Assessing the Influence of Intermittent Alcohol Access on Acrylamide-Induced Neuronal Toxicity in an Experimental Rat Model
Abstract
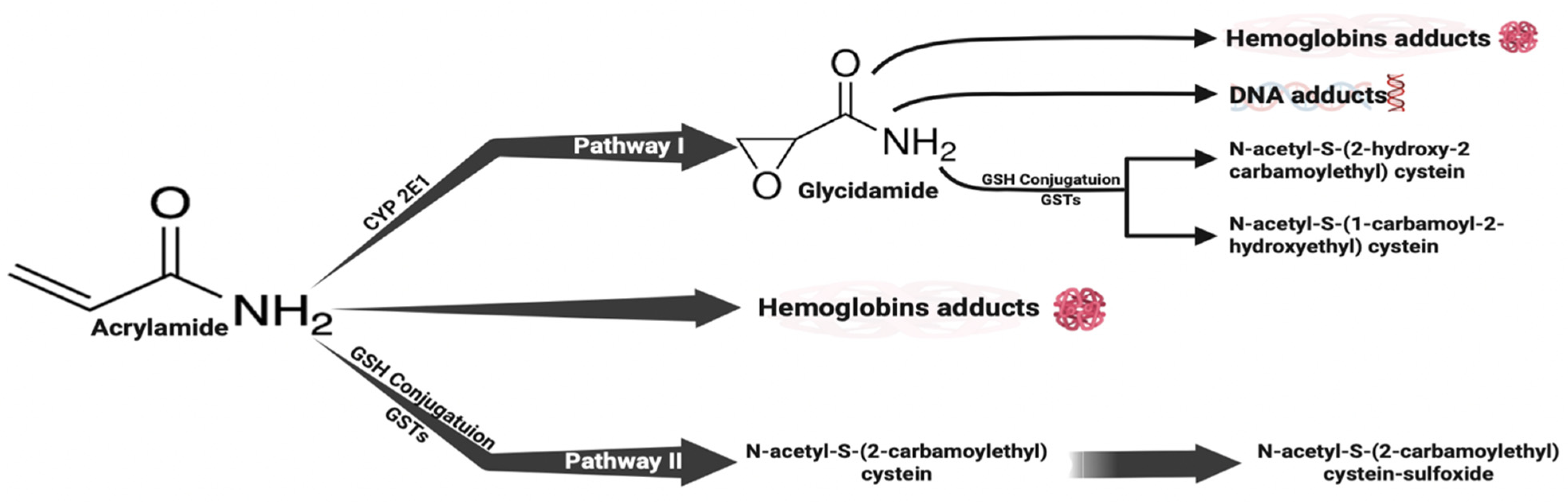
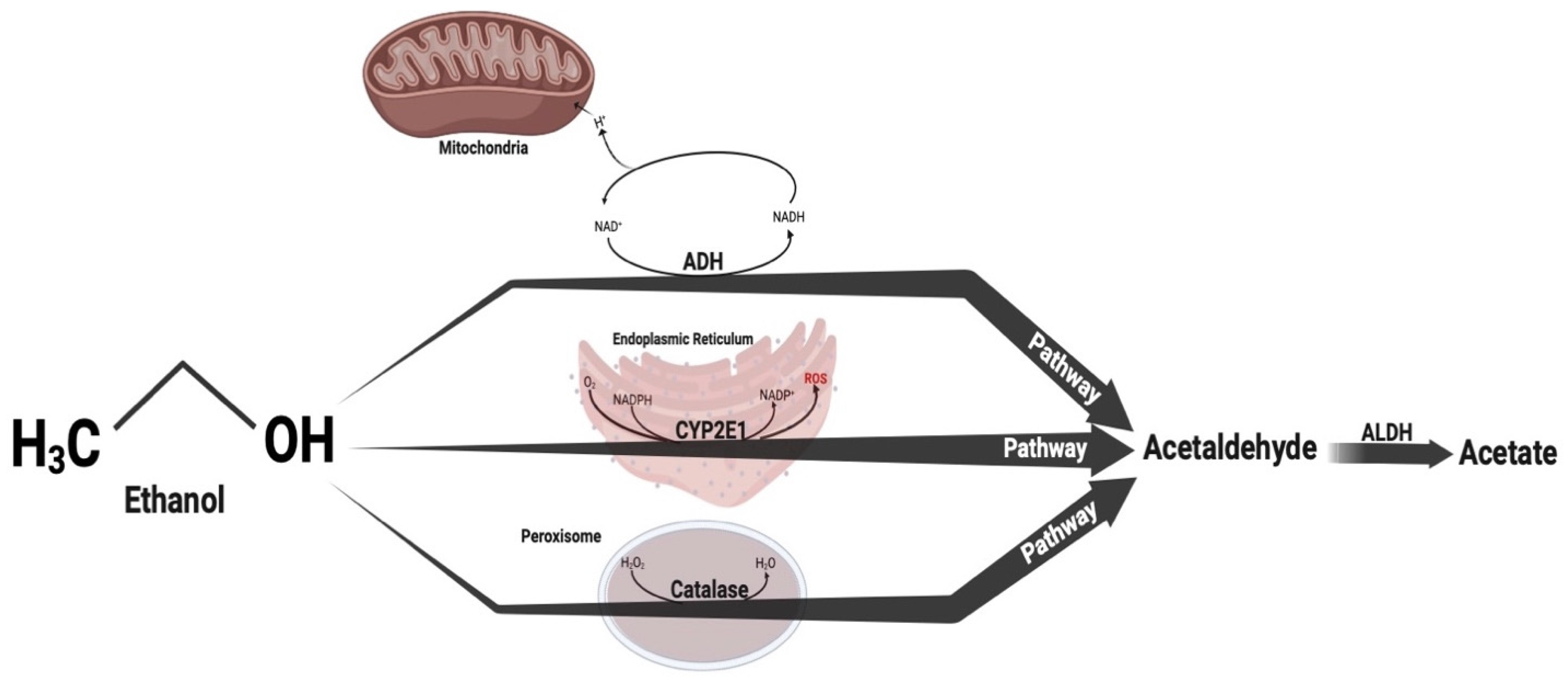
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Treatment Groups and Schedule
2.3. Cognitive Assessments
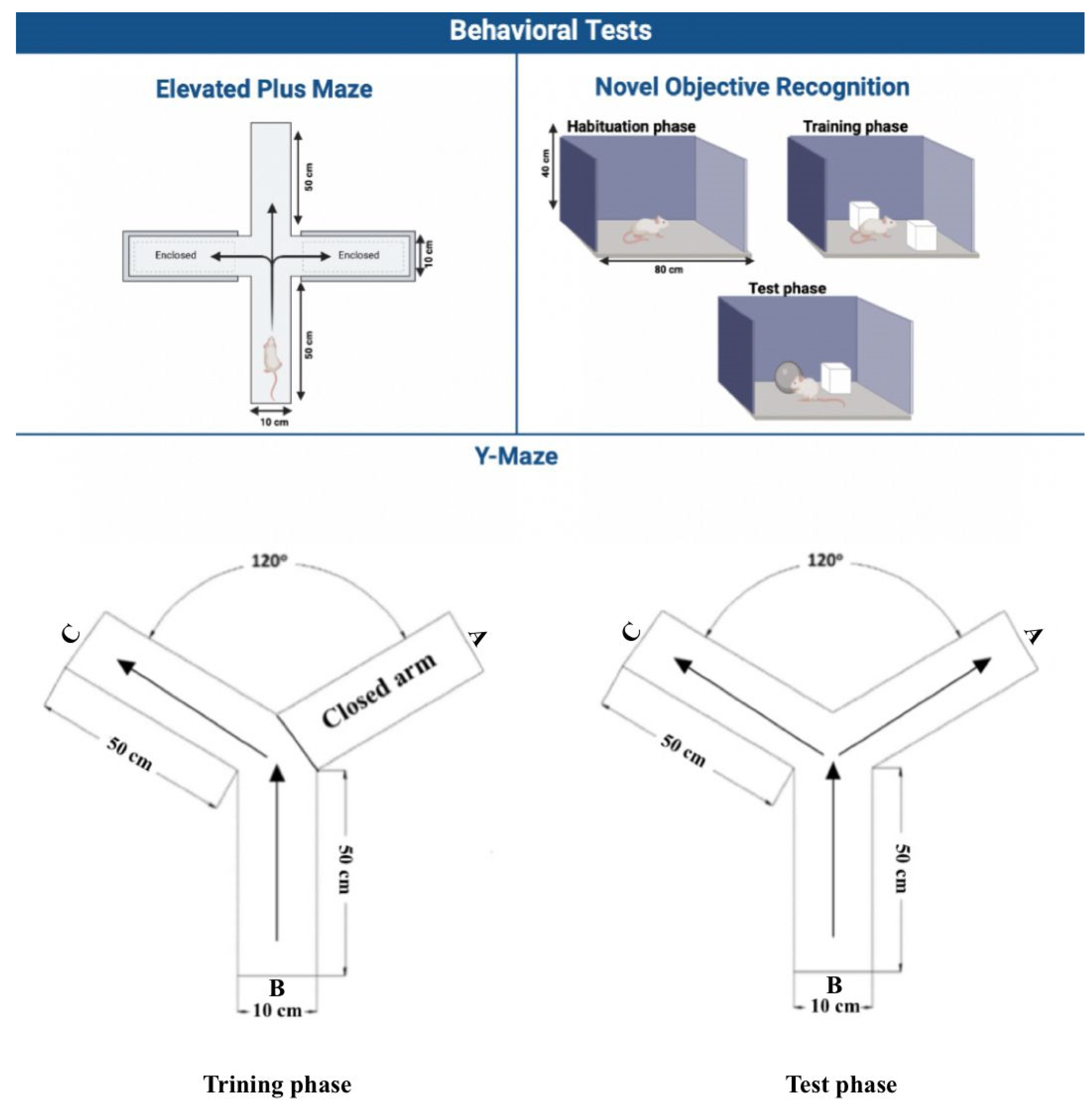
2.3.1. Elevated Plus Maze (EPM)
2.3.2. Novel Object Recognition (NOR)
2.3.3. Y-Maze
2.4. Biochemical Analysis—Enzyme-Linked Immunosorbent (ELISA) Assay
2.4.1. Brain Isolation
2.4.2. Neuronal Inflammation
2.4.3. Neuronal Apoptosis
2.4.4. Neuronal Oxidative Stress
2.4.5. Histopathological Analysis
2.5. Statistical Analysis
3. Results
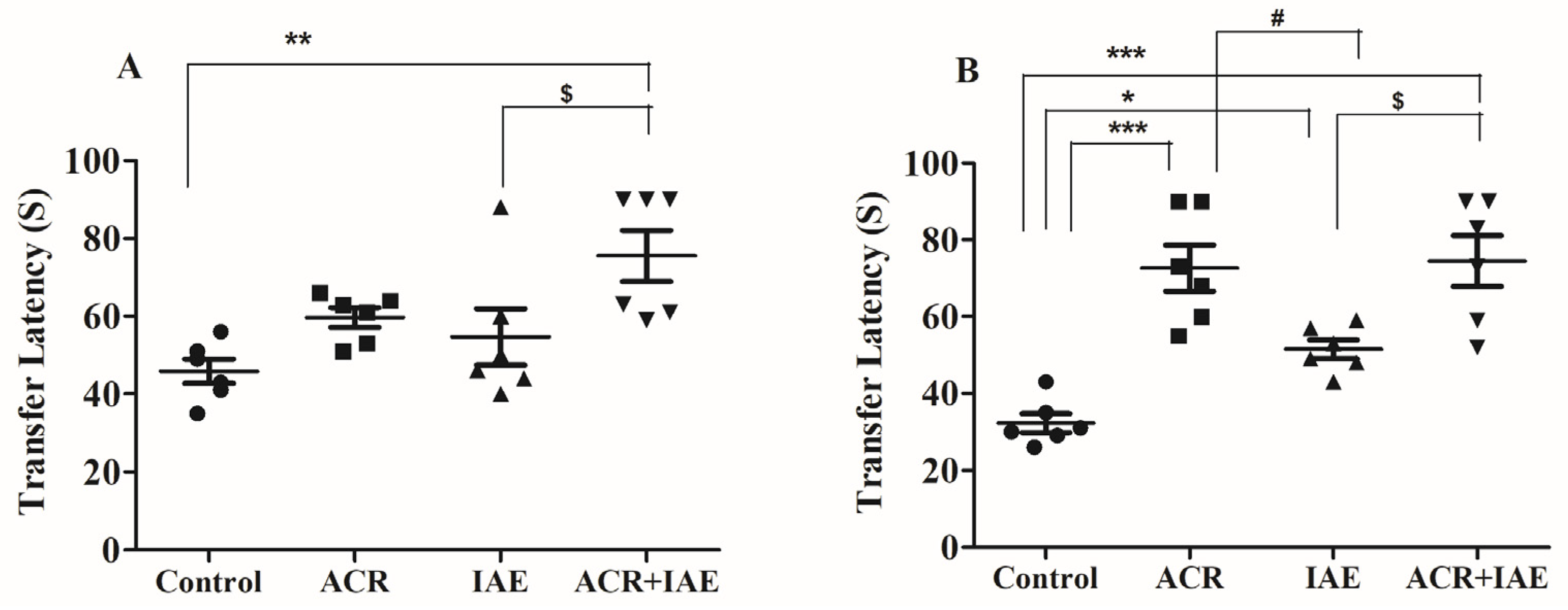
3.1. Effect of ACR and IAE on Transfer Latency (TL) Time of Elevated Plus-Maze (EPM) Test
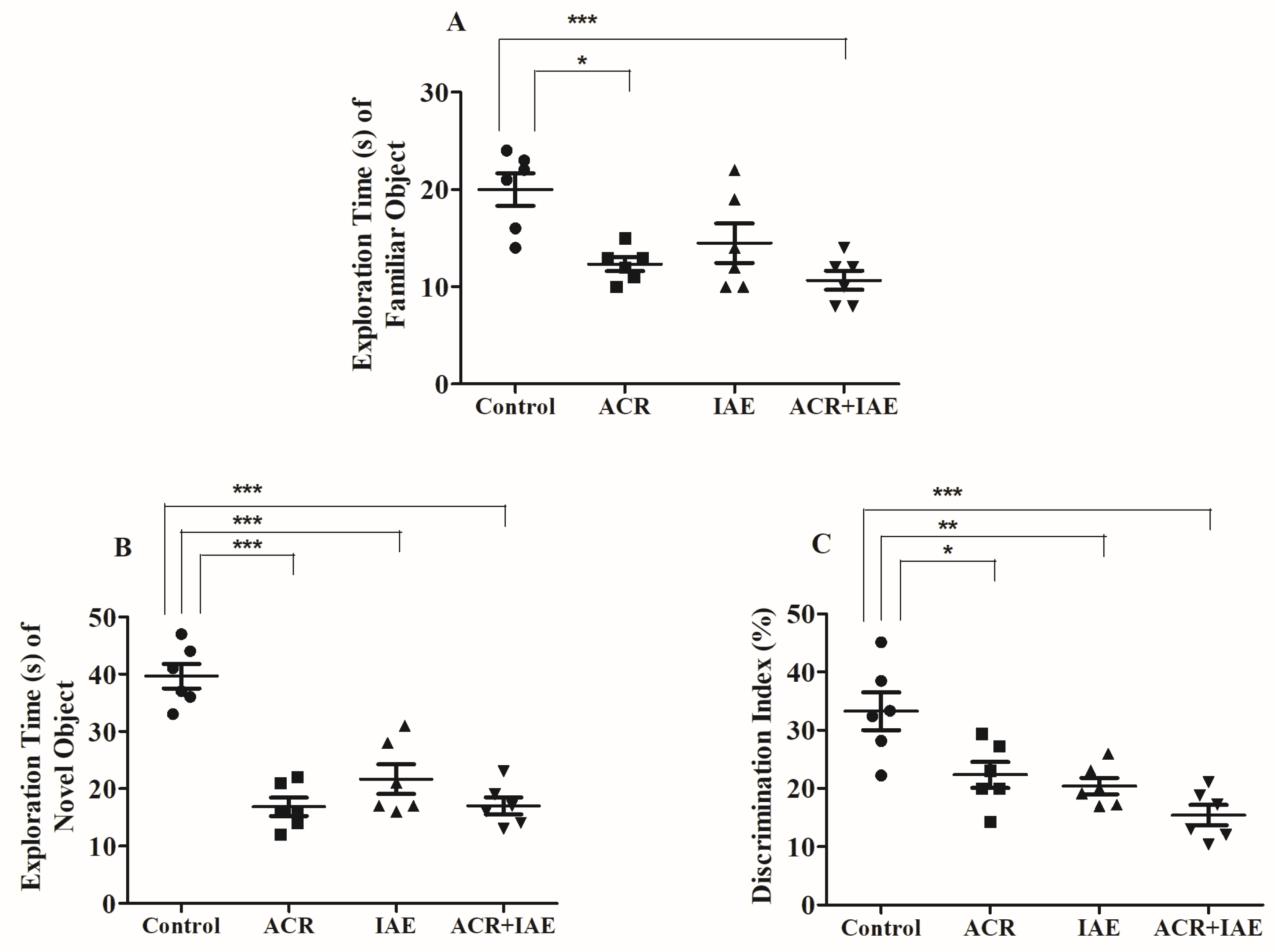
3.2. Effect of ACR and IAE on Targeted Cognitive Parameters in the Novel Object Recognition (NOR) Test
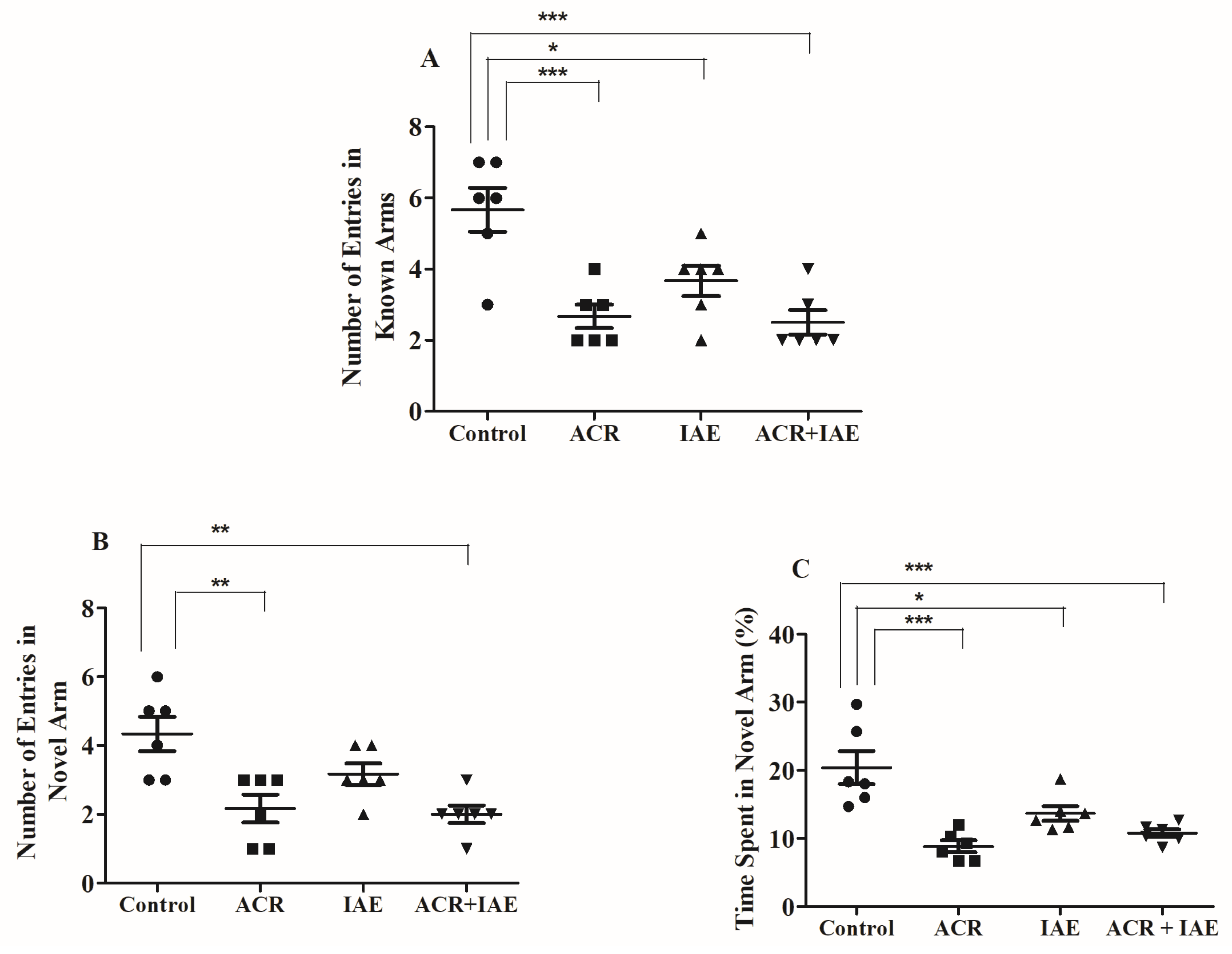
3.3. Effect of ACR and IAE on Targeted Cognitive Parameters in Y-Maze Test
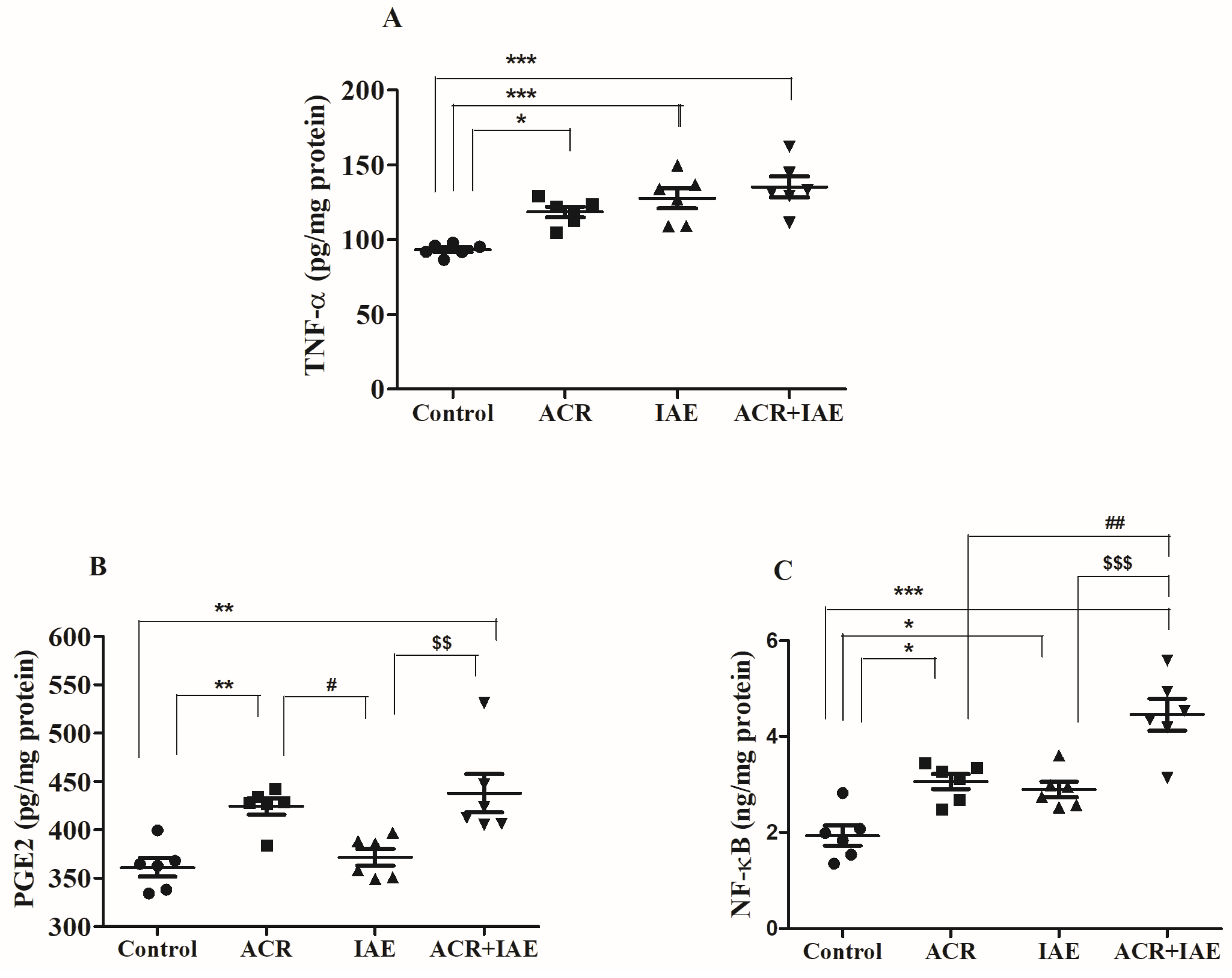
3.4. Effect of ACR and IAE on Neuro-Inflammatory Mediators in the Rat Brain
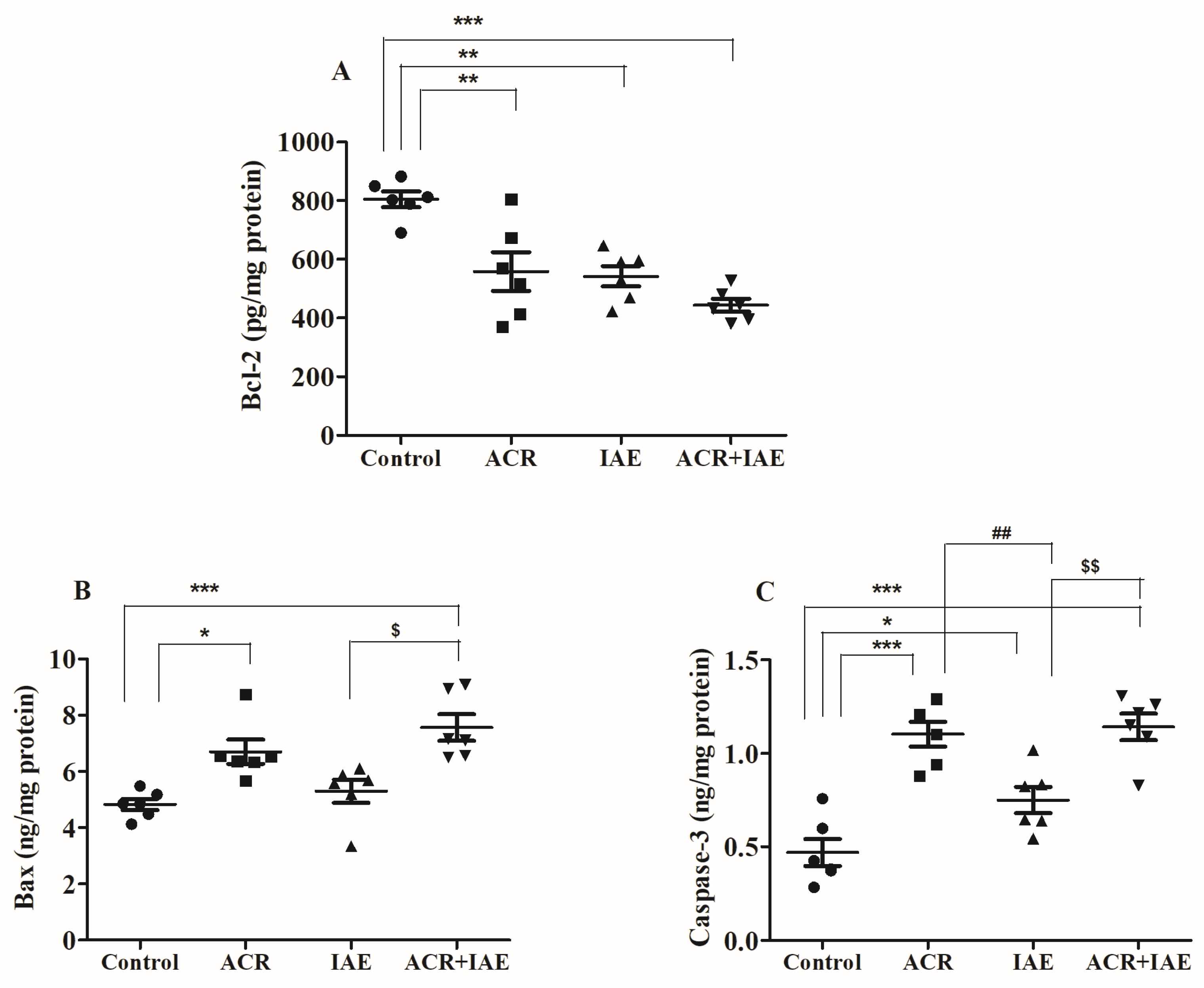
3.5. Effect of ACR and IAE on Apoptosis Parameters in the Rat Brain
3.6. Effect of ACR and IAE on Oxidative Parameters in the Rat Brain
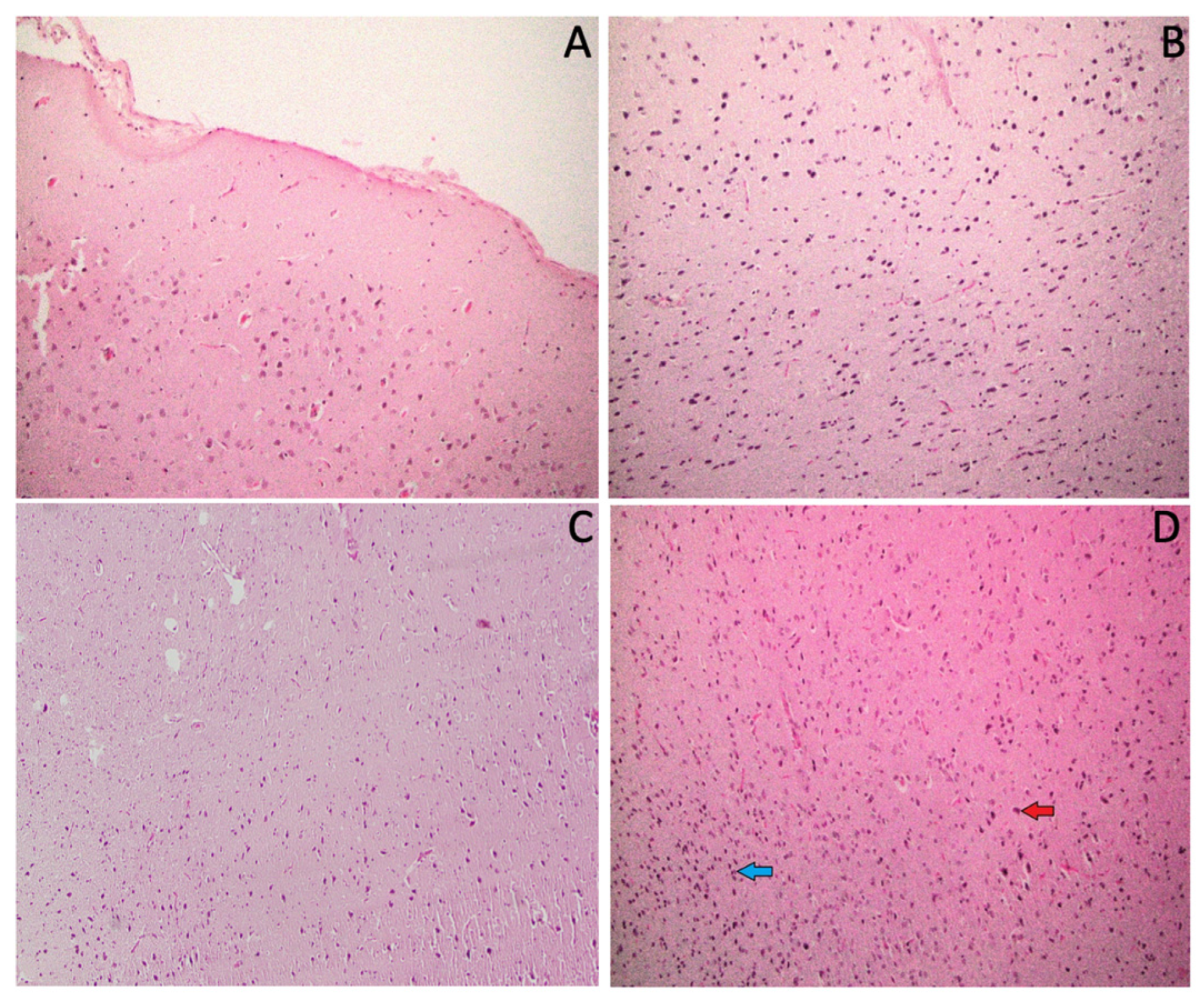
3.7. Effect of ACR and IAE on the Histopathology of the Rat Brain
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Report on the Global Tobacco Epidemic, 2023: Protect People from Tobacco Smoke; The MPOWER Package 2023; World Health Organization: Geneva, Switzerland, 2023; p. 248. Available online: https://www.who.int/publications/i/item/9789240077164 (accessed on 8 March 2024).
- World Health Organization. WHO Mortalidad Atribuíble al Tabaco; WHO Global Report 2012; World Health Organization: Geneva, Switzerland, 2012; Available online: https://www.who.int/publications/i/item/9789241564434 (accessed on 8 March 2024).
- Black, G.B.; Janes, S.M.; Callister, M.E.J.; van Os, S.; Whitaker, K.L.; Quaife, S.L. The Role of Smoking Status in Making Risk-Informed Diagnostic Decisions in the Lung Cancer Pathway: A Qualitative Study of Health Care Professionals and Patients. Med. Decis. Mak. 2024, 44, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Vourliotis, T.; Twyman, L.; Trigg, J.; Fairweather, A.K.; Disney, G.; Lawn, S.; Kavanagh, A.; Bonevski, B. High Tobacco Smoking Rates in People with Disability: An Unaddressed Public Health Issue. Aust. N. Z. J. Public Health 2024, 48, 100110. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.D.; Nilsson, L.G.; Nyberg, L.; Bäckman, L. Cigarette Smoking and Cognitive Performance in Healthy Swedish Adults. Age Ageing 2003, 32, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Yang, X.; Yeung, S.C.; Chiu, K.; Lau, C.F.; Tsang, A.W.T.; Mak, J.C.W.; Chang, R.C.C. Cigarette Smoking Accelerated Brain Aging and Induced Pre-Alzheimer-Like Neuropathology in Rats. PLoS ONE 2012, 7, e36752. [Google Scholar] [CrossRef] [PubMed]
- Thun, M.; Peto, R.; Boreham, J.; Lopez, A.D. Stages of the Cigarette Epidemic on Entering Its Second Century. Tob. Control 2012, 21, 96–101. [Google Scholar] [CrossRef] [PubMed]
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2014; pp. 1–36.
- Kenwood, B.M.; Zhu, W.; Zhang, L.; Bhandari, D.; Blount, B.C. Cigarette Smoking Is Associated with Acrylamide Exposure among the U.S. Population: NHANES 2011–2016. Environ. Res. 2022, 209, 112774. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, S.C.; Gerardi, A.R. Acrylamide Analysis in Tobacco, Alternative Tobacco Products, and Cigarette Smoke. J. Chromatogr. Sci. 2011, 49, 234–242. [Google Scholar] [CrossRef]
- Lee, H.W.; Baek, C.H.; Ma, Y.; Lee, J.; Moon, B.K.; Lee, K.W.; Jung, M.Y. Identifying High-Risk Factors and Mitigation Strategies for Acrylamide Formation in Air-Fried Lotus Root Chips: Impact of Cooking Parameters, Including Temperature, Time, Presoaking, and Seasoning. J. Food Sci. 2024, 89, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Koszucka, A.; Nowak, A.; Nowak, I.; Motyl, I. Acrylamide in Human Diet, Its Metabolism, Toxicity, Inactivation and the Associated European Union Legal Regulations in Food Industry. Crit. Rev. Food Sci. Nutr. 2020, 60, 1677–1692. [Google Scholar] [CrossRef]
- Katen, A.L.; Roman, S.D. The Genetic Consequences of Paternal Acrylamide Exposure and Potential for Amelioration. Mutat. Res. 2015, 777, 91–100. [Google Scholar] [CrossRef]
- Rifai, L.; Saleh, F.A. A Review on Acrylamide in Food: Occurrence, Toxicity, and Mitigation Strategies. Int. J. Toxicol. 2020, 39, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, B.; Deng, L. The Mechanism of Acrylamide-Induced Neurotoxicity: Current Status and Future Perspectives. Front. Nutr. 2022, 9, 859189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y. Formation and Reduction of Acrylamide in Maillard Reaction: A Review Based on the Current State of Knowledge. Crit. Rev. Food Sci. Nutr. 2007, 47, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Zamani, E.; Shokrzadeh, M.; Ziar, A.; Abedian-Kenari, S.; Shaki, F. Acrylamide Attenuated Immune Tissues’ Function via Induction of Apoptosis and Oxidative Stress: Protection by l-Carnitine. Hum. Exp. Toxicol. 2018, 37, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Lewis Wang, F.S.; Hu, X.; Chen, F.; Chan, H.M. Acrylamide-Induced Neurotoxicity in Primary Astrocytes and Microglia: Roles of the Nrf2-ARE and NF-ΚB Pathways. Food Chem. Toxicol. 2017, 106, 25–35. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2018; p. 478. Available online: https://www.who.int/publications/i/item/9789241565639 (accessed on 8 March 2024).
- Manthey, J.; Shield, K.D.; Rylett, M.; Hasan, O.S.M.; Probst, C.; Rehm, J. Global Alcohol Exposure between 1990 and 2017 and Forecasts until 2030: A Modelling Study. Lancet 2019, 393, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, M.; Menon, A.; Oto, M.; Fullerton, N.E.; Leach, J.P. Alcohol and the Central Nervous System. Pract. Neurol. 2023, 23, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Sabzali, M.; Eidi, A.; Khaksari, M.; Khastar, H. Anti-Inflammatory, Antioxidant, and Antiapoptotic Action of Metformin Attenuates Ethanol Neurotoxicity in the Animal Model of Fetal Alcohol Spectrum Disorders. Neurotox. Res. 2022, 40, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.N.; Wang, F.; Fan, Y.X.; Ping, G.F.; Yang, J.Y.; Wu, C.F. Activated Microglia Are Implicated in Cognitive Deficits, Neuronal Death, and Successful Recovery Following Intermittent Ethanol Exposure. Behav. Brain Res. 2013, 236, 270–282. [Google Scholar] [CrossRef]
- Pascual, M.; Baliño, P.; Alfonso-Loeches, S.; Aragón, C.M.G.; Guerri, C. Impact of TLR4 on Behavioral and Cognitive Dysfunctions Associated with Alcohol-Induced Neuroinflammatory Damage. Brain Behav. Immun. 2011, 25 (Suppl. S1), S80–S91. [Google Scholar] [CrossRef]
- Pascual, M.; Blanco, A.M.; Cauli, O.; Miñarro, J.; Guerri, C. Intermittent Ethanol Exposure Induces Inflammatory Brain Damage and Causes Long-Term Behavioural Alterations in Adolescent Rats. Eur. J. Neurosci. 2007, 25, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I. Alcohol Metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Mortality and Burden of Disease Attributable to Selected Major Risks; WHO Library Cataloguing-in-Publication Data Global; World Health Organization: Geneva, Switzerland, 2009; p. 70. Available online: https://www.who.int/publications/i/item/9789241563871 (accessed on 8 March 2024).
- Pelucchi, C.; Gallus, S.; Garavello, W.; Bosetti, C.; Vecchia, C.L. Alcohol and Tobacco Use, and Cancer Risk for Upper Aerodigestive Tract and Liver. Eur. J. Cancer Prev. 2008, 17, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Madero-Cabib, I.; Bambs, C. Association between Lifetime Tobacco Use and Alcohol Consumption Trajectories and Cardiovascular and Chronic Respiratory Diseases among Older People. Int. J. Environ. Res. Public Health 2021, 18, 11275. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.M.; Quintal, C.; Lourenço. Killing Two Birds with One Stone? Association between Tobacco and Alcohol Consumption. Public Health 2018, 154, 136–143. [Google Scholar] [CrossRef]
- Lamy, E.; Völkel, Y.; Roos, P.H.; Kassie, F.; Mersch-Sundermann, V. Ethanol Enhanced the Genotoxicity of Acrylamide in Human, Metabolically Competent HepG2 Cells by CYP2E1 Induction and Glutathione Depletion. Int. J. Hyg. Environ. Health 2008, 211, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Vesper, H.W.; Slimani, N.; Hallmans, G.; Tjønneland, A.; Agudo, A.; Benetou, V.; Bingham, S.; Boeing, H.; Boutron-Ruault, M.C.; Bueno-De-Mesquita, H.B.; et al. Cross-Sectional Study on Acrylamide Hemoglobin Adducts in Subpopulations from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. J. Agric. Food Chem. 2008, 56, 6046–6053. [Google Scholar] [CrossRef]
- Vikström, A.C.; Wilson, K.M.; Paulsson, B.; Athanassiadis, I.; Grönberg, H.; Adami, H.O.; Adolfsson, J.; Mucci, L.A.; Bälter, K.; Törnqvist, M. Alcohol Influence on Acrylamide to Glycidamide Metabolism Assessed with Hemoglobin-Adducts and Questionnaire Data. Food Chem. Toxicol. 2010, 48, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, M.; Mombeini, M.A.; Fatemi, I.; Aminzadeh, A.; Kalantari, H.; Nesari, A.; Najafzadehvarzi, H.; Mehrzadi, S. Neuroprotective Effects of Ellagic Acid against Acrylamide-Induced Neurotoxicity in Rats. Neurol. Res. 2019, 41, 419–428. [Google Scholar] [CrossRef]
- Rahangadale, S.; Kurkure, N.; Prajapati, B.; Hedaoo, V.; Bhandarkar, A.G. Neuroprotective Effect of Vitamin E Supplementation in Wistar Rat Treated with Acrylamide. Toxicol. Int. 2012, 19, 1. [Google Scholar] [CrossRef][Green Version]
- Simms, J.A.; Steensland, P.; Medina, B.; Abernathy, K.E.; Chandler, L.J.; Wise, R.; Bartlett, S.E. Intermittent Access to 20% Ethanol Induces High Ethanol Consumption in Long-Evans and Wistar Rats. Alcohol. Clin. Exp. Res. 2008, 32, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.C.; Kulkarni, S.K. Evaluation of Learning and Memory Mechanisms Employing Elevated Plus-Maze in Rats and Mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 1992, 16, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Rabbani, S.I.; Shariq, A.; Amirthalingam, P.; Arfeen, M. Piracetam as a Therapeutic Agent for Doxorubicin-Induced Cognitive Deficits by Enhancing Cholinergic Functions and Reducing Neuronal Inflammation, Apoptosis, and Oxidative Stress in Rats. Pharmaceuticals 2022, 15, 1563. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Arfeen, M.; Rabbani, S.I.; Shariq, A.; Amirthalingam, P. Levetiracetam Ameliorates Doxorubicin-Induced Chemobrain by Enhancing Cholinergic Transmission and Reducing Neuroinflammation Using an Experimental Rat Model and Molecular Docking Study. Molecules 2022, 27, 7364. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Ye, J.; Liu, W.; Zhao, B.; Shi, X.; Zhang, C.; Liu, Z.; Liu, X. Acrylamide Aggravates Cognitive Deficits at Night Period via the Gut-Brain Axis by Reprogramming the Brain Circadian Clock. Arch. Toxicol. 2019, 93, 467–486. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Xiong, F.; Li, Z.; Dong, G.; Sun, X.; Yin, W.; Cai, H. The Effect of Chronic Alcohol Exposure on Spatial Memory and BDNF–TrkB- PLCγ1 Signaling in the Hippocampus of Male and Female Mice. Heliyon 2023, 9, e16660. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Arfeen, M.; Dhaked, D.K.; Mohammed, H.A.; Amirthalingam, P.; Elsisi, H.A. Neuroprotective Effect of Methanolic Ajwa Seed Extract on Lipopolysaccharide-Induced Memory Dysfunction and Neuroinflammation: In Vivo, Molecular Docking and Dynamics Studies. Plants 2023, 12, 934. [Google Scholar] [CrossRef] [PubMed]
- Lueptow, L.M. Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J. Vis. Exp. 2017, 2017, 55718. [Google Scholar] [CrossRef] [PubMed]
- Mathiasen, J.R.; Dicamillo, A. Novel Object Recognition in the Rat: A Facile Assay for Cognitive Function. Curr. Protoc. Pharmacol. 2010, 5 (Suppl. S49), 5–59. [Google Scholar] [CrossRef]
- Mani, V. Betahistine Protects Doxorubicin-Induced Memory Deficits via Cholinergic and Anti-Inflammatory Pathways in Mouse Brain. Int. J. Pharmacol. 2021, 17, 584–595. [Google Scholar] [CrossRef]
- Mani, V.; Arfeen, M.; Mohammed, H.A.; Elsisi, H.A.; Sajid, S.; Almogbel, Y.; Aldubayan, M.; Dhanasekaran, M.; Alhowail, A. Sukkari Dates Seed Improves Type-2 Diabetes Mellitus-Induced Memory Impairment by Reducing Blood Glucose Levels and Enhancing Brain Cholinergic Transmission: In Vivo and Molecular Modeling Studies. Saudi Pharm. J. 2022, 30, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Marco, E.M.; Peñasco, S.; Hernández, M.D.; Gil, A.; Borcel, E.; Moya, M.; Giné, E.; López-Moreno, J.A.; Guerri, C.; López-Gallardo, M.; et al. Long-Term Effects of Intermittent Adolescent Alcohol Exposure in Male and Female Rats. Front. Behav. Neurosci. 2017, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Ajibare, A.J.; Oriyomi, A.I.; Asuku, A.O. Neuroprotective Potential of Virgin Coconut Oil in Abrogating Acrylamide-Induced Neurobehavioural Impairment Via NRF-2/NFK-B Signaling and BDNF Upregulation. Preprint 2023. [Google Scholar] [CrossRef]
- Contreras, A.; Morales, L.; Del Olmo, N. The Intermittent Administration of Ethanol during the Juvenile Period Produces Changes in the Expression of Hippocampal Genes and Proteins and Deterioration of Spatial Memory. Behav. Brain Res. 2019, 372, 112033. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Karin, M. NF-ΚB, Inflammation, Immunity and Cancer: Coming of Age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Pan, X.; Yao, J.; Wang, D.; Wu, X.; Chen, X.; Shi, N.; Yan, H. MAPKs and NF-ΚB-Mediated Acrylamide-Induced Neuropathy in Rat Striatum and Human Neuroblastoma Cells SY5Y. J. Cell Biochem. 2019, 120, 3898–3910. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, C.; Temel, Y.; Kandemir, F.M.; Yildirim, S.; Kucukler, S. Naringin Protects against Cyclophosphamide-Induced Hepatotoxicity and Nephrotoxicity through Modulation of Oxidative Stress, Inflammation, Apoptosis, Autophagy, and DNA Damage. Environ. Sci. Pollut. Res. Int. 2018, 25, 20968–20984. [Google Scholar] [CrossRef]
- Kandemir, F.M.; Kucukler, S.; Caglayan, C.; Gur, C.; Batil, A.A.; Gülçin, İ. Therapeutic Effects of Silymarin and Naringin on Methotrexate-Induced Nephrotoxicity in Rats: Biochemical Evaluation of Anti-Inflammatory, Antiapoptotic, and Antiautophagic Properties. J. Food Biochem. 2017, 41, e12398. [Google Scholar] [CrossRef]
- Temel, Y.; Kucukler, S.; Yıldırım, S.; Caglayan, C.; Kandemir, F.M. Protective Effect of Chrysin on Cyclophosphamide-Induced Hepatotoxicity and Nephrotoxicity via the Inhibition of Oxidative Stress, Inflammation, and Apoptosis. Naunyn. Schmiedebergs Arch. Pharmacol. 2020, 393, 325–337. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abd Eldaim, M.A.; Hassan, A.G.A. Trigonella Foenum-Graecum Ameliorates Acrylamide-Induced Toxicity in Rats: Roles of Oxidative Stress, Proinflammatory Cytokines, and DNA Damage. Biochem. Cell Biol. 2015, 93, 192–198. [Google Scholar] [CrossRef]
- Nassar, A.; Sharon-Granit, Y.; Azab, A.N. Psychotropic Drugs Attenuate Lipopolysaccharide-Induced Hypothermia by Altering Hypothalamic Levels of Inflammatory Mediators in Rats. Neurosci. Lett. 2016, 626, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Song, G.; Zou, C.; Liu, G.; Wu, W.; Yuan, T.; Liu, X. Acrylamide Induces Mitochondrial Dysfunction and Apoptosis in BV-2 Microglial Cells. Free Radic. Biol. Med. 2015, 84, 42–53. [Google Scholar] [CrossRef]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and Oxidative Stress in Neurodegenerative Diseases. J. Alzheimers Dis. 2014, 42 (Suppl. S3), S125–S152. [Google Scholar] [CrossRef]
- Idrus, N.M.; Napper, R.M.A. Acute and Long-Term Purkinje Cell Loss Following a Single Ethanol Binge During the Early Third Trimester Equivalent in the Rat. Alcohol. Clin. Exp. Res. 2012, 36, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.S.; Gautam, P.; Kushwaha, P. Lead and Ethanol Co-Exposure Lead to Blood Oxidative Stress and Subsequent Neuronal Apoptosis in Rats. Alcohol. Alcohol. 2012, 47, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Vasudevan, D.M. Alcohol-Induced Oxidative Stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.W.; Ishimaru, M.J.; Bittigau, P.; Ikonomidou, C. Ethanol-Induced Apoptotic Neurodegeneration in the Developing Brain. Apoptosis 2000, 5, 515–521. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Sudha, K.; Rao, A.V.; Rao, A. Oxidative Stress and Antioxidants in Epilepsy. Clin. Chim. Acta 2001, 303, 19–24. [Google Scholar] [CrossRef]
- Johansen, J.S.; Harris, A.K.; Rychly, D.J.; Ergul, A. Oxidative Stress and the Use of Antioxidants in Diabetes: Linking Basic Science to Clinical Practice. Cardiovasc. Diabetol. 2005, 4, 5. [Google Scholar] [CrossRef]
- Schulz, J.B.; Lindenau, J.; Seyfried, J.; Dichgans, J. Glutathione, Oxidative Stress and Neurodegeneration. Eur. J. Biochem. 2000, 267, 4904–4911. [Google Scholar] [CrossRef] [PubMed]
- Tsermpini, E.E.; Plemenitaš Ilješ, A.; Dolžan, V. Alcohol-Induced Oxidative Stress and the Role of Antioxidants in Alcohol Use Disorder: A Systematic Review. Antioxidants 2022, 11, 1374. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.M.; Deeny, M.A.; Shaner, C.A.; Nixon, K. Determining the Threshold for Alcohol-Induced Brain Damage: New Evidence with Gliosis Markers. Alcohol. Clin. Exp. Res. 2013, 37, 425. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshammari, A.A.A.; Almutairi, A.B.; Arfeen, M.; Alkhamiss, A.S.; Aldubayan, M.A.; Alhowail, A.H.; Mani, V. Assessing the Influence of Intermittent Alcohol Access on Acrylamide-Induced Neuronal Toxicity in an Experimental Rat Model. Brain Sci. 2024, 14, 574. https://doi.org/10.3390/brainsci14060574
Alshammari AAA, Almutairi AB, Arfeen M, Alkhamiss AS, Aldubayan MA, Alhowail AH, Mani V. Assessing the Influence of Intermittent Alcohol Access on Acrylamide-Induced Neuronal Toxicity in an Experimental Rat Model. Brain Sciences. 2024; 14(6):574. https://doi.org/10.3390/brainsci14060574
Chicago/Turabian StyleAlshammari, Abdulaziz Arif A., Awyed Batah Almutairi, Minhajul Arfeen, Abdullah Saleh Alkhamiss, Maha A. Aldubayan, Ahmad H. Alhowail, and Vasudevan Mani. 2024. "Assessing the Influence of Intermittent Alcohol Access on Acrylamide-Induced Neuronal Toxicity in an Experimental Rat Model" Brain Sciences 14, no. 6: 574. https://doi.org/10.3390/brainsci14060574
APA StyleAlshammari, A. A. A., Almutairi, A. B., Arfeen, M., Alkhamiss, A. S., Aldubayan, M. A., Alhowail, A. H., & Mani, V. (2024). Assessing the Influence of Intermittent Alcohol Access on Acrylamide-Induced Neuronal Toxicity in an Experimental Rat Model. Brain Sciences, 14(6), 574. https://doi.org/10.3390/brainsci14060574







