Neural Similarity and Synchrony among Friends
Abstract
1. Introduction
2. Methodology Used to Assess Neural Similarity and Synchrony
3. Specificity of Neural Similarity and Synchrony among Friends
3.1. Neural Similarity in Non-Interactive Situations
3.2. Neural Synchrony in Interactive Situations
4. Situational Modulations on Neural Synchrony among Friends
4.1. Neural Synchrony during Free Communication
4.2. Neural Synchrony during Goal-Directed Tasks
5. Discussion
5.1. Stimuli and Tasks Used in Previous Studies
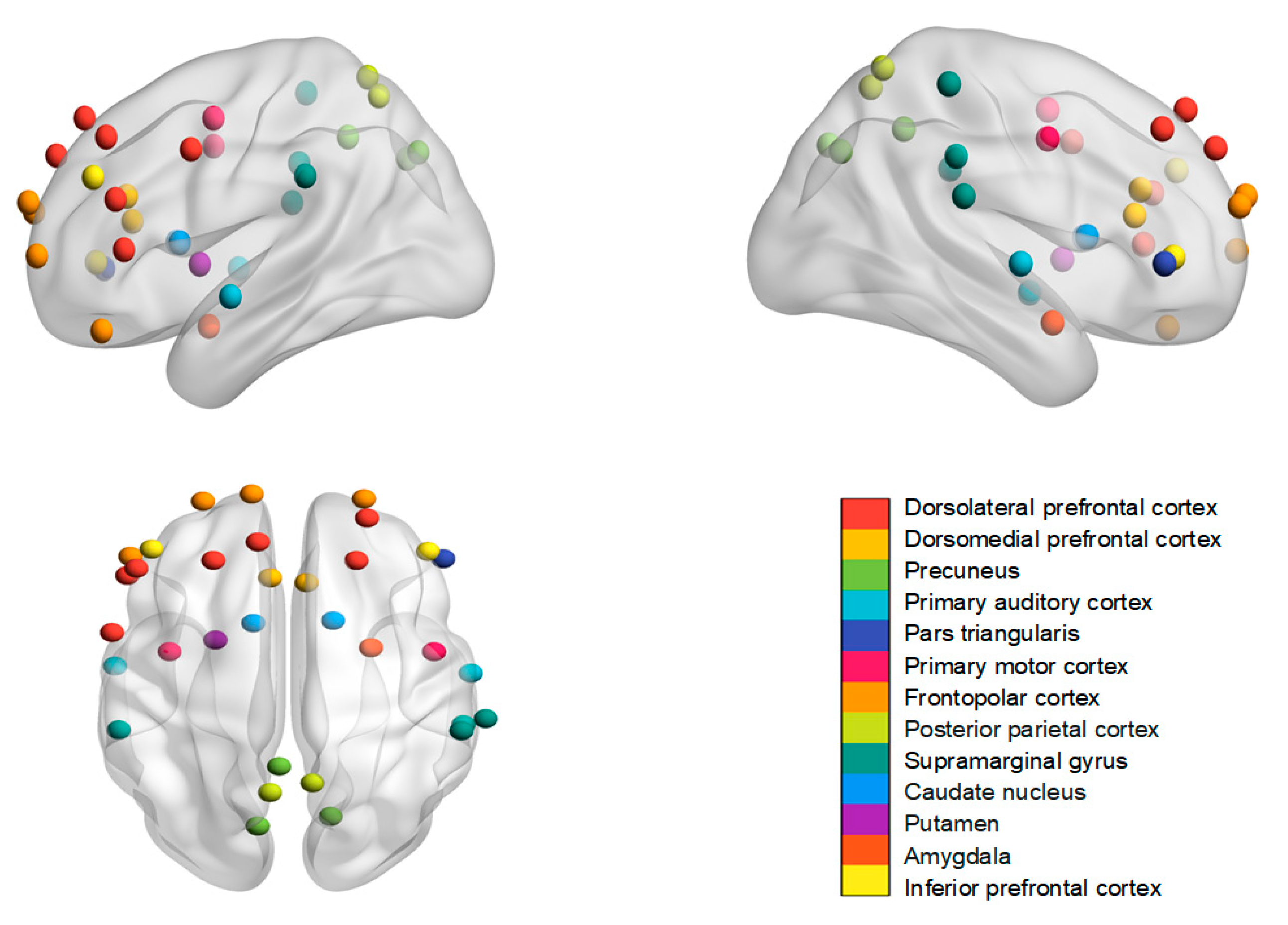
5.2. Brain Regions and Their Functional Meanings
5.3. Distinctions between Neural Similarity and Neural Synchrony
5.4. Comparisons between Research Methods
6. Limitations and Future Directions
- (1)
- Confirming conclusions: The current findings suggest that the specificity of neural similarity when viewing videos is robust, whereas the specificity of neural synchrony during interactive tasks is elusive and dependent on interactive contexts. Future research needs to clarify the contextual moderators on the specificity of neural synchrony. For example, exploring the factors that differ between friends and strangers, such as empathy, can be beneficial in identifying the specificity of neural synchrony among friends. In addition, although positive social cues appear to promote neural synchrony, the evidence comes from various manipulations (e.g., eye contact and gift exchange) and under different tasks (e.g., tangram puzzle and time reproduction tasks). More comparable and converging evidence is needed.
- (2)
- Verifying explanations: The current explanations for neural synchrony often rely on post hoc interpretations based on the experimental task or the common functions of the involved brain regions. Future studies could develop new interactive paradigms that precisely manipulate these situations to test which behavioral or psychological processes drive neural synchrony. Moreover, although the existing studies have established a link between neural similarity and friendship, the causal relationship remains unclear, due to the cross-sectional nature of most research. Longitudinal studies could help trace the developmental trajectory of neural similarity between friends, exploring whether this similarity exists before friendship formation and how it evolves over time.
- (3)
- Standardizing definitions of friendship: The definition of ‘friend’ varies across studies. In studies focusing on neural similarity in non-interactive contexts, friends are defined within a specific social network [8,12,13,22], whereas in interactive studies friendship is self-reported without objective measures of closeness, such as duration or quality [9,10,11,14,15,16,17,18,19,21,23,24,25,26]. Moreover, the existing studies on the specificity of neural similarity or synchrony commonly use strangers rather than acquaintances as controls. Future research should clearly define friendship and consider incorporating measures of friendship quality like perceived closeness or mutual support, and replace the stranger control group with acquaintances to better capture the social attachment nature of friendships. In addition, future studies should compare the neural similarities between same-gender [9,16,24] and opposite-gender [11,17,18,21] friends to enhance the understanding of social relationships from a gender perspective.
- (4)
- Integrating multiple research methods: Different technologies, i.e., fMRI, fNIRS, and EEG, employ distinct analytical methods making direct comparisons between studies challenging. For example, fMRI technology focuses on analyzing time series and spatial response patterns, while fNIRS calculates the coherence coefficients between time series for each dyad based on frequency and time, and EEG measures the phase locking of the EEG signals across time. To overcome these challenges and provide a more comprehensive understanding of neural similarity and synchrony among friends, future research should use multiple methods and conduct multi-faceted analyses to obtain converging evidence.
Funding
Conflicts of Interest
References
- Byrne, D.; Gouaux, C.; Griffitt, W.; Lamberth, J.; Murakawa, N.; Prasad, M.; Prasad, A.; Ramirez, M. The ubiquitous relationship: Attitude similarity and attraction. Hum. Relations 1971, 24, 201–207. [Google Scholar] [CrossRef]
- McPherson, M.; Smith-Lovin, L.; Cook, J.M. Birds of a feather: Homophily in social networks. Annu. Rev. Sociol. 2001, 27, 415–444. [Google Scholar] [CrossRef]
- Feiler, D.C.; Kleinbaum, A.M. Popularity, Similarity, and the Network Extraversion Bias. Psychol. Sci. 2015, 26, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Winslow, C.N. A study of the extent of agreement between friends’ opinions and their ability to estimate the opinions of each other. J. Soc. Psychol. 1937, 8, 433–442. [Google Scholar] [CrossRef]
- Han, X.; Wang, L.; Crespi, N.; Park, S.; Cuevas, Á. Alike people, alike interests? Inferring interest similarity in online social networks. Decis. Support Syst. 2015, 69, 92–106. [Google Scholar] [CrossRef]
- Christakis, N.A.; Fowler, J.H. Friendship and natural selection. Proc. Natl. Acad. Sci. USA 2014, 111, 10796–10801. [Google Scholar] [CrossRef] [PubMed]
- Brent, L.J.; Chang, S.W.; Gariépy, J.; Platt, M.L. The neuroethology of friendship. Ann. N. Y. Acad. Sci. 2013, 1316, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Baek, E.C.; Hyon, R.; López, K.; Finn, E.S.; Porter, M.A.; Parkinson, C. In-degree centrality in a social network is linked to coordinated neural activity. Nat. Commun. 2022, 13, 1118. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Fronda, G. The "gift effect" on functional brain connectivity. Inter-brain synchronization when prosocial behavior is in action. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Fronda, G.; Vanutelli, M.E. When gratitude and cooperation between friends affect inter-brain connectivity for EEG. BMC Neurosci. 2020, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Djalovski, A.; Dumas, G.; Kinreich, S.; Feldman, R. Human attachments shape interbrain synchrony toward efficient performance of social goals. NeuroImage 2021, 226, 117600. [Google Scholar] [CrossRef] [PubMed]
- Hyon, R.; Kleinbaum, A.M.; Parkinson, C. Social network proximity predicts similar trajectories of psychological states: Evidence from multi-voxel spatiotemporal dynamics. NeuroImage 2020, 216, 116492. [Google Scholar] [CrossRef] [PubMed]
- Hyon, R.; Youm, Y.; Kim, J.; Chey, J.; Kwak, S.; Parkinson, C. Similarity in functional brain connectivity at rest predicts interpersonal closeness in the social network of an entire village. Proc. Natl. Acad. Sci. USA 2020, 117, 33149–33160. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Meng, Y.; Gao, Y.; Ao, L.; Yang, L.; Wang, H.; Liu, Y. Romantic relationships attenuated competition between lovers: Evidence from brain synchronization. Cereb. Cortex 2024, 34, bhae028. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Sun, J.; McGeady, C.; Ji, L.; Li, C. Enhancing brain–computer interface performance by incorporating brain-to-brain coupling. Cyborg Bionic Syst. 2024, 5, 0116. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, K.; Hao, N.; Wang, Y. Cognitive reappraisal and expressive suppression evoke distinct neural connections during interpersonal emotion regulation. J. Neurosci. 2023, 43, 8456–8471. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Zheng, L.; Zhao, H.; Zhou, S.; Zhai, Y.; Lu, C. Interpersonal neural synchronization during interpersonal touch underlies Affiliative pair bonding between romantic couples. Cereb. Cortex 2020, 31, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Chen, C.; Wu, K.; Zhou, S.; Zhou, F.; Zheng, L.; Zhao, H.; Zhai, Y.; Lu, C. Interpersonal conflict increases interpersonal neural synchronization in romantic couples. Cereb. Cortex 2021, 32, 3254–3268. [Google Scholar] [CrossRef]
- Luft, C.D.B.; Zioga, I.; Giannopoulos, A.; Di Bona, G.; Binetti, N.; Civilini, A.; Latora, V.; Mareschal, I. Social synchronization of brain activity increases during eye-contact. Commun. Biol. 2022, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- McNabb, C.B.; Burgess, L.G.; Fancourt, A.; Mulligan, N.; FitzGibbon, L.; Riddell, P.; Murayama, K. No evidence for a relationship between social closeness and similarity in resting-state functional brain connectivity in schoolchildren. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Cheng, X.; Zhang, Z.; Li, X.; Hu, Y. Cooperation in lovers: An fNIRS-based hyperscanning study. Hum. Brain Mapp. 2017, 38, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, C.; Kleinbaum, A.M.; Wheatley, T. Similar neural responses predict friendship. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Dong, M.; Feng, K.; Li, J.; Hu, X.; Liu, T. Influence of interpersonal distance on collaborative performance in the joint Simon task—An fNIRS-based hyperscanning study. NeuroImage 2024, 285, 120473. [Google Scholar] [CrossRef] [PubMed]
- Spiegelhalder, K.; Ohlendorf, S.; Regen, W.; Feige, B.; Van Elst, L.T.; Weiller, C.; Hennig, J.; Berger, M.; Tüscher, O. Interindividual synchronization of brain activity during live verbal communication. Behav. Brain Res. 2014, 258, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yin, Z.; Zhang, X.; Zhang, H.; Bao, M.; Xuan, B. Neural mechanisms distinguishing two types of cooperative problem-solving approaches: An fNIRS hyperscanning study. NeuroImage 2024, 291, 120587. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, C.; Tao, R.; Duan, H.; Xu, S. Distinct inter-brain synchronization patterns underlying group decision-making under uncertainty with partners in different interpersonal relationships. NeuroImage 2023, 272, 120043. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cheng, T.; Guo, Z. A review of EEG acquisition, processing and application. J. Phys. Conf. Ser. 2021, 1907, 012045. [Google Scholar] [CrossRef]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. NeuroImage 2012, 63, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Geethanath, S.; Vaughan, J.T., Jr. Accessible magnetic resonance imaging: A review. J. Magn. Reson. Imaging 2019, 49, e65–e77. [Google Scholar] [CrossRef] [PubMed]
- Burle, B.; Spieser, L.; Roger, C.; Casini, L.; Hasbroucq, T.; Vidal, F. Spatial and temporal resolutions of EEG: Is it really black and white? A scalp current density view. Int. J. Psychophysiol. 2015, 97, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Scarapicchia, V.; Brown, C.; Mayo, C.; Gawryluk, J.R. Functional magnetic resonance imaging and functional near-infrared spectroscopy: Insights from combined recording studies. Front. Hum. Neurosci. 2017, 11, 419. [Google Scholar] [CrossRef] [PubMed]
- Nastase, S.A.; Gazzola, V.; Hasson, U.; Keysers, C. Measuring shared responses across subjects using intersubject correlation. Soc. Cogn. Affect. Neurosci. 2019, 14, 669–687. [Google Scholar] [CrossRef] [PubMed]
- Léné, P.; Karran, A.J.; Labonté-Lemoyne, E.; Sénécal, S.; Fredette, M.; Johnson, K.J.; Léger, P. Wavelet transform coherence: An innovative method to investigate social interaction in NeuroIS. In Information Systems and Neuroscience; Springer: Berlin/Heidelberg, Germany, 2019; pp. 147–154. [Google Scholar] [CrossRef]
- Nam, C.S.; Choo, S.; Huang, J.; Park, J. Brain-to-brain neural synchrony during social interactions: A systematic review on Hyperscanning studies. Appl. Sci. 2020, 10, 6669. [Google Scholar] [CrossRef]
- Jiang, J.; Zheng, L.; Lu, C. A hierarchical model for interpersonal verbal communication. Soc. Cogn. Affect. Neurosci. 2020, 16, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.P. On the interpretation of synchronization in EEG hyperscanning studies: A cautionary note. Front. Hum. Neurosci. 2013, 7, 881. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Bryant, D.M.; Reiss, A.L. NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. NeuroImage 2012, 59, 2430–2437. [Google Scholar] [CrossRef]
| Reference | Subjects | Techniques | Task | Independent Variables | Neural Synchrony or Similarity Index | Brain Regions | Specificity | Situational Modulations on INS |
|---|---|---|---|---|---|---|---|---|
| Baek et al., 2022 [8] | 63 participants from a social network (1953 dyads) | fMRI successive scanning | Non-interaction (watching videos) | Individuals’ centrality within a social network | Temporal INS | Dorsomedial prefrontal cortex and precuneus | More central individuals > less central individuals (paired with all other members) | |
| Balconi and Fronda, 2020 [9] | 15 friend dyads | fNIRS hyperscanning | Interaction (attentional cooperative task) | Before a gift exchange vs. after a gift exchange | Time-aligned temporal INS | Dorsolateral prefrontal cortex | Before a gift exchange < after a gift exchange | |
| Balconi et al., 2020 [10] | 14 friend dyads | EEG hyperscanning | Interaction (attentional cooperative task) | Before a gift exchange vs. after a gift exchange | Time-aligned temporal INS | Dorsolateral prefrontal cortex (delta and theta bands) | Before a gift exchange < after a gift exchange | |
| Djalovski et al., 2021 [11] | 34 friend dyads 52 stranger dyads (46 romantic partners) | EEG hyperscanning | Interaction (cooperative drawing; empathy giving task) | Friends vs. strangers | PLV | Motor task: central regions Empathy giving task: central and bilateral temporal regions | Friends = strangers | |
| Hyon et al., 2020a [12] | 42 subjects from a social network (861 dyads) | fMRI successive scanning | Non-interaction (watching a series of videos) | Social distance within a social network | Spatial INS Spatio-temporal INS | Posterior parietal cortex | (1) Spatial INS: neural similarity is not correlated with social distance (2) Spatio-temporal INS: neural similarity decreases with increasing social distance | |
| Hyon et al., 2020b [13] | 57 subjects from a social network (1596 dyads) | fMRI successive scanning | Non-interaction (rest) | Social distance within a social network | Functional connectomic INS | 13 functional brain networks | Neural similarity decreases with increasing social distance | |
| Jia et al., 2024a [14] | 26 friend dyads 26 stranger dyads (26 romantic partners) | EEG hyperscanning | Interaction (competitive button press task) | Friends vs. strangers | PLV | Frontal and occipital regions (Theta band) | Strangers > friends (400–600 ms time window); friends > strangers (800–1000 ms time window) | |
| Jia et al., 2024b [15] | 12 friend dyads 10 stranger dyads | EEG hyperscanning | Interaction (motor imagery task) | No contact vs. eye contact vs. hand contact vs. eye and hand contact | CCorr | Frontal, parietal, and occipital; frontocentral regions | Theta band: eye and hand contact > other conditions; eye contact > no contact Alpha band: hand contact > no contact; eye and hand contact > other conditions | |
| Liu et al., 2023 [16] | 35 friend dyads | fNIRS hyperscanning | Interaction (interpersonal emotion regulation) | Cognitive reappraisal vs. expressive suppression | Time-aligned temporal INS | During the sharing stage: supramarginal gyrus and dorsolateral prefrontal cortex During the regulation stage: prefrontal cortex | During the sharing stage: cognitive reappraisal > expressive suppression During the regulation stage: cognitive reappraisal < expressive suppression | |
| Long et al., 2020 [17] | 22 friend dyads (22 romantic partners) | fNIRS hyperscanning | Interaction (free discussion) | Free discussion vs. touch | Time-lagged (2 s) WTC | Anterior temporal lobe in women and the temporoparietal junction and sensorimotor cortex in men | Free discussion > touch | |
| Long et al., 2021 [18] | 22 friend dyads (22 romantic partners) | fNIRS hyperscanning | Interaction (free discussion) | Supportive vs. conflictual vs. neutral topics | Time-lagged (4 s) WTC | Sensorimotor cortex | Supportive > conflictual Supportive > neutral | |
| Luft et al., 2022 [19] | 27 friend dyads 29 stranger dyads | EEG hyperscanning | Interaction (time reproduction task) | Friends vs. strangers Eye contact vs. no eye contact | PLV | Whole-brain network (Gamma band) | Friends > strangers | Eye contact > no eye contact |
| McNabb et al., 2020 [20] | 68 subjects (school age; 767 dyads) | fMRI successive scanning | Non-interaction (rest) | Social distance within a social network | Functional connectomic INS | Whole-brain and default mode network, salience network, and bilateral fronto-parietal networks | Neural similarity is not correlated with social distance | |
| Pan et al., 2017 [21] | 16 friend dyads 16 stranger dyads (17 romantic partners) | fNIRS hyperscanning | Interaction (cooperative button press task) | Friends vs. strangers | Time-aligned WTC | Right superior frontal cortex | Friends=strangers | |
| Parkinson et al., 2018 [22] | 42 subjects from a social network (861 dyads) | fMRI successive scanning | Non-interaction (watching videos) | Social distance within a social network | Temporal INS | Superior and inferior parietal lobe, nucleus accumbens, amygdala, putamen, and caudate nucleus. | Neural similarity decreases with increasing social distance | |
| Song et al., 2024 [23] | 23 friend dyads 24 stranger dyads | fNIRS hyperscanning | Interaction (joint Simon task) | Friends vs. strangers | Time-aligned temporal INS | Dorsolateral and medial parts of the prefrontal cortex | Friends > strangers | |
| Spiegelhalder et al., 2014 [24] | 11 friend dyads | fMRI hyperscanning | Interaction (discussions) | Real conversational friends vs. not actually conversational dyads | Temporal INS | Speech production areas synchronized with auditory cortex and posterior cingulate cortex | Real conversational friends > not actually conversational dyads | |
| Zhang et al., 2024 [25] | 18 friend dyads 18 stranger dyads | fNIRS hyperscanning | Interaction (tangram puzzle task) | Friends vs. strangers Collaborative cooperation vs. division of labor cooperation | Time-aligned WTC | Specificity: bilateral dorsolateral prefrontal cortex and the right temporoparietal junction Situational modulations left dorsolateral prefrontal cortex, right pars triangularis, and right supramarginal gyrus | Friends = strangers | Collaborative cooperation > division of labor cooperation |
| Zhao et al., 2023 [26] | 30 friend dyads 30 stranger dyads | fNIRS hyperscanning | Interaction (Balloon Analogue Risk Task) | Friend dyads vs. stranger dyads Negative feedback vs. positive feedback | Time-aligned WTC | Right dorsolateral prefrontal cortex, bilateral inferior prefrontal gyrus, and frontopolar cortex | Friend dyads: negative feedback > positive feedback |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Liu, Y. Neural Similarity and Synchrony among Friends. Brain Sci. 2024, 14, 562. https://doi.org/10.3390/brainsci14060562
Ma C, Liu Y. Neural Similarity and Synchrony among Friends. Brain Sciences. 2024; 14(6):562. https://doi.org/10.3390/brainsci14060562
Chicago/Turabian StyleMa, Chao, and Yi Liu. 2024. "Neural Similarity and Synchrony among Friends" Brain Sciences 14, no. 6: 562. https://doi.org/10.3390/brainsci14060562
APA StyleMa, C., & Liu, Y. (2024). Neural Similarity and Synchrony among Friends. Brain Sciences, 14(6), 562. https://doi.org/10.3390/brainsci14060562






