patients with depression. Researchers in the field of yoga, particularly in those centers where research is most active, should view these results as a clarion call to improve the quality and replicability of their work. A key first step in this process is to identify and address the limitations of the existing research, and it is hoped that the data summarized and analyzed in this review will be of use to them in this endeavor.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/brainsci14060543/s1, Table S1: PRISMA-ScR checklist for the current review.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No original data were generated for the purpose of this review.
Conflicts of Interest
The author declares no conflict of interest.
References
- Kessler, R.C. The costs of depression. Psychiatr. Clin. N. Am. 2012, 35, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Uher, R.; Payne, J.L.; Pavlova, B.; Perlis, R.H. Major depressive disorder in DSM-5: Implications for clinical practice and research of changes from DSM-IV. Depress. Anxiety 2014, 31, 459–471. [Google Scholar] [CrossRef]
- Liu, Q.; He, H.; Yang, J.; Feng, X.; Zhao, F.; Lyu, J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J. Psychiatr. Res. 2020, 126, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, S.; McLean, L.; Fritz, K.; Lampe, L.; Malhi, G.S. Getting depression clinical practice guidelines right: Time for change? Acta Psychiatr. Scand. Suppl. 2013, 444, 24–30. [Google Scholar] [CrossRef] [PubMed]
- de Silva, V.A.; Hanwella, R. Efficacy and tolerability of venlafaxine versus specific serotonin reuptake inhibitors in treatment of major depressive disorder: A meta-analysis of published studies. Int. Clin. Psychopharmacol. 2012, 27, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.J.; Trivedi, M.; Fava, M.; Thase, M.; Wisniewski, S. The STAR*D Data Remain Strong: Reply to Pigott et al. Am. J. Psychiatry 2023, 180, 919–920. [Google Scholar] [CrossRef]
- Sakurai, H.; Noma, H.; Watanabe, K.; Uchida, H.; Furukawa, T.A. Cumulative remission rate after sequential treatments in depression: Reappraisal of the STAR*D trial data. World Psychiatry 2024, 23, 156–157. [Google Scholar] [CrossRef]
- Pigott, H.E.; Kim, T.; Xu, C.; Kirsch, I.; Amsterdam, J. What are the treatment remission, response and extent of improvement rates after up to four trials of antidepressant therapies in real-world depressed patients? A reanalysis of the STAR*D study’s patient-level data with fidelity to the original research protocol. BMJ Open 2023, 13, e063095. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, S.N. Why antidepressants are not antidepressants: STEP-BD, STAR*D, and the return of neurotic depression. Bipolar Disord. 2008, 10, 957–968. [Google Scholar] [CrossRef]
- Chen, C.; Shan, W. Pharmacological and non-pharmacological treatments for major depressive disorder in adults: A systematic review and network meta-analysis. Psychiatry Res. 2019, 281, 112595. [Google Scholar] [CrossRef]
- Levy, L.B.; O’Hara, M.W. Psychotherapeutic interventions for depressed, low-income women: A review of the literature. Clin. Psychol. Rev. 2010, 30, 934–950. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, S.N.; Vöhringer, P.A.; Vergne, D.E. The varieties of depressive experience: Diagnosing mood disorders. Psychiatr. Clin. N. Am. 2012, 35, 73–86. [Google Scholar] [CrossRef]
- Furukawa, T.A.; Shinohara, K.; Sahker, E.; Karyotaki, E.; Miguel, C.; Ciharova, M.; Bockting, C.L.H.; Breedvelt, J.J.F.; Tajika, A.; Imai, H.; et al. Initial treatment choices to achieve sustained response in major depression: A systematic review and network meta-analysis. World Psychiatry 2021, 20, 387–396. [Google Scholar] [CrossRef]
- Warnick, S.J.; Mehdi, L.; Kowalkowski, J. Wait-there’s evidence for that? Integrative medicine treatments for major depressive disorder. Int. J. Psychiatry Med. 2021, 56, 334–343. [Google Scholar] [CrossRef]
- Asher, G.N.; Gartlehner, G.; Gaynes, B.N.; Amick, H.R.; Forneris, C.; Morgan, L.C.; Coker-Schwimmer, E.; Boland, E.; Lux, L.J.; Gaylord, S.; et al. Comparative Benefits and Harms of Complementary and Alternative Medicine Therapies for Initial Treatment of Major Depressive Disorder: Systematic Review and Meta-Analysis. J. Altern. Complement. Med. 2017, 23, 907–919. [Google Scholar] [CrossRef]
- Ng, J.Y.; Nazir, Z.; Nault, H. Complementary and alternative medicine recommendations for depression: A systematic review and assessment of clinical practice guidelines. BMC Complement. Med. Ther. 2020, 20, 299. [Google Scholar] [CrossRef]
- Cutler, J.B.R.; Pane, O.; Panesar, S.K.; Updike, W.; Moore, T.R. Treatment of Mood and Depressive Disorders With Complementary and Alternative Medicine: Efficacy Review. J. Midwifery Women’s Health 2023, 68, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Introduction to Yoga—Vikaspedia. Available online: https://vikaspedia.in/health/ayush/yoga-1/introduction-to-yoga (accessed on 28 February 2024).
- Douglass, L. How did we get here? A history of yoga in America, 1800–1970. Int. J. Yoga Ther. 2007, 17, 35–42. [Google Scholar] [CrossRef]
- Srinivasan, T. Is yoga an intervention? Int. J. Yoga 2012, 5, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Bhavanani, A.B. Yoga is not an intervention but may be yogopathy is. Int. J. Yoga 2012, 5, 157–158. [Google Scholar] [CrossRef]
- Janakiramaiah, N.; Gangadhar, B.N.; Murthy, P.J.N.V.; Harish, M.G.; Shetty, K.T.; Subbakrishna, D.K.; Meti, B.L.; Raju, T.R.; Vedamurthachar, A. Therapeutic efficacy of Sudarshan Kriya Yoga (SKY) in dysthymic disorder. Nimhans J. 1998, 1, 21–28. [Google Scholar]
- Janakiramaiah, N.; Gangadhar, B.N.; Naga Venkatesha Murthy, P.J.; Harish, M.G.; Subbakrishna, D.K.; Vedamurthachar, A. Antidepressant efficacy of Sudarshan Kriya Yoga (SKY) in melancholia: A randomized comparison with electroconvulsive therapy (ECT) and imipramine. J. Affect. Disord. 2000, 57, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Cramer, H.; Anheyer, D.; Lauche, R.; Dobos, G. A systematic review of yoga for major depressive disorder. J. Affect. Disord. 2017, 213, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yan, D.; Yang, J. Effectiveness of yoga for major depressive disorder: A systematic review and meta-analysis. Front. Psychiatry 2023, 14, 1138205. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Subramaniam, E.; Bhavanani, A.B.; Sarkar, S.; Balasundaram, S. Effect of adjunct yoga therapy in depressive disorders: Findings from a randomized controlled study. Indian J. Psychiatry 2019, 61, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Kuppili, P.P.; Gupta, T.; Nebhinani, N.; Chandani, A. Kriya Yoga in Patients with Depressive Disorders: A Pilot Study. J. Neurosci. Rural. Pract. 2021, 12, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Bieber, M.; Görgülü, E.; Schmidt, D.; Zabel, K.; Etyemez, S.; Friedrichs, B.; Prvulovic, D.; Reif, A.; Oertel, V. Effects of body-oriented yoga: A RCT study for patients with major depressive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 1217–1229. [Google Scholar] [CrossRef] [PubMed]
- Vollbehr, N.K.; Stant, A.D.; Hoenders, H.J.R.; Bartels-Velthuis, A.A.; Nauta, M.H.; Castelein, S.; Schroevers, M.J.; de Jong, P.J.; Ostafin, B.D. Cost-effectiveness of a mindful yoga intervention added to treatment as usual for young women with major depressive disorder versus treatment as usual only: Cost-effectiveness of yoga for young women with depression. Psychiatry Res. 2024, 333, 115692. [Google Scholar] [CrossRef] [PubMed]
- Uebelacker, L.A.; Broughton, M.K. Yoga for depression and anxiety: A review of published research and implications for healthcare providers. Focus 2018, 16, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, A.V.; Balneaves, L.G.; Faulkner, G.; Ortiz, A.; McIntosh, D.; Morehouse, R.L.; Ravindran, L.; Yatham, L.N.; Kennedy, S.H.; Lam, R.W.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 5. Complementary and Alternative Medicine Treatments. Can. J. Psychiatry 2016, 61, 576–587. [Google Scholar] [CrossRef]
- Gautam, S.; Jain, A.; Gautam, M.; Vahia, V.N.; Grover, S. Clinical Practice Guidelines for the management of Depression. Indian. J. Psychiatry 2017, 59, S34–S50. [Google Scholar] [CrossRef] [PubMed]
- Nauphal, M.; Mischoulon, D.; Uebelacker, L.; Streeter, C.; Nyer, M. Yoga for the treatment of depression: Five questions to move the evidence-base forward. Complement. Ther. Med. 2019, 46, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Kinser, P.A.; Elswick, R.K.; Kornstein, S. Potential long-term effects of a mind-body intervention for women with major depressive disorder: Sustained mental health improvements with a pilot yoga intervention. Arch. Psychiatr. Nurs. 2014, 28, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Farias, M.; Maraldi, E.; Wallenkampf, K.C.; Lucchetti, G. Adverse events in meditation practices and meditation-based therapies: A systematic review. Acta Psychiatr. Scan 2020, 142, 374–393. [Google Scholar] [CrossRef] [PubMed]
- Lundt, A.; Jentschke, E. Long-term changes of symptoms of anxiety, depression, and fatigue in cancer patients 6 months after the end of yoga therapy. Integr. Cancer Ther. 2019, 18, 1534735418822096. [Google Scholar] [CrossRef] [PubMed]
- Gaynes, B.N.; Warden, D.; Trivedi, M.H.; Wisniewski, S.R.; Fava, M.; Rush, A.J. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr. Serv. 2009, 60, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Hurst, S.; Maiya, M.; Casteel, D.; Sarkin, A.J.; Libretto, S.; Elwy, A.R.; Park, C.L.; Groessl, E.J. Yoga therapy for military personnel and veterans: Qualitative perspectives of yoga students and instructors. Complement. Ther. Med. 2018, 40, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Bressington, D.; Mui, J.; Yu, C.; Leung, S.F.; Cheung, K.; Wu, C.S.T.; Bollard, M.; Chien, W.T. Feasibility of a group-based laughter yoga intervention as an adjunctive treatment for residual symptoms of depression, anxiety and stress in people with depression. J. Affect. Disord. 2019, 248, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Vollbehr, N.K.; Hoenders, H.J.R.; Bartels-Velthuis, A.A.; Nauta, M.H.; Castelein, S.; Schroevers, M.J.; Stant, A.D.; Albers, C.J.; de Jong, P.J.; Ostafin, B.D. Mindful yoga intervention as add-on to treatment as usual for young women with major depressive disorder: Results from a randomized controlled trial. J. Consult. Clin. Psychol. 2022, 90, 925–941. [Google Scholar] [CrossRef]
- veGangadhar, B.N.; Naveen, G.H.; Rao, M.G.; Thirthalli, J.; Varambally, S. Positive antidepressant effects of generic yoga in depressive out-patients: A comparative study. Indian J. Psychiatry 2013, 55, S369–S373. [Google Scholar] [CrossRef]
- Schmidt, H.D.; Shelton, R.C.; Duman, R.S. Functional biomarkers of depression: Diagnosis, treatment, and pathophysiology. Neuropsychopharmacology 2011, 36, 2375–2394. [Google Scholar] [CrossRef] [PubMed]
- Ivanets, N.N.; Svistunov, A.A.; Chubarev, V.N.; Kinkulkina, M.A.; Tikhonova, Y.G.; Syzrantsev, N.S.; Sologova, S.S.; Ignatyeva, N.V.; Mutig, K.; Tarasov, V.V. Can Molecular Biology Propose Reliable Biomarkers for Diagnosing Major Depression? Curr. Pharm. Des. 2021, 27, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Rocha, K.K.F.; Ribeiro, A.M.; Rocha, K.C.F.; Sousa, M.B.C.; Albuquerque, F.S.; Ribeiro, S.; Silva, R.H. Improvement in physiological and psychological parameters after 6 months of yoga practice. Conscious. Cogn. 2012, 21, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Wadden, K.P.; Snow, N.J.; Sande, P.; Slawson, S.; Waller, T.; Boyd, L.A. Yoga practitioners uniquely activate the superior parietal lobule and supramarginal gyrus during emotion regulation. Front. Integr. Neurosci. 2018, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Kinser, P.A.; Goehler, L.E.; Taylor, A.G. How might yoga help depression? A neurobiological perspective. Explore 2012, 8, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Tolahunase, M.R.; Gautam, S.; Sagar, R.; Kumar, M.; Dada, R. Yoga in major depressive disorder: Molecular mechanisms and clinical utility. Front. Biosci. 2021, 13, 56–81. [Google Scholar] [CrossRef] [PubMed]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource; Food and Drug Administration (US): Silver Spring, MD, USA; National Institutes of Health (US): Bethesda, MD, USA, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK326791/ (accessed on 11 May 2024).
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Busch, Y.; Menke, A. Blood-based biomarkers predicting response to antidepressants. J. Neural Transm. 2019, 126, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Thase, M.E. Using biomarkers to predict treatment response in major depressive disorder: Evidence from past and present studies. Dialogues Clin. Neurosci. 2014, 16, 539–544. [Google Scholar] [CrossRef]
- Voegeli, G.; Clery-Melin, M.L.; Ramoz, N.; Gorwood, P. Progress in elucidating biomarkers of antidepressant pharmacological treatment response: A systematic review and meta-analysis of the last 15 years. Drugs 2017, 77, 1967–1986. [Google Scholar] [CrossRef]
- Leuchter, A.F.; Cook, I.A.; Hamilton, S.P.; Narr, K.L.; Toga, A.; Hunter, A.M.; Faull, K.; Whitelegge, J.; Andrews, A.M.; Loo, J.; et al. Biomarkers to predict antidepressant response. Curr. Psychiatry Rep. 2010, 12, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Dwivedi, Y. An insight into the sprawling microverse of microRNAs in depression pathophysiology and treatment response. Neurosci. Biobehav. Rev. 2023, 146, 105040. [Google Scholar] [CrossRef] [PubMed]
- Maffioletti, E.; Silva, R.C.; Bortolomasi, M.; Baune, B.T.; Gennarelli, M.; Minelli, A. Molecular biomarkers of electroconvulsive therapy effects and clinical response: Understanding the present to shape the future. Brain Sci. 2021, 11, 1120. [Google Scholar] [CrossRef] [PubMed]
- Shamabadi, A.; Karimi, H.; Cattarinussi, G.; Moghaddam, H.S.; Akhondzadeh, S.; Sambataro, F.; Schiena, G.; Delvecchio, G. Neuroimaging correlates of treatment response to transcranial magnetic stimulation in bipolar depression: A systematic review. Brain Sci. 2023, 13, 801. [Google Scholar] [CrossRef] [PubMed]
- Lester, K.J.; Eley, T.C. Therapygenetics: Using genetic markers to predict response to psychological treatment for mood and anxiety disorders. Biol. Mood Anxiety Disord. 2013, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Mazza, E.; Poletti, S.; Benedetti, F. White matter integrity and pro-inflammatory cytokines as predictors of antidepressant response in MDD. J. Psychiatr. Res. 2023, 159, 22–32. [Google Scholar] [CrossRef]
- Gasparini, A.; Callegari, C.; Lucca, G.; Bellini, A.; Caselli, I.; Ielmini, M. Inflammatory biomarker and response to antidepressant in major depressive disorder: A systematic review and meta-analysis. Psychopharmacol. Bull. 2022, 52, 36–52. [Google Scholar] [PubMed]
- Mohammad, A.; Thakur, P.; Kumar, R.; Kaur, S.; Saini, R.V.; Saini, A.K. Biological markers for the effects of yoga as a complementary and alternative medicine. J. Complement. Integr. Med. 2019, 16, 20180094. [Google Scholar] [CrossRef]
- van Aalst, J.; Ceccarini, J.; Demyttenaere, K.; Sunaert, S.; Van Laere, K. What has neuroimaging taught us on the neurobiology of yoga? A review. Front. Integr. Neurosci. 2020, 14, 34. [Google Scholar] [CrossRef]
- Malhotra, V.; Pathak, T.; Javed, D.; Wakode, S.; Thakare, A.; Shrivastava, R.; Chouhan, S.; Filho, F.J.C. Comparative analysis of heart rate variability parameters between Surya Namaskar and stationary bike exercise groups. Int. J. Yoga 2023, 16, 202–209. [Google Scholar] [CrossRef]
- Mishra, B.; Agarwal, A.; George, J.A.; Upadhyay, A.D.; Nilima, N.; Mishra, R.; Kuthiala, N.; Basheer, A.; Vishnu, V.Y.; Srivastava, V.P. Effectiveness of yoga in modulating markers of immunity and inflammation: A systematic review and meta-analysis. Cureus 2024, 16, e57541. [Google Scholar] [CrossRef] [PubMed]
- Meister, K.; Juckel, G. A systematic review of mechanisms of change in body-oriented yoga in major depressive disorders. Pharmacopsychiatry 2018, 51, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Shreekantiah, U.; Goyal, N. Nerve growth factor in psychiatric disorders: A scoping review. Indian. J. Psychol. Med. 2023, 45, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Hiller, J.K.; Jangmo, A.; Tesli, M.S.; Jaholkowski, P.P.; Hoseth, E.Z.; Steen, N.E.; Haram, M. Lipid biomarker research in bipolar disorder: A scoping review of trends, challenges, and future directions. Biol. Psychiatry Glob. Open Sci. 2023, 3, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Wirsching, J.; Graßmann, S.; Eichelmann, F.; Harms, L.M.; Schenk, M.; Barth, E.; Berndzen, A.; Olalekan, M.; Sarmini, L.; Zuberer, H.; et al. Development and reliability assessment of a new quality appraisal tool for cross-sectional studies using biomarker data (BIOCROSS). BMC Med. Res. Methodol. 2018, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldrige, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, D.; Cook, I.A.; Davydov, D.M.; Ottaviani, C.; Leuchter, A.F.; Abrams, M. Yoga as a complementary treatment of depression: Effects of traits and moods on treatment outcome. eCAM 2007, 4, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Streeter, C.C.; Whitfield, T.H.; Owen, L.; Rein, T.; Karri, S.K.; Yakhkind, A.; Perlmutter, R.; Prescot, A.; Renshaw, P.F.; Ciraulo, D.A.; et al. Effects of yoga versus walking on mood, anxiety, and brain GABA levels: A randomized controlled MRS study. J. Altern. Complement. Med. 2010, 16, 1145–1152. [Google Scholar] [CrossRef]
- Murthy, P.J.; Gangadhar, B.N.; Janakiramaiah, N.; Subbakrishna, D.K. Normalization of P300 amplitude following treatment in dysthymia. Biol. Psychiatry 1997, 42, 740–743. [Google Scholar] [CrossRef]
- Murthy, P.J.N.V.; Janakiramaiah, N.; Gangadhar, B.N.; Subbakrishna, D.K. P300 amplitude and antidepressant response to Sudarshan Kriya Yoga (SKY). J. Affect. Disord. 1998, 50, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Jain, F.A.; Cook, I.A.; Leuchter, A.F.; Hunter, A.M.; Davydov, D.M.; Ottaviani, C.; Tartter, M.; Crump, C.; Shapiro, D. Heart rate variability and treatment outcome in major depression: A pilot study. Int. J. Psychophysiol. 2014, 93, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Tolahunase, M.R.; Sagar, R.; Dada, R. 5-HTTLPR and MTHFR 677C>T polymorphisms and response to yoga-based lifestyle intervention in major depressive disorder: A randomized active-controlled trial. Indian J. Psychiatry 2018, 60, 410–426. [Google Scholar] [CrossRef] [PubMed]
- Streeter, C.C.; Gerbarg, P.L.; Brown, R.P.; Scott, T.M.; Nielsen, G.H.; Owen, L.; Sakai, O.; Sneider, J.T.; Nyer, M.B.; Silveri, M.M. Thalamic gamma aminobutyric acid level changes in major depressive disorder after a 12-week Iyengar yoga and coherent breathing intervention. J. Altern. Complement. Med. 2020, 26, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Aditi Devi, N.; Philip, M.; Varambally, S.; Christopher, R.; Gangadhar, B.N. Yoga as a monotherapy alters proBDNF—Mature BDNF ratio in patients with major depressive disorder. Asian J. Psychiatry 2023, 81, 103429. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Das, S.; Mondal, S.; Goswami, U.; Gandhi, A. Effect of Sahaj Yoga on neuro-cognitive functions in patients suffering from major depression. Indian J. Physiol. Pharmacol. 2006, 50, 375–383. [Google Scholar] [PubMed]
- Sharma, V.K.; Das, S.; Mondal, S.; Goswami, U. Comparative effect of Sahaj Yoga on EEG in patients of major depression and healthy subjects. Biomedicine 2007, 27, 95–99. [Google Scholar]
- Sharma, V.K.; Das, S.; Mondal, S.; Goswami, U. Effect of Sahaj Yoga on autonomic functions in healthy subjects and patients of major depression. Biomedicine 2008, 28, 139–141. [Google Scholar]
- Naveen, G.H.; Thirthalli, J.; Rao, M.G.; Varambally, S.; Christopher, R.; Gangadhar, B.N. Positive therapeutic and neurotropic effects of yoga in depression: A comparative study. Indian J. Psychiatry 2013, 55, 400–404. [Google Scholar] [CrossRef]
- Halappa, N.G.; Thirthalli, J.; Varambally, S.; Rao, M.; Christopher, R.; Nanjundaiah, G.B. Improvement in neurocognitive functions and serum brain-derived neurotrophic factor levels in patients with depression treated with antidepressants and yoga. Indian J. Psychiatry 2018, 60, 32–37. [Google Scholar] [CrossRef]
- Sarubin, N.; Nothdurfter, C.; Schule, C.; Lieb, M.; Uhr, M.; Born, C.; Zimmermann, R.; Buhner, M.; Konopka, K.; Ruppercht, R.; et al. The influence of Hatha yoga as add-on treatment in major depression on hypothalamic-pituitary-adrenal axis activity: A randomized trial. J. Psychiatr. Res. 2014, 53, 76–83. [Google Scholar] [CrossRef]
- Naveen, G.H.; Varambally, S.; Thirthalli, J.; Rao, M.; Christopher, R.; Gangadhar, B.N. Serum cortisol and BDNF in patients with major depression—Effect of yoga. Int. Rev. Psychiatry 2016, 28, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Toschi-Dias, E.; Tobaldini, E.; Solbiati, M.; Costantino, G.; Sanlorenzo, R.; Doria, S.; Irtelli, F.; Mencacci, C.; Montano, N. Sudarshan Kriya Yoga improves cardiac autonomic control in patients with anxiety-depression disorders. J. Affect. Disord. 2017, 214, 74–80. [Google Scholar] [CrossRef]
- Tolahunase, M.R.; Sagar, R.; Faiq, M.; Dada, R. Yoga- and meditation-based lifestyle intervention increases neuroplasticity and reduces severity of major depressive disorder: A randomized controlled trial. Restor. Neurol. Neurosci. 2018, 36, 423–442. [Google Scholar] [CrossRef] [PubMed]
- Bhargav, P.H.; Reddy, P.V.; Govindaraj, R.; Gulati, K.; Ravindran, A.; Gayathri, D.; Karmani, S.J.; Udupa, K.; Venkatasubramanian, G.; Philip, M.; et al. Impact of a course of add-on supervised yoga on cortical inhibition in major depressive disorder: A randomized controlled trial. Can. J. Psychiatry 2021, 66, 179–181. [Google Scholar] [CrossRef]
- Gulati, K.; Bhargav, P.H.; Reddy, P.V.; Govindaraj, R.; Ravindran, A.; Gayathri, D.; Karmani, S.J.; Udupa, K.; Philip, M.; Debnath, M.; et al. Adjunct yoga therapy: Influence on heart rate variability in major depressive disorder—A randomized controlled trial. Asian J. Psychiatry 2021, 65, 102832. [Google Scholar] [CrossRef]
- Nugent, N.R.; Brick, L.; Armey, M.F.; Tyrka, A.R.; Ridout, K.K.; Uebelacker, L.A. Benefits of yoga on IL-6: Findings from a randomized controlled trial of yoga for depression. Behav. Med. 2021, 47, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Subbanna, M.; Talukdar, P.M.; Abdul, F.; Debnath, M.; Reddy, P.V.; Arasappa, R.; Venkatasubramanian, G.; Muralidharan, K.; Gangadhar, B.N.; Bhargav, P.H.; et al. Long-term add-on Yoga therapy offers clinical benefits in major depressive disorder by modulating the complement pathway: A randomized controlled trial. Asian J. Psychiatry 2021, 66, 102876. [Google Scholar] [CrossRef]
- Naveen, G.H.; Rao, M.G.; Vishal, V.; Thirthalli, J.; Varambally, S.; Gangadhar, B.N. Development and feasibility of yoga therapy module for out-patients with depression in India. Indian J. Psychiatry 2013, 55, S350–S356. [Google Scholar] [CrossRef]
- Shi, Y.; Luan, D.; Song, R.; Zhang, Z. Value of peripheral neurotrophin levels for the diagnosis of depression and response to treatment: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2020, 41, 40–51. [Google Scholar] [CrossRef]
- Haapakoski, R.; Mathieu, J.; Ebmeier, K.P.; Alenius, H.; Kivimaki, M. Cumulative meta-analysis of interleukins 6 and 1β, tumor necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015, 49, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav. Immun. 2020, 87, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Duran, N.L.; Kovacs, M.; George, C.J. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology 2009, 34, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Stetler, C.; Miller, C.E. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom. Med. 2011, 73, 114–126. [Google Scholar] [CrossRef]
- Cakici, N.; Sutterland, A.L.; Penninx, B.W.J.H.; de Haan, L.; van Beveren, N.J.M. Changes in peripheral blood compounds following psychopharmacological treatment in drug-naïve first-episode patients with either schizophrenia or major depressive disorder: A meta-analysis. Psychol. Med. 2021, 51, 538–549. [Google Scholar] [CrossRef]
- Claudino, F.C.A.; Goncalves, L.; Schuch, F.B.; Martins, H.R.S.; da Rocha, N.S. The effects of individual psychotherapy in BDNF levels of patients with mental disorders: A systematic review. Front. Psychiatry 2020, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Permoda-Pachuta, A.; Malewska-Kasprzak, M.; Skibinska, M.; Rzepski, K.; Dmitrzak-Weglarz, M. Changes in adipokine, resistin, and BDNF concentrations in treatment-resistant depression after electroconvulsive therapy. Brain Sci. 2023, 13, 1358. [Google Scholar] [CrossRef]
- Zajkowska, Z.; Gullett, N.; Walsh, A.; Zonca, V.; Pedersen, G.A.; Souza, L.; Kieling, C.; Fisher, H.L.; Kohrt, B.A.; Mondelli, V. Cortisol and development of depression in adolescence and young adulthood—A systematic review and meta-analysis. Psychoneuroendocrinology 2022, 136, 105625. [Google Scholar] [CrossRef]
- Wang, R.; Kogler, L.; Derntl, B. Sex differences in cortisol levels in depression: A systematic review and meta-analysis. Front. Neuroendocrinol. 2024, 72, 101118. [Google Scholar] [CrossRef]
- Perrin, A.J.; Horowitz, M.A.; Roelofs, J.; Zunszain, P.A.; Pariante, C.M. Glucocorticoid resistance: Is it a requisite for increased cytokine production in depression? A systematic review and meta-analysis. Front. Psychiatry 2019, 28, 423. [Google Scholar] [CrossRef]
- Calabrese, F.; Rossetti, A.C.; Racagni, G.; Gass, P.; Riva, M.A.; Molteni, R. Brain-derived neurotrophic factor: A bridge between inflammation and neuroplasticity. Front. Cell. Neurosci. 2014, 8, 430. [Google Scholar] [CrossRef] [PubMed]
- Bocchio-Chiavetto, L.; Bagnardi, V.; Zanardini, R.; Molteni, R.; Nielsen, M.G.; Placentino, A.; Giovannini, C.; Rillosi, L.; Ventriglia, M.; Riva, M.A.; et al. Serum and plasma BDNF levels in major depression: A replication study and meta-analyses. World J. Biol. Psychiatry 2010, 11, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Estevao, C. The role of yoga in inflammatory markers. Brain Behav. Immun. Health 2022, 20, 100421. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, M.C.; Bauer, I.E. A systematic review of randomized control trials on the effects of yoga on stress measures and mood. J. Psychiatr. Res. 2015, 68, 270–2872. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhardwaj, S.; Gupta, A.; Katoch, V.M.; Sharma, K.K.; Gupta, R. Influence of 24-week yoga intervention on cardiovascular risk factors and inflammatory markers in type 2 diabetes. Int. J. Yoga 2023, 16, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhong, S.; Liao, X.; Chen, J.; He, T.; Lai, S.; Jia, Y. A meta-analysis of oxidative stress markers in depression. PLoS ONE 2015, 10, e0138904. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, M.; Wada, M.; Nakajima, S.; Tsugawa, S.; Nakahara, T.; Blumberger, D.M.; Mimura, M.; Noda, Y. Transcranial magnetic stimulation neurophysiology of patients with major depressive disorder: A systematic review and meta-analysis. Psychol. Med. 2021, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, Y.; Zhang, Y.; Chen, L.; Zou, Y.; Xiao, J.; Min, W.; Yuan, C.; Ye, Y.; Li, M.; et al. Heart rate variability in generalized anxiety disorder, major depressive disorder and panic disorder: A network meta-analysis and systematic review. J. Affect. Disord. 2023, 330, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Romeo, B.; Choucha, W.; Fossati, P.; Rotge, J.Y. Meta-analysis of central and peripheral γ-aminobutyric acid levels in patients with unipolar and bipolar depression. J. Psychiatr. Neurosci. 2018, 43, 58–66. [Google Scholar] [CrossRef]
- Shobana, R.; Maheshkumar, K.; Venkateswaran, S.T.; Geetha, M.B.; Padmavathi, R. Effects of long-term yoga training on autonomic function among the healthy adults. J. Family Med. Prim. Care 2023, 11, 3471–3475. [Google Scholar] [CrossRef]
- Fitzgerald, I.; Sahm, L.J.; Byrne, A.; O’Connell, J.; Ensor, J.; Ni Dhubhlaing, C.; O’Dwyer, S.; Crowley, E.K. Predicting antipsychotic-induced weight gain in first episode psychosis—A field-wide systematic review and meta-analysis of non-genetic prognostic factors. Eur. Psychiatry 2023, 66, e42. [Google Scholar] [CrossRef] [PubMed]
- Tsermpini, E.E.; Serretti, A.; Dolzan, V. Precision medicine in antidepressants treatment. Handb. Exp. Pharmacol. 2023, 280, 131–186. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.J.; Guimaraes, F.S.; Joca, S.R.L. DNA methylation in stress and depression: From biomarker to therapeutics. Acta Neuropsychiatr. 2021, 33, 217–241. [Google Scholar] [CrossRef]
- Siegel, J.S.; Pearson, C.; Lenze, E.J. Better biomarkers, faster drugs, stronger models: Progress towards precision psychiatry. MO Med. 2023, 120, 292–298. [Google Scholar] [PubMed]
- Curtiss, J.; Smoller, J.W.; Pedrelli, P. Optimizing precision medicine for second-step depression treatment: A machine learning approach. Psychol. Med. 2024, Mar 27, 1–8. [Google Scholar] [CrossRef]
- Grassbaugh, J.A. Editorial commentary: Clinical trial subgroup analyses and investigation of secondary outcome measures should be limited in number to avoid false findings. Arthroscopy 2024, 40, 397–399. [Google Scholar] [CrossRef]
- Baxter, K.; Krokosky, A.; Terry, S.F. Risky business: The need for hypothesis-generating research. Genet. Test. Mol. Biomarkers 2011, 15, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.H.; Wright, J.; Bissett, P.G.; Poldrack, R.A. Dataset decay and the problem of sequential analyses on open datasets. eLife 2020, 9, e53498. [Google Scholar] [CrossRef] [PubMed]
- Forstmeier, W.; Wagenmakers, E.-J.; Parker, T.H. Detecting and avoiding likely false-positive findings—A practical guide. Biol. Rev. Camb. Philos. Soc. 2017, 92, 1941–1968. [Google Scholar] [CrossRef]
- Telles, S.; Agnihotri, S.; Sharma, S.K.; Balkrishna, A. Patients’ choices for yoga therapy: An exploratory cross-sectional, convenience-sampling survey from India. Int. J. Yoga Ther. 2023, 33, 15. [Google Scholar] [CrossRef]
- Moerman, D.E.; Jonas, W.B. Deconstructing the placebo effect and finding the meaning response. Ann. Intern. Med. 2002, 136, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, A.R. Yoga research and public health: Is research aligned with the stakeholders’ needs? J. Prim. Care Community Health 2017, 8, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Gwee, X.; Chua, D.Q.; Tan, C.T.; Yap, K.B.; Larbi, A.; Lu, Y.; Ng, T.P. Inflammatory markers and incident depression: Evidence in a population-based prospective study. Psychoneuroendocrinology 2022, 142, 105806. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, G.; Kaufmann, F.N.; Manosso, L.; Platt, N.; Ghisleni, G.; Rodrigues, A.L.S.; Rieger, D.K.; Kaster, M.P. Depression and peripheral inflammatory profile of patients with obesity. Psychoneuroendocrinology 2018, 91, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Balshem, H.; Helfrand, M.; Schunemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of the evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Xiao, H. Exercise for mental well-being: Exploring neurobiological advances and intervention effects for depression. Life 2023, 13, 1505. [Google Scholar] [CrossRef] [PubMed]
- Rubia, K. The neurobiology of meditation and its clinical effectiveness in psychiatric disorders. Biol. Psychol. 2009, 82, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Botschek, T.; Husslein, V.; Peters, E.M.J.; Brosig, B. Hair cortisol as outcome parameter for psychological and neuropsychiatric interventions—A literature review. Front. Psychiatry 2023, 14, 1227153. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.; Chan, J.S.M.; Cheung, J.C.; Zou, L. Qigong and Tai-Chi for mood regulation. Focus 2018, 16, 40–47. [Google Scholar] [CrossRef]
- Vickers, A.; Goyal, N.; Harland, R.; Rees, R. Do certain countries produce only positive results? A systematic review of controlled trials. Control Clin. Trials 1998, 19, 159–166. [Google Scholar] [CrossRef]
- Cramer, H.; Lauche, R.; Langhorst, J.; Dobos, G. Are Indian yoga trials more likely to be positive than those from other countries? A systematic review of randomized controlled trials. Contemp. Clin. Trials 2015, 41, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Cramer, H.; Langhorst, J.; Dobos, G.; Lauche, R. Associated factors and consequences of risk of bias in randomized controlled trials of yoga: A systematic review. PLoS ONE 2015, 10, e0144125. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Dhawan, A. Methodological issues in conducting yoga- and meditation-based research: A narrative review and research implications. J. Ayurveda Integr. Med. 2022, 13, 100620. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, C.; Goldacre, B.; Mahtani, K.R. Why clinical trial outcomes fail to translate into benefits for patients. Trials 2017, 18, 122. [Google Scholar] [CrossRef]
- Li, G.; Taljaard, M.; Van den Heuvel, E.R.; Levine, M.A.H.; Cook, D.J.; Wells, G.A.; Devereaux, P.J.; Thabane, L. An introduction to multiplicity issues in clinical trials: The what, why, when and how. Int. J. Epidemiol. 2017, 46, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Karri, R.R.; Bhavanani, A.B.; Ramanathan, M.; Mopidevi, V.G. Methodological issues in yoga therapy research among psychiatric patients. Indian J. Psychiatry 2023, 65, 12–17. [Google Scholar] [CrossRef]
- Udupa, K.; Ramanathan, M.; Bhavanani, A.B. SWOT analysis in yoga research. SBV J. Basic Clin. Appl. Health Sci. 2021, 4, 26–30. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).