The Utility of Heartrate and Heartrate Variability Biofeedback for the Improvement of Interoception across Behavioural, Physiological and Neural Outcome Measures: A Systematic Review
Abstract
:1. Introduction
1.1. Interoception
1.2. Measuring Interoception
1.3. Heartrate (Variability) Biofeedback
1.4. The Current Review
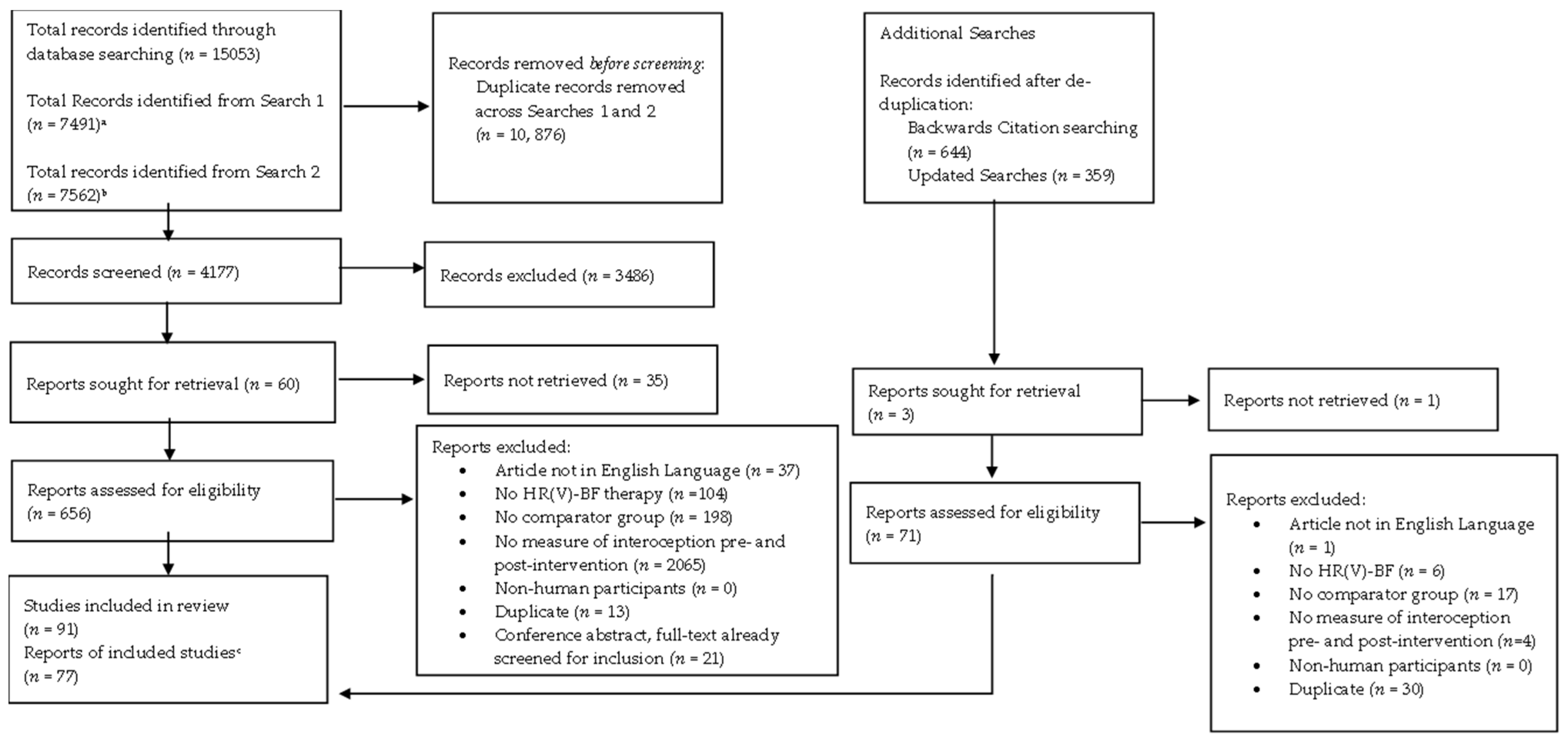
2. Methodology
2.1. Transparency and Openness
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Selection Process
2.5. Quality Assessment
2.6. Data Extraction and Data Synthesis
3. Results
3.1. Behavioural Measures
Conclusions—Behavioural Measures
3.2. Physiological Measures
3.2.1. High Frequency HRV (HF-HRV)
3.2.2. Low-Frequency HRV (LF-HRV)
3.2.3. Root Mean Square of Successive Differences (RMSSD)
| Features of Included Studies | Effect of Intervention (n = 13) | No Effect of Intervention (n = 28) | Other Effect (n = 1) (i.e., Time or Effect of Comparator) |
|---|---|---|---|
| Clinical population (%) | 53.85 | 42.86 | 0.00 |
| Comparator Group (%) a | NI—23.57 TAU—15.38 AC—42.86 S/P—14.29 | NI—36.37 TAU—18.19 AC—30.30 S/P—15.16 | NI—0.00 TAU—0.00 AC—100.00 S/P—0.00 |
| Intervention Intensity (%) | Mild—15.39 Moderate—23.08 Intense—61.54 | Mild—57.14 Moderate—25.00 Intense—17.86 | Mild—0.00 Moderate—0.00 Intense—100.00 |
| Resonance Frequency Breathing (%) | Overall RF—53.85 Optimal—30.77 Individual—23.08 Paced/preset—23.08 Unclear—23.08 | Overall RF—57.14 Optimal—46.43 Individual—10.71 Paced/preset—35.71 Unclear—7.14 | Overall RF– 100.00 Optimal—0.00 Individual—100.00 Paced/preset—0.00 Unclear—0.00 |
| Risk of Bias | 14.45 b | 14.39 | 12.00 |
3.2.4. Standard Deviation of N-N Intervals (SDNN)
3.2.5. Percentage of Successive N-N Interval Pairs Differing by >50 ms (pNN50)
3.2.6. Total Power (TP)
3.2.7. Coherence Measures
3.2.8. Baroreflex Sensitivity (BRS)
3.2.9. Respiratory Sinus Arrhythmia (RSA)
3.2.10. Conclusions—Physiological Measures
3.3. Neural Measures
Conclusions—Neural Measures
3.4. Overall Results
| Outcome | Included Studies (n) | Overall Summary Statement |
|---|---|---|
| Behavioural | 1 | No effect of HR(V)-BF |
| Physiological | ||
| HF-HRV | 46 | Trend towards no effect of HR(V)-BF |
| LF-HRV | 41 | Split results but trend for effects to be more frequently observed in healthy populations. |
| RMSSD | 42 | Trend towards no effect of HR(V)-BF |
| SDNN | 51 | Mixed results appear to be affected by covariance and compliance. |
| pNN50 | 12 | Trend towards no effect of HR(V)-BF. |
| TP | 14 | Mixed findings |
| Coherence | 5 | Mixed findings but trend towards an effect of HR(V)-BF. |
| BRS | 5 | Mixed results. |
| RSA | 5 | Split results, but trend favouring more towards no effect. |
| Neural | 4 | Trend towards an effect of HR(V)-BF amongst healthy populations. |
4. Discussion and Conclusions
4.1. Behavioural Measures
4.2. Physiological Measures
4.3. Neural Measures
4.4. A Proposed Mechanism
- Over the course of HR(V)-BF therapy, improvements will first occur in indices of central autonomic activity, such as SDNN and TP, followed by changes in neural connectivity and behavioural measures and then by indices of autonomic regulation.
- Higher compliance with resonance breathing will be associated with an increased likelihood of intervention effects at each of these stages.
- The greater the number of total intervention sessions, and the greater intensity with which they are delivered, the more likely there is for changes to be observed across all interoceptive indices.
4.5. Directions for Future Research
4.6. Limitations of Included Studies
- Overall, the average quality score of included studies was ‘Fair’ and this may have contributed to the observed heterogeneity in findings. However, we note that we expected (and found) the majority of studies to employ a clinical trial structure and therefore chose a bias assessment tool designed to evaluate these studies. Consequently, this may have affected the quality scores of studies not employing this structure.
- Some studies did not adhere to The European Society of Cardiology and The North American Society of Pacing and Electrophysiology Task Force [49] standards of HRV measurement and reporting and therefore heterogeneity in findings may arise from inconsistencies in the degree to which potential confounds were controlled for across studies. For example, though medications such as antipsychotic and antidepressant medications do not impact HRV, others such as tricyclic antidepressants and clozapine reduce HRV below normal levels (see [205] for a review), whereas medications such as beta-blockers can induce positive changes in HRV [206]. Therefore, medications may confound treatment effects. Future studies should ensure all medications are clearly reported and, where possible, controlled for in analyses.
- Only one study incorporated HR-BF [169], rather than HRV-BF. Furthermore, in this study, HR-BF was combined with the biofeedback of cerebral blood flow from the rostrolateral prefrontal cortex to the frontopolar cortex. Accordingly, there is currently limited evidence regarding the efficacy of HR-BF as an interoceptive intervention.
- As has been previously noted [207], we observed a lack of standardised intervention protocols, including the type of HR(V)-BF used (e.g., some studies used games, apps, or HR(V)-BF in combination with other interventions), and the comparator used which thus limits the conclusions that can be drawn. Hence, there is a pertinent need for studies to adhere to a standardised procedure for conducting HR(V)-BF.
- Variability in whether a transformation was applied to HRV data, and the type of transform applied, was observed between studies. Therefore, it may be important for future studies to investigate whether the type of transformation applied changes the result observed.
- Many studies did not report the treatment protocol in sufficient detail to facilitate replication, particularly with respect to the breathing techniques used (e.g., no reporting of the ratio of inhalation to exhalation used). Consequently, we echo the call by Lalanza, et al. [84] for more standardised and transparent reporting in investigations of HR(V)-BF.
4.7. Limitations of the Review Process
- As this review assessed the current state of the literature on the effects of HR(V)-BF on interoception, we used broad inclusion criteria. Consequently, there was heterogeneity amongst included studies, meaning a quantitative synthesis was not conducted and therefore conclusions do not have statistical support. Moreover, as most studies did not report effect sizes, our conclusions are based on p-values which may be inflated by sample sizes. Therefore, we encourage future reviews to employ more stringent inclusion criteria in order to facilitate the conducting of meta-analyses which could provide further evidence for, or against, our conclusions.
- Joint screening of only 10 percent of papers at the level of full-text may also be viewed as a limitation. Yet, inter-rater agreement at both title-and-abstract and full-text screening was substantial; therefore, it is unlikely that papers will have been missed using this approach. Relatedly, as only one author conducted data extraction, this may have increased the propensity for errors. However, the data extraction table was thoroughly checked by both an independent reviewer, and the reviewer responsible for data extraction. Therefore, errors in this table are unlikely.
- Conference abstracts included within this review could not be assessed for risk of bias and hence the quality of the evidence obtained in these investigations is unclear. Nevertheless, including these studies allowed us to provide a comprehensive overview of the literature, including the grey literature sources.
4.8. Conclusions
4.8.1. Behavioural Measures
4.8.2. Physiological Measures
4.8.3. Neural Measures
4.8.4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garfinkel, S.N.; Seth, A.K.; Barrett, A.B.; Suzuki, K.; Critchley, H.D. Knowing Your Own Heart: Distinguishing Interoceptive Accuracy from Interoceptive Awareness. Biol. Psychol. 2015, 104, 65–74. [Google Scholar] [CrossRef]
- Quadt, L.; Critchley, H.D.; Garfinkel, S.N. The Neurobiology of Interoception in Health and Disease. Ann. N. Y. Acad. Sci. 2018, 1428, 112–128. [Google Scholar] [CrossRef]
- Critchley, H.D.; Garfinkel, S.N. Interoception and Emotion. Curr. Opin. Psychol. 2017, 17, 7–14. [Google Scholar] [CrossRef]
- Tsakiris, M.; Preester, H.D. The Interoceptive Mind: From Homeostasis to Awareness; Oxford University Press: Oxford, UK, 2018; ISBN 978-0-19-254005-8. [Google Scholar]
- Carvalho, G.B.; Damasio, A. Interoception and the Origin of Feelings: A New Synthesis. BioEssays 2021, 43, 2000261. [Google Scholar] [CrossRef]
- Di Lernia, D.; Serino, S.; Riva, G. Pain in the Body. Altered Interoception in Chronic Pain Conditions: A Systematic Review. Neurosci. Biobehav. Rev. 2016, 71, 328–341. [Google Scholar] [CrossRef]
- Locatelli, G.; Matus, A.; James, R.; Salmoirago-Blotcher, E.; Ausili, D.; Vellone, E.; Riegel, B. What Is the Role of Interoception in the Symptom Experience of People with a Chronic Condition? A Systematic Review. Neurosci. Biobehav. Rev. 2023, 148, 105142. [Google Scholar] [CrossRef]
- Paulus, M.P.; Stein, M.B. Interoception in Anxiety and Depression. Brain Struct. Funct. 2010, 214, 451–463. [Google Scholar] [CrossRef]
- Herbert, B.M. Interoception and Its Role for Eating, Obesity, and Eating Disorders. Eur. J. Health Psychol. 2020, 27, 188–205. [Google Scholar] [CrossRef]
- Desmedt, O.; Luminet, O.; Maurage, P.; Corneille, O. Discrepancies in the Definition and Measurement of Human Interoception: A Comprehensive Discussion and Suggested Ways Forward. Perspect. Psychol. Sci. 2023, 17456916231191537. [Google Scholar] [CrossRef]
- Murphy, J. Interoception: Where Do We Go from Here? Q. J. Exp. Psychol. 2023, 77, 17470218231172725. [Google Scholar] [CrossRef]
- Chen, W.G.; Schloesser, D.; Arensdorf, A.M.; Simmons, J.M.; Cui, C.; Valentino, R.; Gnadt, J.W.; Nielsen, L.; Hillaire-Clarke, C.S.; Spruance, V.; et al. The Emerging Science of Interoception: Sensing, Integrating, Interpreting, and Regulating Signals within the Self. Trends Neurosci. 2021, 44, 3–16. [Google Scholar] [CrossRef]
- Khalsa, S.S.; Adolphs, R.; Cameron, O.G.; Critchley, H.D.; Davenport, P.W.; Feinstein, J.S.; Feusner, J.D.; Garfinkel, S.N.; Lane, R.D.; Mehling, W.E.; et al. Interoception and Mental Health: A Roadmap. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 501–513. [Google Scholar] [CrossRef]
- Suksasilp, C.; Garfinkel, S.N. Towards a Comprehensive Assessment of Interoception in a Multi-Dimensional Framework. Biol. Psychol. 2022, 168, 108262. [Google Scholar] [CrossRef]
- Berntson, G.G.; Khalsa, S.S. Neural Circuits of Interoception. Trends Neurosci. 2021, 44, 17–28. [Google Scholar] [CrossRef]
- Craig, A. Interoception: The Sense of the Physiological Condition of the Body. Curr. Opin. Neurobiol. 2003, 13, 500–505. [Google Scholar] [CrossRef]
- Fermin, A.S.R.; Friston, K.; Yamawaki, S. An Insula Hierarchical Network Architecture for Active Interoceptive Inference. R. Soc. Open Sci. 2022, 9, 220226. [Google Scholar] [CrossRef]
- Chong, J.S.X.; Ng, G.J.P.; Lee, S.C.; Zhou, J. Salience Network Connectivity in the Insula Is Associated with Individual Differences in Interoceptive Accuracy. Brain Struct. Funct. 2017, 222, 1635–1644. [Google Scholar] [CrossRef]
- Schulz, S.M. Neural Correlates of Heart-Focused Interoception: A Functional Magnetic Resonance Imaging Meta-Analysis. Phil. Trans. R. Soc. B 2016, 371, 20160018. [Google Scholar] [CrossRef]
- Petzschner, F.H.; Weber, L.A.; Wellstein, K.V.; Paolini, G.; Do, C.T.; Stephan, K.E. Focus of Attention Modulates the Heartbeat Evoked Potential. NeuroImage 2019, 186, 595–606. [Google Scholar] [CrossRef]
- Coll, M.-P.; Hobson, H.; Bird, G.; Murphy, J. Systematic Review and Meta-Analysis of the Relationship between the Heartbeat-Evoked Potential and Interoception. Neurosci. Biobehav. Rev. 2021, 122, 190–200. [Google Scholar] [CrossRef]
- Schandry, R. Heart Beat Perception and Emotional Experience. Psychophysiology 1981, 18, 483–488. [Google Scholar] [CrossRef]
- Whitehead, W.E.; Drescher, V.M.; Heiman, P.; Blackwell, B. Relation of Heart Rate Control to Heartbeat Perception. Biofeedback Self-Regul. 1977, 2, 371–392. [Google Scholar] [CrossRef]
- Murphy, J.; Brewer, R.; Plans, D.; Khalsa, S.S.; Catmur, C.; Bird, G. Testing the Independence of Self-Reported Interoceptive Accuracy and Attention. Q. J. Exp. Psychol. 2020, 73, 115–133. [Google Scholar] [CrossRef]
- Strigo, I.A.; Craig, A.D. Interoception, Homeostatic Emotions and Sympathovagal Balance. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160010. [Google Scholar] [CrossRef]
- Tsakiris, M.; Critchley, H. Interoception beyond Homeostasis: Affect, Cognition and Mental Health. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160002. [Google Scholar] [CrossRef]
- Quigley, K.S.; Kanoski, S.; Grill, W.M.; Barrett, L.F.; Tsakiris, M. Functions of Interoception: From Energy Regulation to Experience of the Self. Trends Neurosci. 2021, 44, 29–38. [Google Scholar] [CrossRef]
- Benarroch, E.E. The Central Autonomic Network: Functional Organization, Dysfunction, and Perspective. Mayo Clin. Proc. 1993, 68, 988–1001. [Google Scholar] [CrossRef]
- Sklerov, M.; Dayan, E.; Browner, N. Functional Neuroimaging of the Central Autonomic Network: Recent Developments and Clinical Implications. Clin. Auton. Res. 2019, 29, 555–566. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. A Model of Neurovisceral Integration in Emotion Regulation and Dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef]
- Battipaglia, I.; Lanza, G.A. The Autonomic Nervous System of the Heart. In Autonomic Innervation of the Heart: Role of Molecular Imaging; Slart, R.H.J.A., Tio, R.A., Elsinga, P.H., Schwaiger, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–12. ISBN 978-3-662-45074-1. [Google Scholar]
- Ellis, R.J.; Thayer, J.F. Music and Autonomic Nervous System (Dys)function. Music. Percept. 2010, 27, 317–326. [Google Scholar] [CrossRef]
- Lin, F.V.; Heffner, K.L. Autonomic Nervous System Flexibility for Understanding Brain Aging. Ageing Res. Rev. 2023, 90, 102016. [Google Scholar] [CrossRef]
- Ernst, G. Heart Rate Variability; Springer: London, UK, 2014. [Google Scholar]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A Healthy Heart Is Not a Metronome: An Integrative Review of the Heart’s Anatomy and Heart Rate Variability. Front. Psychol. 2014, 5, 108292. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T., Jr. Heart Rate Variability: Measurement and Clinical Utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef]
- Vigo, D.E.; Siri, L.N.; Cardinali, D.P. Heart Rate Variability: A Tool to Explore Autonomic Nervous System Activity in Health and Disease. In Psychiatry and Neuroscience Update: From Translational Research to a Humanistic Approach—Volume III; Gargiulo, P.Á., Mesones Arroyo, H.L., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 113–126. ISBN 978-3-319-95360-1. [Google Scholar]
- Lischke, A.; Pahnke, R.; Mau-Moeller, A.; Weippert, M. Heart Rate Variability Modulates Interoceptive Accuracy. Front. Neurosci. 2021, 14, 612445. [Google Scholar] [CrossRef]
- Owens, A.P.; Friston, K.J.; Low, D.A.; Mathias, C.J.; Critchley, H.D. Investigating the Relationship between Cardiac Interoception and Autonomic Cardiac Control Using a Predictive Coding Framework. Auton. Neurosci. 2018, 210, 65–71. [Google Scholar] [CrossRef]
- Wu, Q.; Miao, X.; Cao, Y.; Chi, A.; Xiao, T. Heart Rate Variability Status at Rest in Adult Depressed Patients: A Systematic Review and Meta-Analysis. Front. Public Health 2023, 11, 1243213. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.; Kim, J.-H. Diminished Autonomic Neurocardiac Function in Patients with Generalized Anxiety Disorder. Neuropsychiatr. Dis. Treat. 2016, 12, 3111–3118. [Google Scholar] [CrossRef]
- Schneider, M.; Schwerdtfeger, A. Autonomic Dysfunction in Posttraumatic Stress Disorder Indexed by Heart Rate Variability: A Meta-Analysis. Psychol. Med. 2020, 50, 1937–1948. [Google Scholar] [CrossRef]
- Bourdillon, N.; Jeanneret, F.; Nilchian, M.; Albertoni, P.; Ha, P.; Millet, G.P. Sleep Deprivation Deteriorates Heart Rate Variability and Photoplethysmography. Front. Neurosci. 2021, 15, 642548. [Google Scholar] [CrossRef]
- Acharya, R.U.; Kannathal, N.; Mei Hua, L.; Mei Yi, L. Study of Heart Rate Variability Signals at Sitting and Lying Postures. J. Bodyw. Mov. Ther. 2005, 9, 134–141. [Google Scholar] [CrossRef]
- Soer, R.; Six Dijkstra, M.W.M.C.; Bieleman, H.J.; Oosterveld, F.G.J.; Rijken, N.H.M. Influence of Respiration Frequency on Heart Rate Variability Parameters: A Randomized Cross-Sectional Study. J. Back Musculoskelet. Rehabil. 2021, 34, 1063–1068. [Google Scholar] [CrossRef]
- Molfino, A.; Fiorentini, A.; Tubani, L.; Martuscelli, M.; Fanelli, F.R.; Laviano, A. Body Mass Index Is Related to Autonomic Nervous System Activity as Measured by Heart Rate Variability. Eur. J. Clin. Nutr. 2009, 63, 1263–1265. [Google Scholar] [CrossRef]
- Koenig, J.; Jarczok, M.N.; Kuhn, W.; Morsch, K.; Schäfer, A.; Hillecke, T.K.; Thayer, J.F. Impact of Caffeine on Heart Rate Variability: A Systematic Review. J. Caffeine Res. 2013, 3, 22–37. [Google Scholar] [CrossRef]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Boswell, J.F.; Anderson, L.M.; Oswald, J.M.; Reilly, E.E.; Gorrell, S.; Anderson, D.A. A Preliminary Naturalistic Clinical Case Series Study of the Feasibility and Impact of Interoceptive Exposure for Eating Disorders. Behav. Res. Ther. 2019, 117, 54–64. [Google Scholar] [CrossRef]
- de Lima-Araujo, G.L.; Júnior, G.M.d.S.; Mendes, T.; Demarzo, M.; Farb, N.; de Araujo, D.B.; de Sousa, M.B.C. The Impact of a Brief Mindfulness Training on Interoception: A Randomized Controlled Trial. PLoS ONE 2022, 17, e0273864. [Google Scholar] [CrossRef]
- Heim, N.; Bobou, M.; Tanzer, M.; Jenkinson, P.M.; Steinert, C.; Fotopoulou, A. Psychological Interventions for Interoception in Mental Health Disorders: A Systematic Review of Randomized-Controlled Trials. Psychiatry Clin. Neurosci. 2023, 77, 530–540. [Google Scholar] [CrossRef]
- Frank, D.L.; Khorshid, L.; Kiffer, J.F.; Moravec, C.S.; McKee, M.G. Biofeedback in Medicine: Who, When, Why and How? Ment. Health Fam. Med. 2010, 7, 85–91. [Google Scholar]
- McKee, M.G. Biofeedback: An Overview in the Context of Heart-Brain Medicine. Clevel. Clin. J. Med. 2008, 75, S31. [Google Scholar] [CrossRef]
- Rockstroh, C.; Blum, J.; Göritz, A.S. Virtual Reality in the Application of Heart Rate Variability Biofeedback. Int. J. Hum.-Comput. Stud. 2019, 130, 209–220. [Google Scholar] [CrossRef]
- Chang, W.-L.; Lee, J.-T.; Li, C.-R.; Davis, A.H.T.; Yang, C.-C.; Chen, Y.-J. Effects of Heart Rate Variability Biofeedback in Patients With Acute Ischemic Stroke: A Randomized Controlled Trial. Biol. Res. For. Nurs. 2020, 22, 34–44. [Google Scholar] [CrossRef]
- Lehrer, P.M.; Vaschillo, E.; Vaschillo, B. Resonant Frequency Biofeedback Training to Increase Cardiac Variability: Rationale and Manual for Training. Appl. Psychophysiol. Biofeedback 2000, 15, 177–191. [Google Scholar] [CrossRef]
- Vaschillo, E.; Lehrer, P.; Rishe, N.; Konstantinov, M. Heart Rate Variability Biofeedback as a Method for Assessing Baroreflex Function: A Preliminary Study of Resonance in the Cardiovascular System. Appl. Psychophysiol. Biofeedback 2002, 27, 1–27. [Google Scholar] [CrossRef]
- Berntson, G.G.; Cacioppo, J.T.; Quigley, K.S. Respiratory Sinus Arrhythmia: Autonomic Origins, Physiological Mechanisms, and Psychophysiological Implications. Psychophysiology 1993, 30, 183–196. [Google Scholar] [CrossRef]
- Lehrer, P.M.; Gevirtz, R. Heart Rate Variability Biofeedback: How and Why Does It Work? Front. Psychol. 2014, 5, 104242. [Google Scholar] [CrossRef]
- Lehrer, P. How Does Heart Rate Variability Biofeedback Work? Resonance, the Baroreflex, and Other Mechanisms. Biofeedback 2013, 41, 26–31. [Google Scholar] [CrossRef]
- Lehrer, P.; Kaur, K.; Sharma, A.; Shah, K.; Huseby, R.; Bhavsar, J.; Sgobba, P.; Zhang, Y. Heart Rate Variability Biofeedback Improves Emotional and Physical Health and Performance: A Systematic Review and Meta Analysis. Appl. Psychophysiol. Biofeedback 2020, 45, 109–129. [Google Scholar] [CrossRef]
- Vaschillo, E.G.; Vaschillo, B.; Lehrer, P.M. Characteristics of Resonance in Heart Rate Variability Stimulated by Biofeedback. Appl. Psychophysiol. Biofeedback 2006, 31, 129–142. [Google Scholar] [CrossRef]
- Lehrer, P.M.; Vaschillo, E.; Vaschillo, B.; Lu, S.-E.; Eckberg, D.L.; Edelberg, R.; Shih, W.J.; Lin, Y.; Kuusela, T.A.; Tahvanainen, K.U.O.; et al. Heart Rate Variability Biofeedback Increases Baroreflex Gain and Peak Expiratory Flow. Psychosom. Med. 2003, 65, 796. [Google Scholar] [CrossRef]
- Lehrer, P.M.; Vaschillo, E.G.; Vidali, V. Heart Rate and Breathing Are Not Always in Phase During Resonance Frequency Breathing. Appl. Psychophysiol. Biofeedback 2020, 45, 145–152. [Google Scholar] [CrossRef]
- Grossman, P. Respiratory Sinus Arrhythmia (RSA), Vagal Tone and Biobehavioral Integration: Beyond Parasympathetic Function. Biol. Psychol. 2024, 186, 108739. [Google Scholar] [CrossRef]
- Yuan, H.; Silberstein, S.D. Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part I. Headache J. Head Face Pain 2016, 56, 71–78. [Google Scholar] [CrossRef]
- Garcia, R.G.; Lin, R.L.; Lee, J.; Kim, J.; Barbieri, R.; Sclocco, R.; Wasan, A.D.; Edwards, R.R.; Rosen, B.R.; Hadjikhani, N.; et al. Modulation of Brainstem Activity and Connectivity by Respiratory-Gated Auricular Vagal Afferent Nerve Stimulation (RAVANS) in Migraine Patients. Pain 2017, 158, 1461–1472. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, C.; Falahpour, M.; MacNiven, K.H.; Heit, G.; Sharma, V.; Alataris, K.; Liu, T.T. Effects of Sub-Threshold Transcutaneous Auricular Vagus Nerve Stimulation on Cingulate Cortex and Insula Resting-State Functional Connectivity. Front. Hum. Neurosci. 2022, 16, 862443. [Google Scholar] [CrossRef]
- Poppa, T.; Benschop, L.; Horczak, P.; Vanderhasselt, M.-A.; Carrette, E.; Bechara, A.; Baeken, C.; Vonck, K. Auricular Transcutaneous Vagus Nerve Stimulation Modulates the Heart-Evoked Potential. Brain Stimul. 2022, 15, 260–269. [Google Scholar] [CrossRef]
- Richter, F.; García, A.M.; Rodriguez Arriagada, N.; Yoris, A.; Birba, A.; Huepe, D.; Zimmer, H.; Ibáñez, A.; Sedeño, L. Behavioral and Neurophysiological Signatures of Interoceptive Enhancements Following Vagus Nerve Stimulation. Hum. Brain Mapp. 2021, 42, 1227–1242. [Google Scholar] [CrossRef]
- Ventura-Bort, C.; Weymar, M. Transcutaneous Auricular Vagus Nerve Stimulation Modulates the Processing of Interoceptive Prediction Error Signals and Their Role in Allostatic Regulation. Hum. Brain Mapp. 2024, 45, e26613. [Google Scholar] [CrossRef]
- Huang, C.; Gevirtz, R.N.; Onton, J.; Criado, J.R. Investigation of Vagal Afferent Functioning Using the Heartbeat Event Related Potential. Int. J. Psychophysiol. 2018, 131, 113–123. [Google Scholar] [CrossRef]
- van der Zwan, J.E.; de Vente, W.; Huizink, A.C.; Bögels, S.M.; de Bruin, E.I. Physical Activity, Mindfulness Meditation, or Heart Rate Variability Biofeedback for Stress Reduction: A Randomized Controlled Trial. Appl. Psychophysiol. Biofeedback 2015, 40, 257–268. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Clamor, A.; Koenig, J.; Thayer, J.F.; Lincoln, T.M. A Randomized-Controlled Trial of Heart Rate Variability Biofeedback for Psychotic Symptoms. Behav. Res. Ther. 2016, 87, 207–215. [Google Scholar] [CrossRef]
- Heathers, J.A.J. Everything Hertz: Methodological Issues in Short-Term Frequency-Domain HRV. Front. Physiol. 2014, 5, 177. [Google Scholar] [CrossRef]
- May, R.W.; Seibert, G.S.; Sanchez-Gonzalez, M.A.; Fincham, F.D. Self-Regulatory Biofeedback Training: An Intervention to Reduce School Burnout and Improve Cardiac Functioning in College Students. Stress 2019, 22, 1–8. [Google Scholar] [CrossRef]
- Mehling, W.E.; Acree, M.; Stewart, A.; Silas, J.; Jones, A. The Multidimensional Assessment of Interoceptive Awareness, Version 2 (MAIA-2). PLoS ONE 2018, 13, e0208034. [Google Scholar] [CrossRef]
- Gevirtz, R. The Promise of Heart Rate Variability Biofeedback: Evidence-based Applications. Biofeedback 2013, 41, 110–120. [Google Scholar] [CrossRef]
- Kohl, C.; McIntosh, E.J.; Unger, S.; Haddaway, N.R.; Kecke, S.; Schiemann, J.; Wilhelm, R. Online Tools Supporting the Conduct and Reporting of Systematic Reviews and Systematic Maps: A Case Study on CADIMA and Review of Existing Tools. Environ. Evid. 2018, 7, 8. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The Feasibility of Creating a Checklist for the Assessment of the Methodological Quality Both of Randomised and Non-randomised Studies of Health Care Interventions. J. Epidemil. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Hooper, P.; Jutai, J.W.; Strong, G.; Russell-Minda, E. Age-related Macular Degeneration and Low-vision Rehabilitation: A Systematic Review. Can. J. Opthalmol. 2008, 43, 180–187. [Google Scholar] [CrossRef]
- Lalanza, J.F.; Lorente, S.; Bullich, R.; García, C.; Losilla, J.-M.; Capdevila, L. Methods for Heart Rate Variability Biofeedback (HRVB): A Systematic Review and Guidelines. Appl. Psychophysiol. Biofeedback 2023, 48, 275–297. [Google Scholar] [CrossRef]
- Lehrer, P.; Vaschillo, B.; Zucker, T.; Graves, J.; Katsamanis, M.; Aviles, M.; Wamboldt, F. Protocol for Heart Rate Variability Biofeedback Training. Biofeedback 2013, 41, 98–109. [Google Scholar] [CrossRef]
- Cuijpers, P.; Griffin, J.W.; Furukawa, T.A. The Lack of Statistical Power of Subgroup Analyses in Meta-Analyses: A Cautionary Note. Epidemiol. Psychiatr. Sci. 2021, 30, e78. [Google Scholar] [CrossRef]
- Popay, J.; Roberts, H.; Sowden, A.; Petticrew, M.; Arai, L.; Rodgers, M.; Britten, N. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews; Lancaster University: Lancaster, UK, 2006. [Google Scholar] [CrossRef]
- Kim, H. Heart Rate Variability (Hrv) Biofeedback Training with Young Adult Male Patients in Treatment for Addiction. Ph.D. Thesis, Rutgers University, New Brunswick, NJ, USA, 2014. [Google Scholar]
- Wearne, T.A.; Logan, J.A.; Trimmer, E.M.; Wilson, E.; Filipcikova, M.; Kornfeld, E.; Rushby, J.A.; McDonald, S. Regulating Emotion Following Severe Traumatic Brain Injury: A Randomized Controlled Trial of Heart-Rate Variability Biofeedback Training. Brain Inj. 2021, 35, 1390–1401. [Google Scholar] [CrossRef]
- White, B. The Effects of Heart Rate Variability Biofeedback as an Adjunct to Therapy on Trauma Symptoms. Ph.D. Thesis, Alliant International University, San Diego, CA, USA, 2008. [Google Scholar]
- Limmer, A.; Laser, M.; Schütz, A. Mobile Heart Rate Variability Biofeedback as a Complementary Intervention After Myocardial Infarction: A Randomized Controlled Study. Int. J. Behav. Med. 2022, 29, 230–239. [Google Scholar] [CrossRef]
- Narita, Y.; Shinohara, H.; Kodama, H. Resting Heart Rate Variability and the Effects of Biofeedback Intervention in Women with Low-Risk Pregnancy and Prenatal Childbirth Fear. Appl. Psychophysiol. Biofeedback 2018, 43, 113–121. [Google Scholar] [CrossRef]
- Lin, I.-M.; Fan, S.-Y.; Yen, C.-F.; Yeh, Y.-C.; Tang, T.-C.; Huang, M.-F.; Liu, T.-L.; Wang, P.-W.; Lin, H.-C.; Tsai, H.-Y.; et al. Heart Rate Variability Biofeedback Increased Autonomic Activation and Improved Symptoms of Depression and Insomnia among Patients with Major Depression Disorder. Clin. Psychopharmacol. Neurosci. 2019, 17, 222–232. [Google Scholar] [CrossRef]
- Lin, I.-M.; Fan, S.-Y.; Lu, H.-C.; Lin, T.-H.; Chu, C.-S.; Kuo, H.-F.; Lee, C.-S.; Lu, Y.-H. Randomized Controlled Trial of Heart Rate Variability Biofeedback in Cardiac Autonomic and Hostility among Patients with Coronary Artery Disease. Behav. Res. Ther. 2015, 70, 38–46. [Google Scholar] [CrossRef]
- Caldwell, H.W. Impact of Heart-Rate Variability Biofeedback on Major Depression Disorder in Resting-State fMRI. Ph.D. Thesis, Brigham Young University, Provo, UT, USA, 2015. [Google Scholar]
- Hsieh, H.-F.; Huang, I.-C.; Liu, Y.; Chen, W.-L.; Lee, Y.-W.; Hsu, H.-T. The Effects of Biofeedback Training and Smartphone-Delivered Biofeedback Training on Resilience, Occupational Stress, and Depressive Symptoms among Abused Psychiatric Nurses. Int. J. Environ. Res. Public Health 2020, 17, 2905. [Google Scholar] [CrossRef]
- Eddie, D.; Kim, C.; Lehrer, P.; Deneke, E.; Bates, M.E. A Pilot Study of Brief Heart Rate Variability Biofeedback to Reduce Craving in Young Adult Men Receiving Inpatient Treatment for Substance Use Disorders. Appl. Psychophysiol. Biofeedback 2014, 39, 181–192. [Google Scholar] [CrossRef]
- Siepmann, T.; Ohle, P.; Sedghi, A.; Simon, E.; Arndt, M.; Pallesen, L.-P.; Ritschel, G.; Barlinn, J.; Reichmann, H.; Puetz, V.; et al. Randomized Sham-Controlled Pilot Study of Neurocardiac Function in Patients With Acute Ischaemic Stroke Undergoing Heart Rate Variability Biofeedback. Front. Neurol. 2021, 12, 669843. [Google Scholar] [CrossRef]
- Li, X.; Zhang, T.; Song, L.; Zhang, Y.; Xing, C.; Chen, H. A Control Study on the Effects of HRV Biofeedback Therapy in Patients with Post-Stroke Depression. In Smart Health; Zheng, X., Zeng, D., Chen, H., Zhang, Y., Xing, C., Neill, D.B., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 213–224. [Google Scholar]
- Hallman, D.M.; Olsson, E.M.G.; von Schéele, B.; Melin, L.; Lyskov, E. Effects of Heart Rate Variability Biofeedback in Subjects with Stress-Related Chronic Neck Pain: A Pilot Study. Appl. Psychophysiol. Biofeedback 2011, 36, 71–80. [Google Scholar] [CrossRef]
- Penzlin, A.I.; Siepmann, T.; Illigens, B.M.-W.; Weidner, K.; Siepmann, M. Heart Rate Variability Biofeedback in Patients with Alcohol Dependence: A Randomized Controlled Study. Neuropsychiatr. Dis. Treat. 2015, 11, 2619–2627. [Google Scholar] [CrossRef]
- Nyx, A.E. Investigation of Heart Rate Variability (HRV) Biofeedback to Reduce Substance Cravings in an Outpatient Substance Abuse Treatment Program. Ph.D. Thesis, Alliant International University, San Diego, CA, USA, 2021. [Google Scholar]
- Spray, J.S. Effects of Heart Rate Variability Biofeedback on Patients with Depression and Anxiety in a Community Mental Health Setting. Ph.D. Thesis, Alliant International University, San Diego, CA, USA, 2020. [Google Scholar]
- Meule, A.; Freund, R.; Skirde, A.K.; Vögele, C.; Kübler, A. Heart Rate Variability Biofeedback Reduces Food Cravings in High Food Cravers. Appl. Psychophysiol. Biofeedback 2012, 37, 241–251. [Google Scholar] [CrossRef]
- Krempel, L.; Martin, A. Efficacy of Heart Rate Variability Biofeedback for Somatic Symptom Disorder: A Pilot Randomized Controlled Trial. Psychosom. Med. 2023, 85, 61. [Google Scholar] [CrossRef]
- Herhaus, B.; Siepmann, M.; Kahaly, G.J.; Conrad, R.; Petrowski, K. Effect of a Biofeedback Intervention on Heart Rate Variability in Individuals With Panic Disorder: A Randomized Controlled Trial. Psychosom. Med. 2022, 84, 199. [Google Scholar] [CrossRef]
- Tatschl, J.M.; Hochfellner, S.M.; Schwerdtfeger, A.R. Implementing Mobile HRV Biofeedback as Adjunctive Therapy During Inpatient Psychiatric Rehabilitation Facilitates Recovery of Depressive Symptoms and Enhances Autonomic Functioning Short-Term: A 1-Year Pre–Post-Intervention Follow-Up Pilot Study. Front. Neurosci. 2020, 14, 547394. [Google Scholar] [CrossRef]
- Nolan, R.P.; Floras, J.S.; Harvey, P.J.; Kamath, M.V.; Picton, P.E.; Chessex, C.; Hiscock, N.; Powell, J.; Catt, M.; Hendrickx, H.; et al. Behavioral Neurocardiac Training in Hypertension. Hypertension 2010, 55, 1033–1039. [Google Scholar] [CrossRef]
- Bian, Y.; Liu, F.; Wang, Y.; Wang, X.; Chen, R. Effects of Heart Rate Variability (HRV) Biofeedback for Women Undergoing First-Time In Vitro Fertilization and Embryo Transfer. Altern. Ther. Health Med. 2023, 29, 162–167. [Google Scholar]
- Han, J.; Park, J.; Kang, H.; Lee, H.; Kim, N. The Effect of a Biofeedback-Based Integrated Program on Improving Orthostatic Hypotension in Community-Dwelling Older Adults: A Pilot Study. J. Cardiovasc. Nurs. 2023; advance online publication. [Google Scholar] [CrossRef]
- Solarikova, P.; Blahunkova, S.; Rajcani, J.; Turoňová, D.; Brezina, I. HRV Biofeedback and Controlled Slow Breathing May Have Limited Effectiveness as Stress Reduction Methods in Healthy Subjects. Act. Nerv. Super. Rediviva 2021, 63, 111–117. [Google Scholar]
- Hasuo, H.; Kanbara, K.; Sakuma, H.; Yoshida, K.; Uchitani, K.; Fukunaga, M. Self-Care System for Family Caregivers of Cancer Patients Using Resonant Breathing with a Portable Home Device: A Randomized Open-Label Study. J. Palliat. Med. 2019, 22, 18–24. [Google Scholar] [CrossRef]
- Lin, I.-M. Effects of a Cardiorespiratory Synchronization Training Mobile Application on Heart Rate Variability and Electroencephalography in Healthy Adults. Int. J. Psychophysiol. 2018, 134, 168–177. [Google Scholar] [CrossRef]
- Whited, A.; Larkin, K.T.; Whited, M. Effectiveness of emWave Biofeedback in Improving Heart Rate Variability Reactivity to and Recovery from Stress. Appl. Psychophysiol. Biofeedback 2014, 39, 75–88. [Google Scholar] [CrossRef]
- Solarikova, P.; Blahunkova, S.; Rajcani, J.; Turonova, D.; Brezina, I. The Effect of HRV Biofeedback, Yoga and Mindfulness Training on Autonomic Nervous System, Perceived Stress, and Dispositional Mindfulness. Act. Nerv. Super. Rediviva 2021, 63, 53–62. [Google Scholar]
- de Souza, P.M.; de Cássia Souza, M.; Diniz, L.A.; Araújo, C.R.V.; Lopez, M.; Volchan, E.; Fernandes, O.; Sanchez, T.A.; Souza, G.G.L. Long-Term Benefits of Heart Rate Variability Biofeedback Training in Older Adults with Different Levels of Social Interaction: A Pilot Study. Sci. Rep. 2022, 12, 18795. [Google Scholar] [CrossRef]
- Prinsloo, G.E.; Derman, W.E.; Lambert, M.I.; Laurie Rauch, H.G. The Effect of a Single Session of Short Duration Biofeedback-Induced Deep Breathing on Measures of Heart Rate Variability During Laboratory-Induced Cognitive Stress: A Pilot Study. Appl. Psychophysiol. Biofeedback 2013, 38, 81–90. [Google Scholar] [CrossRef]
- McCraty, R.; Atkinson, M.; Lipsenthal, L.; Arguelles, L. New Hope for Correctional Officers: An Innovative Program for Reducing Stress and Health Risks. Appl. Psychophysiol. Biofeedback 2009, 34, 251–272. [Google Scholar] [CrossRef]
- Kerr, J.I.; Weibel, R.P.; Naegelin, M.; Ferrario, A.; Schinazi, V.R.; La Marca, R.; Hoelscher, C.; Nater, U.M.; von Wangenheim, F. The Effectiveness and User Experience of a Biofeedback Intervention Program for Stress Management Supported by Virtual Reality and Mobile Technology: A Randomized Controlled Study. BMC Digit. Health 2023, 1, 42. [Google Scholar] [CrossRef]
- Park, S.M.; Jung, H.Y. Respiratory Sinus Arrhythmia Biofeedback Alters Heart Rate Variability and Default Mode Network Connectivity in Major Depressive Disorder: A Preliminary Study. Int. J. Psychophysiol. 2020, 158, 225–237. [Google Scholar] [CrossRef]
- Ratajczak, E.; Hajnowski, M.; Stawicki, M.; Duch, W. Novel Methodological Tools for Behavioral Interventions: The Case of HRV-Biofeedback. Sham Control and Quantitative Physiology-Based Assessment of Training Quality and Fidelity. Sensors 2021, 21, 3670. [Google Scholar] [CrossRef]
- Lin, G.; Xiang, Q.; Fu, X.; Wang, S.; Wang, S.; Chen, S.; Shao, L.; Zhao, Y.; Wang, T. Heart Rate Variability Biofeedback Decreases Blood Pressure in Prehypertensive Subjects by Improving Autonomic Function and Baroreflex. J. Altern. Complement. Med. 2012, 18, 143–152. [Google Scholar] [CrossRef]
- Paul, M.; Garg, K. The Effect of Heart Rate Variability Biofeedback on Performance Psychology of Basketball Players. Appl. Psychophysiol. Biofeedback 2012, 37, 131–144. [Google Scholar] [CrossRef]
- Kudo, N.; Shinohara, H.; Kodama, H. Heart Rate Variability Biofeedback Intervention for Reduction of Psychological Stress During the Early Postpartum Period. Appl. Psychophysiol. Biofeedback 2014, 39, 203–211. [Google Scholar] [CrossRef]
- Göçmen, R.; Aktop, A.; Pınar, Y.; Toktaş, N.; Kristýna Jandačková, V. The Effect of Heart Rate Variability Biofeedback on Basketball Performance Tests. Appl. Psychophysiol. Biofeedback 2023, 48, 461–470. [Google Scholar] [CrossRef]
- Lin, I.-M.; Wang, S.-Y.; Fan, S.-Y.; Peper, E.; Chen, S.-P.; Huang, C.-Y. A Single Session of Heart Rate Variability Biofeedback Produced Greater Increases in Heart Rate Variability Than Autogenic Training. Appl. Psychophysiol. Biofeedback 2020, 45, 343–350. [Google Scholar] [CrossRef]
- Caldwell, Y.T.; Steffen, P.R. Adding HRV Biofeedback to Psychotherapy Increases Heart Rate Variability and Improves the Treatment of Major Depressive Disorder. Int. J. Psychophysiol. 2018, 131, 96–101. [Google Scholar] [CrossRef]
- Nashiro, K.; Min, J.; Yoo, H.J.; Cho, C.; Bachman, S.L.; Dutt, S.; Thayer, J.F.; Lehrer, P.M.; Feng, T.; Mercer, N.; et al. Increasing Coordination and Responsivity of Emotion-Related Brain Regions with a Heart Rate Variability Biofeedback Randomized Trial. Cogn. Affect. Behav. Neurosci. 2023, 23, 66–83. [Google Scholar] [CrossRef]
- Dziembowska, I.; Izdebski, P.; Rasmus, A.; Brudny, J.; Grzelczak, M.; Cysewski, P. Effects of Heart Rate Variability Biofeedback on EEG Alpha Asymmetry and Anxiety Symptoms in Male Athletes: A Pilot Study. Appl. Psychophysiol. Biofeedback 2016, 41, 141–150. [Google Scholar] [CrossRef]
- Yu, L.-C.; Lin, I.-M.; Fan, S.-Y.; Chien, C.-L.; Lin, T.-H. One-Year Cardiovascular Prognosis of the Randomized, Controlled, Short-Term Heart Rate Variability Biofeedback Among Patients with Coronary Artery Disease. Int. J. Behav. Med. 2018, 25, 271–282. [Google Scholar] [CrossRef]
- Reyes del Paso, G.A.; Langewitz, W.; Mulder, L.J.M.; van Roon, A.; Duschek, S. The Utility of Low Frequency Heart Rate Variability as an Index of Sympathetic Cardiac Tone: A Review with Emphasis on a Reanalysis of Previous Studies. Psychophysiology 2013, 50, 477–487. [Google Scholar] [CrossRef]
- Rahman, F.; Pechnik, S.; Gross, D.; Sewell, L.; Goldstein, D.S. Low Frequency Power of Heart Rate Variability Reflects Baroreflex Function, Not Cardiac Sympathetic Innervation. Clin. Auton. Res. 2011, 21, 133–141. [Google Scholar] [CrossRef]
- Lewis, G.F.; Hourani, L.; Tueller, S.; Kizakevich, P.; Bryant, S.; Weimer, B.; Strange, L. Relaxation Training Assisted by Heart Rate Variability Biofeedback: Implication for a Military Predeployment Stress Inoculation Protocol. Psychophysiology 2015, 52, 1167–1174. [Google Scholar] [CrossRef]
- Berntson, G.G.; Lozano, D.L.; Chen, Y.-J. Filter Properties of Root Mean Square Successive Difference (RMSSD) for Heart Rate. Psychophysiology 2005, 42, 246–252. [Google Scholar] [CrossRef]
- Thomas, B.L.; Claassen, N.; Becker, P.; Viljoen, M. Validity of Commonly Used Heart Rate Variability Markers of Autonomic Nervous System Function. Neuropsychobiology 2019, 78, 14–26. [Google Scholar] [CrossRef]
- Siepmann, M.; Hennig, U.-D.; Siepmann, T.; Nitzsche, K.; Mück-Weymann, M.; Petrowski, K.; Weidner, K. The Effects of Heart Rate Variability Biofeedback in Patients with Preterm Labour. Appl. Psychophysiol. Biofeedback 2014, 39, 27–35. [Google Scholar] [CrossRef]
- Raaijmakers, S.F.; Steel, F.W.; de Goede, M.; van Wouwe, N.C.; van Erp, J.B.F.; Brouwer, A.-M. Heart Rate Variability and Skin Conductance Biofeedback: A Triple-Blind Randomized Controlled Study. In Proceedings of the 2013 Humaine Association Conference on Affective Computing and Intelligent Interaction, Geneva, Switzerland, 2–5 September 2013; pp. 289–293. [Google Scholar]
- Brinkmann, A.E.; Press, S.A.; Helmert, E.; Hautzinger, M.; Khazan, I.; Vagedes, J. Comparing Effectiveness of HRV-Biofeedback and Mindfulness for Workplace Stress Reduction: A Randomized Controlled Trial. Appl. Psychophysiol. Biofeedback 2020, 45, 307–322. [Google Scholar] [CrossRef]
- Barnable, T. Effects of a Biofeedback Intervention on Student Test Anxiety and Performance. Ph.D. Thesis, University of South Dakota, Vermillion, SD, USA, 2020. [Google Scholar]
- Schumann, A.; de la Cruz, F.; Köhler, S.; Brotte, L.; Bär, K.-J. The Influence of Heart Rate Variability Biofeedback on Cardiac Regulation and Functional Brain Connectivity. Front. Neurosci. 2021, 15, 691988. [Google Scholar] [CrossRef]
- Vagedes, J.; Fazeli, A.; Boening, A.; Helmert, E.; Berger, B.; Martin, D. Efficacy of Rhythmical Massage in Comparison to Heart Rate Variability Biofeedback in Patients with Dysmenorrhea—A Randomized, Controlled Trial. Complement. Ther. Med. 2019, 42, 438–444. [Google Scholar] [CrossRef]
- Schuman, D.L.; Lawrence, K.A.; Boggero, I.; Naegele, P.; Ginsberg, J.P.; Casto, A.; Moser, D.K. A Pilot Study of a Three-Session Heart Rate Variability Biofeedback Intervention for Veterans with Posttraumatic Stress Disorder. Appl. Psychophysiol. Biofeedback 2022, 48, 51–65. [Google Scholar] [CrossRef]
- Del Pozo, J.M.; Gevirtz, R.N.; Scher, B.; Guarneri, E. Biofeedback Treatment Increases Heart Rate Variability in Patients with Known Coronary Artery Disease. Am. Heart J. 2004, 147, 545. [Google Scholar] [CrossRef]
- Vagedes, J.; Mueller, V.; Ranger, A.; Helmert, E.; Schmidt, N.; Schwarze, K.; Kohl, M.; Gevirtz, R.; Andrasik, F. Heart Rate Variability Biofeedback in the Workplace A Randomized Trial. Appl. Psychophysiol. Biofeedback 2016, 41, 450. [Google Scholar]
- Schumann, A.; Köhler, S.; Brotte, L.; Bär, K.-J. Effect of an Eight-Week Smartphone-Guided HRV-Biofeedback Intervention on Autonomic Function and Impulsivity in Healthy Controls. Physiol. Meas. 2019, 40, 064001. [Google Scholar] [CrossRef]
- Siepmann, T.; Wendt, K.; Petrowski, K.; Weidner, K.; Siepmann, M. The Effects of Heart Rate Variability Biofeedback in Unemployed Subjects. Auton. Neurosci. Basic. Clin. 2013, 177, 44–45. [Google Scholar] [CrossRef]
- Prabhu, G.V.; Stanley, L.; Morgan, R.; Shirley, B. Designing and Developing a Nature-Based Virtual Reality with Heart Rate Variability Biofeedback for Surgical Anxiety and Pain Management: Evidence from Total Knee Arthroplasty Patients. Aging Ment. Health 2023, 28, 738–753. [Google Scholar] [CrossRef]
- Tinello, D.; Tarvainen, M.; Zuber, S.; Kliegel, M. Enhancing Inhibitory Control in Older Adults: A Biofeedback Study. Brain Sci. 2023, 13, 335. [Google Scholar] [CrossRef]
- Zucker, T.L.; Samuelson, K.W.; Muench, F.; Greenberg, M.A.; Gevirtz, R.N. The Effects of Respiratory Sinus Arrhythmia Biofeedback on Heart Rate Variability and Posttraumatic Stress Disorder Symptoms: A Pilot Study. Appl. Psychophysiol. Biofeedback 2009, 34, 135–143. [Google Scholar] [CrossRef]
- Schumann, A.; Helbing, N.; Rieger, K.; Suttkus, S.; Bär, K.-J. Depressive Rumination and Heart Rate Variability: A Pilot Study on the Effect of Biofeedback on Rumination and Its Physiological Concomitants. Front. Psychiatry 2022, 13, 961294. [Google Scholar] [CrossRef]
- Climov, D.; Lysy, C.; Berteau, S.; Dutrannois, J.; Dereppe, H.; Brohet, C.; Melin, J. Biofeedback on Heart Rate Variability in Cardiac Rehabilitation: Practical Feasibility and Psycho-Physiological Eff Ects. Acta Cardiol. 2014, 69, 299–307. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gevirtz, R.N.; Brown, M.; Spira, J.; Guarneri, E.; Stoletniy, L. The Effect of Biofeedback on Function in Patients with Heart Failure. Appl. Psychophysiol. Biofeedback 2009, 34, 71–91. [Google Scholar] [CrossRef]
- McCraty, R. New Frontiers in Heart Rate Variability and Social Coherence Research: Techniques, Technologies, and Implications for Improving Group Dynamics and Outcomes. Front. Public Health 2017, 5, 290543. [Google Scholar] [CrossRef]
- McCraty, R.; Zayas, M.A. Cardiac Coherence, Self-Regulation, Autonomic Stability, and Psychosocial Well-Being. Front. Psychol. 2014, 5, 104218. [Google Scholar] [CrossRef]
- Mason, E.B.; Burkhart, K.; Lazebnik, R. Adolescent Stress Management in a Primary Care Clinic. J. Pediatr. Health Care 2019, 33, 178–185. [Google Scholar] [CrossRef]
- Amjadian, M.; Bahrami Ehsan, H.; Saboni, K.; Vahedi, S.; Rostami, R.; Roshani, D. A Pilot Randomized Controlled Trial to Assess the Effect of Islamic Spiritual Intervention and of Breathing Technique with Heart Rate Variability Feedback on Anxiety, Depression and Psycho-Physiologic Coherence in Patients after Coronary Artery Bypass Surgery. Ann. Gen. Psychiatry 2020, 19, 46. [Google Scholar] [CrossRef]
- Berry, M.E.; Chapple, I.T.; Ginsberg, J.P.; Gleichauf, K.J.; Meyer, J.A.; Nagpal, M.L. Non-Pharmacological Intervention for Chronic Pain in Veterans: A Pilot Study of Heart Rate Variability Biofeedback. Glob. Adv. Health Med. 2014, 3, 28–33. [Google Scholar] [CrossRef]
- La Rovere, M.T.; Pinna, G.D.; Raczak, G. Baroreflex Sensitivity: Measurement and Clinical Implications. Ann. Noninvasive Electrocardiol. 2008, 13, 191–207. [Google Scholar] [CrossRef]
- Swenne, C.A. Baroreflex Sensitivity: Mechanisms and Measurement. Neth. Heart J. 2013, 21, 58–60. [Google Scholar] [CrossRef]
- Lehrer, P.; Vaschillo, E. Resonant frequency heart rate variability biofeedback: Effects on cardiovascular and baroreflex function. Biol. Psychol. 2001, 56, 75–76. [Google Scholar]
- Tonhajzerova, I.; Mokra, D.; Visnovcova, Z. Vagal Function Indexed by Respiratory Sinus Arrhythmia and Cholinergic Anti-Inflammatory Pathway. Respir. Physiol. Neurobiol. 2013, 187, 78–81. [Google Scholar] [CrossRef]
- Clemson, P.T.; Hoag, J.B.; Cooke, W.H.; Eckberg, D.L.; Stefanovska, A. Beyond the Baroreflex: A New Measure of Autonomic Regulation Based on the Time-Frequency Assessment of Variability, Phase Coherence and Couplings. Front. Netw. Physiol. 2022, 2, 891604. [Google Scholar] [CrossRef]
- Berntson, G.G.; Thomas Bigger, J., Jr.; Eckberg, D.L.; Grossman, P.; Kaufmann, P.G.; Malik, M.; Nagaraja, H.N.; Porges, S.W.; Saul, J.P.; Stone, P.H.; et al. Heart Rate Variability: Origins, Methods, and Interpretive Caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef]
- Eckberg, D.L. Human Sinus Arrhythmia as an Index of Vagal Cardiac Outflow. J. Appl. Physiol. 1983, 54, 961–966. [Google Scholar] [CrossRef]
- Porges, S.W.; Bohrer, R.E. The Analysis of Periodic Processes in Psychophysiological Research. In Principles of Psychophysiology: Physical, Social, and Inferential Elements; Cambridge University Press: New York, NY, USA, 1990; pp. 708–753. ISBN 978-0-521-34432-6. [Google Scholar]
- Patron, E.; Messerotti Benvenuti, S.; Favretto, G.; Valfrè, C.; Bonfà, C.; Gasparotto, R.; Palomba, D. Biofeedback Assisted Control of Respiratory Sinus Arrhythmia as a Biobehavioral Intervention for Depressive Symptoms in Patients After Cardiac Surgery: A Preliminary Study. Appl. Psychophysiol. Biofeedback 2013, 38, 1–9. [Google Scholar] [CrossRef]
- Munafò, M.; Patron, E.; Palomba, D. Improving Managers’ Psychophysical Well-Being: Effectiveness of Respiratory Sinus Arrhythmia Biofeedback. Appl. Psychophysiol. Biofeedback 2016, 41, 129–139. [Google Scholar] [CrossRef]
- Hjelland, I.E.; Svebak, S.; Berstad, A.; Flatabø, G.; Hausken, T. Breathing Exercises with Vagal Biofeedback May Benefit Patients with Functional Dyspepsia. Scand. J. Gastroenterol. 2007, 42, 1054–1062. [Google Scholar] [CrossRef]
- Kotozaki, Y.; Takeuchi, H.; Sekiguchi, A.; Yamamoto, Y.; Shinada, T.; Araki, T.; Takahashi, K.; Taki, Y.; Ogino, T.; Kiguchi, M.; et al. Biofeedback-Based Training for Stress Management in Daily Hassles: An Intervention Study. Brain Behav. 2014, 4, 566–579. [Google Scholar] [CrossRef]
- Laird, A.R.; Fox, P.M.; Eickhoff, S.B.; Turner, J.A.; Ray, K.L.; McKay, D.R.; Glahn, D.C.; Beckmann, C.F.; Smith, S.M.; Fox, P.T. Behavioral Interpretations of Intrinsic Connectivity Networks. J. Cogn. Neurosci. 2011, 23, 4022–4037. [Google Scholar] [CrossRef]
- zu Eulenburg, P.; Baumgärtner, U.; Treede, R.-D.; Dieterich, M. Interoceptive and Multimodal Functions of the Operculo-Insular Cortex: Tactile, Nociceptive and Vestibular Representations. NeuroImage 2013, 83, 75–86. [Google Scholar] [CrossRef]
- Desmedt, O.; Corneille, O.; Luminet, O.; Murphy, J.; Bird, G.; Maurage, P. Contribution of Time Estimation and Knowledge to Heartbeat Counting Task Performance under Original and Adapted Instructions. Biol. Psychol. 2020, 154, 107904. [Google Scholar] [CrossRef]
- Ferentzi, E.; Wilhelm, O.; Köteles, F. What Counts When Heartbeats Are Counted. Trends Cogn. Sci. 2022, 26, 832–835. [Google Scholar] [CrossRef]
- Leganes-Fonteneau, M.; Bates, M.E.; Muzumdar, N.; Pawlak, A.; Islam, S.; Vaschillo, E.; Buckman, J.F. Cardiovascular Mechanisms of Interoceptive Awareness: Effects of Resonance Breathing. Int. J. Psychophysiol. 2021, 169, 71–87. [Google Scholar] [CrossRef]
- Schuette, S.A.; Zucker, N.L.; Smoski, M.J. Do Interoceptive Accuracy and Interoceptive Sensibility Predict Emotion Regulation? Psychol. Res. 2021, 85, 1894–1908. [Google Scholar] [CrossRef]
- Critchley, H.; Ewing, D.L.; Gould van Praag, C.; Habash-Bailey, H.; Eccles, J.; Meeten, F.; Garfinkel, S.N. Transdiagnostic Expression of Interoceptive Abnormalities in Psychiatric Conditions. MedRxiv 2019, 19012393. [Google Scholar] [CrossRef]
- Desdentado, L.; Miragall, M.; Llorens, R.; Baños, R.M. Disentangling the Role of Interoceptive Sensibility in Alexithymia, Emotion Dysregulation, and Depression in Healthy Individuals. Curr. Psychol. 2022, 42, 20570–20582. [Google Scholar] [CrossRef]
- Heiss, S.; Vaschillo, B.; Vaschillo, E.G.; Timko, C.A.; Hormes, J.M. Heart Rate Variability as a Biobehavioral Marker of Diverse Psychopathologies: A Review and Argument for an “Ideal Range”. Neurosci. Biobehav. Rev. 2021, 121, 144–155. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 238557. [Google Scholar] [CrossRef]
- Hill, L.K.; Siebenbrock, A.; Sollers, J.J. All Are Measures Created Equal? Heart Rate Variability and Respiration. Biomed. Sci. Instrum. 2009, 45, 71–76. [Google Scholar]
- Zhang, M.; Peng, Y. Anterior Insula and Dorsal Anterior Cingulate Cortex as a Hub of Self-Regulation: Combining Activation Likelihood Estimation Meta-Analysis and Meta-Analytic Connectivity Modeling Analysis. Brain Struct. Funct. 2023, 228, 1329–1345. [Google Scholar] [CrossRef]
- Smith, R.; Thayer, J.F.; Khalsa, S.S.; Lane, R.D. The Hierarchical Basis of Neurovisceral Integration. Neurosci. Biobehav. Rev. 2017, 75, 274–296. [Google Scholar] [CrossRef]
- Sliz, D.; Hayley, S. Major Depressive Disorder and Alterations in Insular Cortical Activity: A Review of Current Functional Magnetic Imaging Research. Front. Hum. Neurosci. 2012, 6, 323. [Google Scholar] [CrossRef]
- Li, W.; Chen, Z.; Wu, M.; Zhu, H.; Gu, L.; Zhao, Y.; Kuang, W.; Bi, F.; Kemp, G.J.; Gong, Q. Characterization of Brain Blood Flow and the Amplitude of Low-Frequency Fluctuations in Major Depressive Disorder: A Multimodal Meta-Analysis. J. Affect. Disord. 2017, 210, 303–311. [Google Scholar] [CrossRef]
- Wolf, V.; Kühnel, A.; Teckentrup, V.; Koenig, J.; Kroemer, N.B. Does Transcutaneous Auricular Vagus Nerve Stimulation Affect Vagally Mediated Heart Rate Variability? A Living and Interactive Bayesian Meta-Analysis. Psychophysiology 2021, 58, e13933. [Google Scholar] [CrossRef]
- Soltani, D.; Azizi, B.; Sima, S.; Tavakoli, K.; Hosseini Mohammadi, N.S.; Vahabie, A.-H.; Akbarzadeh-Sherbaf, K.; Vasheghani-Farahani, A. A Systematic Review of the Effects of Transcutaneous Auricular Vagus Nerve Stimulation on Baroreflex Sensitivity and Heart Rate Variability in Healthy Subjects. Clin. Auton. Res. 2023, 33, 165–189. [Google Scholar] [CrossRef]
- De Couck, M.; Cserjesi, R.; Caers, R.; Zijlstra, W.P.; Widjaja, D.; Wolf, N.; Luminet, O.; Ellrich, J.; Gidron, Y. Effects of Short and Prolonged Transcutaneous Vagus Nerve Stimulation on Heart Rate Variability in Healthy Subjects. Auton. Neurosci. 2017, 203, 88–96. [Google Scholar] [CrossRef]
- Friston, K.; Kiebel, S. Predictive Coding under the Free-Energy Principle. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1211–1221. [Google Scholar] [CrossRef]
- Seth, A.K. Interoceptive Inference, Emotion, and the Embodied Self. Trends Cogn. Sci. 2013, 17, 565–573. [Google Scholar] [CrossRef]
- Friston, K.; Mattout, J.; Kilner, J. Action Understanding and Active Inference. Biol. Cybern. 2011, 104, 137–160. [Google Scholar] [CrossRef]
- Limanowski, J.; Blankenburg, F. Minimal Self-Models and the Free Energy Principle. Front. Hum. Neurosci. 2013, 7, 547. [Google Scholar] [CrossRef]
- Friston, K. The Free-Energy Principle: A Unified Brain Theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef]
- Giotakos, O. Emotional Intentionality and Predictive Processing. Dialogues Clin. Neurosci. Ment. Health 2021, 4, 5–17. [Google Scholar] [CrossRef]
- Owens, A.P.; Allen, M.; Ondobaka, S.; Friston, K.J. Interoceptive Inference: From Computational Neuroscience to Clinic. Neurosci. Biobehav. Rev. 2018, 90, 174–183. [Google Scholar] [CrossRef]
- Friston, K.; FitzGerald, T.; Rigoli, F.; Schwartenbeck, P.; O’Doherty, J.; Pezzulo, G. Active Inference and Learning. Neurosci. Biobehav. Rev. 2016, 68, 862–879. [Google Scholar] [CrossRef]
- Tumati, S.; Paulus, M.P.; Northoff, G. Out-of-Step: Brain-Heart Desynchronization in Anxiety Disorders. Mol. Psychiatry 2021, 26, 1726–1737. [Google Scholar] [CrossRef]
- Sevoz-Couche, C.; Laborde, S. Heart Rate Variability and Slow-Paced Breathing:When Coherence Meets Resonance. Neurosci. Biobehav. Rev. 2022, 135, 104576. [Google Scholar] [CrossRef]
- Martin, L.R.; Williams, S.L.; Haskard, K.B.; DiMatteo, M.R. The Challenge of Patient Adherence. Ther. Clin. Risk Manag. 2005, 1, 189–199. [Google Scholar]
- Sabaté, E. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003; ISBN 978-92-4-154599-0. [Google Scholar]
- Mehling, W.E.; Gopisetty, V.; Daubenmier, J.; Price, C.J.; Hecht, F.M.; Stewart, A. Body Awareness: Construct and Self-Report Measures. PLoS ONE 2009, 4, e5614. [Google Scholar] [CrossRef]
- Raimo, S.; Ferrazzano, G.; Vita, A.D.; Gaita, M.; Satriano, F.; Veneziano, M.; Torchia, V.; Zerella, M.P.; Malimpensa, L.; Signoriello, E.; et al. The Multidimensional Assessment of Body Representation and Interoception in Multiple Sclerosis. Mult. Scler. Relat. Disord. 2024, 87, 105692. [Google Scholar] [CrossRef]
- Parma, C.; Doria, F.; Zulueta, A.; Lanzone, J.; Boscarino, M.; Giani, L.; Lunetta, C.; Vassallo, M.; Parati, E.A.; Picozzi, M.; et al. An Overview of the Bodily Awareness Representation and Interoception: Insights and Progress in the Field of Neurorehabilitation Research. Brain Sci. 2024, 14, 386. [Google Scholar] [CrossRef]
- Uraguchi, M.; Maulina, V.V.R.; Ohira, H. Interoceptive Accuracy Correlates with Precision of Time Perception in the Millisecond Range. Front. Neurosci. 2022, 16, 993491. [Google Scholar] [CrossRef]
- Di Lernia, D.; Serino, S.; Pezzulo, G.; Pedroli, E.; Cipresso, P.; Riva, G. Feel the Time. Time Perception as a Function of Interoceptive Processing. Front. Hum. Neurosci. 2018, 12, 74. [Google Scholar] [CrossRef]
- Alvares, G.A.; Quintana, D.S.; Hickie, I.B.; Guastella, A.J. Autonomic Nervous System Dysfunction in Psychiatric Disorders and the Impact of Psychotropic Medications: A Systematic Review and Meta-Analysis. J. Psychiatry Neurosci. 2016, 41, 89–104. [Google Scholar] [CrossRef]
- Niemelä, M.J.; Airaksinen, K.E.J.; Huikuri, H.V. Effect of Beta-Blockade on Heart Rate Variability in Patients with Coronary Artery Disease. J. Am. Coll. Cardiol. 1994, 23, 1370–1377. [Google Scholar] [CrossRef]
- Wheat, A.L.; Larkin, K.T. Biofeedback of Heart Rate Variability and Related Physiology: A Critical Review. Appl. Psychophysiol. Biofeedback 2010, 35, 229–242. [Google Scholar] [CrossRef]
- Valenza, G.; Sclocco, R.; Duggento, A.; Passamonti, L.; Napadow, V.; Barbieri, R.; Toschi, N. The Central Autonomic Network At Rest: Uncovering Functional MRI Correlates Of Time-Varying Autonomic Outflow. NeuroImage. [CrossRef]
- Pagani, M.; Lombardi, F.; Guzzetti, S.; Sandrone, G.; Rimoldi, O.; Malfatto, G.; Cerutti, S.; Malliani, A. Power Spectral Density of Heart Rate Variability as an Index of Sympatho-vagal Interaction in Normal and Hypertensive Subjects. J. Hypertens. 1984, 2, S383–S385. [Google Scholar]
- Montano, N.; Porta, A.; Cogliati, C.; Costantino, G.; Tobaldini, E.; Casali, K.R.; Iellamo, F. Heart Rate Variability Explored in the Frequency Domain: A Tool to Investigate the Link Between Heart and Behavior. Neurosci. Biobehav. Rev. 2009, 33, 71–80. [Google Scholar] [CrossRef]
- Billman, G.E. The LF/HF Ratio Does Not Accurately Measure Cardiac Sympatho-Vagal Balance. Front. Physiol. 2013, 4, 45733. [Google Scholar] [CrossRef]
- Pham, T.; Lau, Z.J.; Chen, S.H.A.; Makowski, D. Heart Rate Variability in Psychology: A Review of HRV Indices and an Analysis Tutorial. Brain Sci. 2021, 21, 3998. [Google Scholar] [CrossRef]
- Bouzida, N.; Bendada, A.; Maldague, X. Visualization of Body Thermoregulation by Infrared Imaging. J. Therm. Biol. 2009, 34, 120–126. [Google Scholar] [CrossRef]
- Machado, C.; Estevez, M.; Perez-Nellar, J.; Schiavi, A. Residual Vasomotor Activity Assessed by Heart Rate Variability in a Brain-dead Case. BMJ Case Rep. 2015, bcr2014205677. [Google Scholar] [CrossRef]
- Serrador, J.M.; Finlayson, H.C.; Hughson, R.L. Physical Activity is a Major Contributor to the Ultra Low Frequency Components of Heart Rate Variability. Heart 1999, 82, e9. [Google Scholar] [CrossRef]
- Critchley, H.D.; Garfinkel, S.N. Interactions Between Visceral Afferent Signaling and Stimulus Processing. Front. Neurosci. 2015, 9, 153864. [Google Scholar] [CrossRef]
- Billman, G.E. Heart Rate Variability—A Historical Perspective. Front. Physiol. 2011, 2, 86. [Google Scholar] [CrossRef]
- Noteboom, J.T.; Fleshner, M.; Enoka, R.M. Activation of the Arousal Response Can Impair Performance on a Simple Motor Task. J. Appl. Physiol. 2001, 91, 821–831. [Google Scholar] [CrossRef]
- Mayet, J.; Hughes, A. Cardiac and Vascular Pathophysiology in Hypertension. Heart 2003, 89, 1104–1109. [Google Scholar] [CrossRef]
- Wehrwein, E.A.; Joyner, M.J.; Hart, E.C.J.; Wallin, G.; Karlsson, T.; Charkoudian, N. Calculation of an “Error Signal” in Control of Sympathetic Nerve Activity. Hypertension 2010, 55, 264–269. [Google Scholar] [CrossRef]
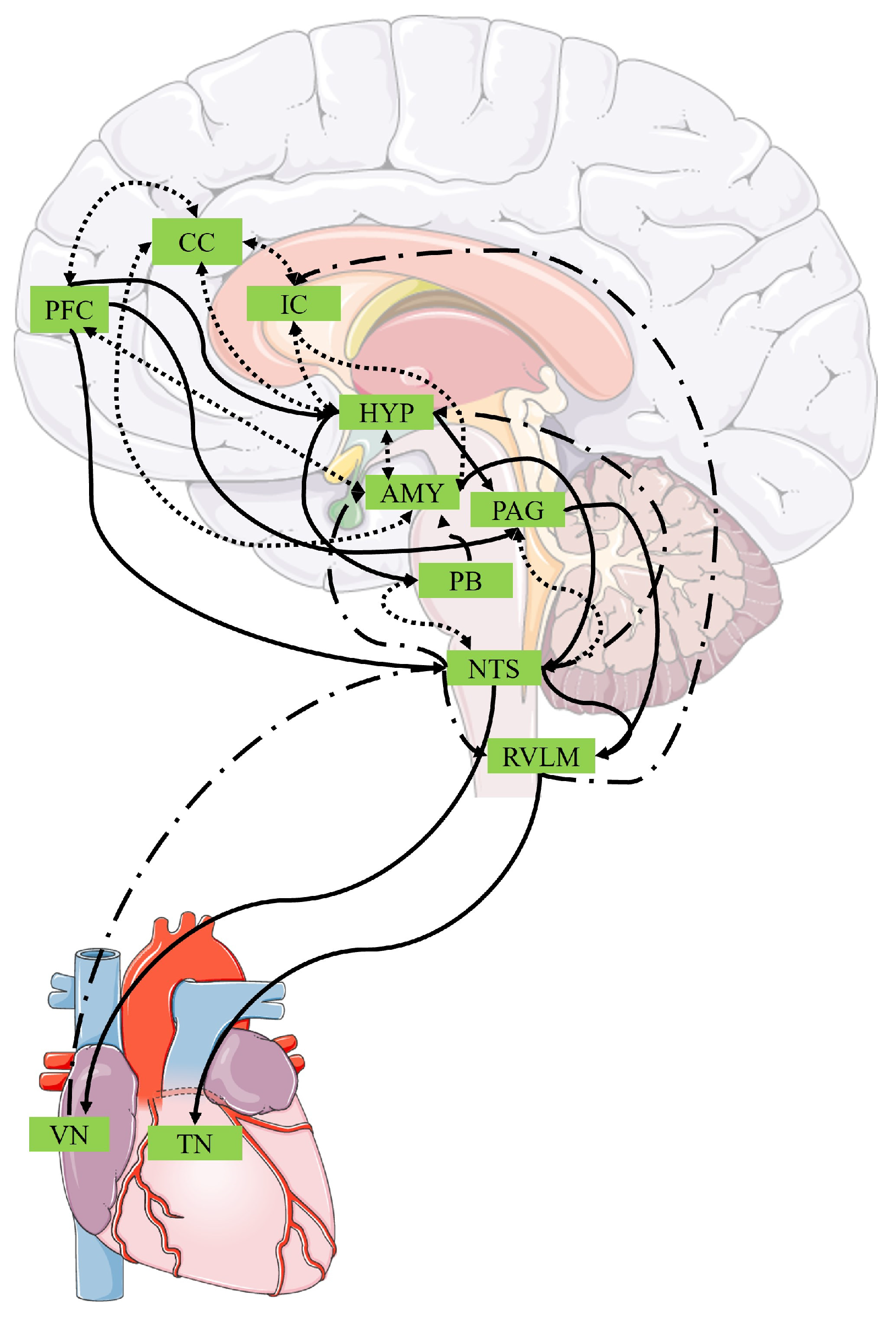
| Interoceptive Measurement Modality | Definition | Included Measures |
|---|---|---|
| Behavioural | Any measure or task assessing individuals’ conscious experience of interoceptive signals including measures of interoceptive accuracy, awareness, or sensibility. | Heartbeat counting task, heartbeat discrimination task, heartbeat detection task, validated questionnaire measures of interoceptive awareness, or sensibility (e.g., the Multidimensional Assessment of Interoceptive Awareness; [79]), measures of interoceptive sensibility or awareness (e.g., confidence ratings of performance on behavioural tasks). |
| Physiological | Measures of cardiac visceral afferent signalling which reflect autonomic regulation by the central autonomic network, or improvements in afferent communication to the brain. | HRV frequency and time-domain indices, indices of baroreflex functioning (e.g., baroreflex sensitivity), indices of resonance or coherence (e.g., coherence index), measures of RSA. |
| Neural | Measures of the neural representation of interoceptive information. This includes the representation of physiological afferents in the brain, patterns of brain activation during attention to interoceptive signals, functional connectivity of interoceptive brain regions, or the relation of brain structure to interoception at other processing levels. | Functional connectivity of the central autonomic network or interoceptive brain regions, activation of interoceptive brain regions (e.g., the insular cortex), electrical activity representing the interoceptive signal (e.g., the heartbeat-evoked potential). |
| Features of Included Studies | Effect of Intervention (n = 0) | No Effect of Intervention (n = 1) | Other Effect (i.e., Time or Effect of Comparator) |
|---|---|---|---|
| Clinical population (%) | - | 0 | - |
| Comparator Group (%) | - | NI—0 TAU—0 AC—100 S/P—0 | - |
| Intervention Intensity (%) | - | Mild—100 Moderate—0 Intense—0 | - |
| Resonance Frequency Breathing (%) | - | Overall RF—100.00 Optimal—100.00 Individual—0.00 Paced/preset—0.00 Unclear—0.00 | - |
| Risk of Bias (Mean) | - | 19 | - |
| Features of Included Studies | Effect of Intervention (n = 8) | No Effect of Intervention (n = 33) | Other Effect (n = 5) (i.e., Time or Effect of Comparator) |
|---|---|---|---|
| Clinical population (%) | 50.00 | 69.70 | 40.00 |
| Comparator Group (%) a | NI—30.00 TAU—20.00 AC—30.00 S/P—20.00 | NI—41.67 TAU—25.00 AC—25.00 S/P—8.33 | NI—20.00 TAU—40.00 AC—20.00 S/P—20.00 |
| Intervention Intensity (%) | Mild—0.00 Moderate—0.00 Intense—100.00 | Mild—30.30 Moderate—36.36 Intense—33.33 | Mild—40.00 Moderate—40.00 Intense—20.00 |
| Resonance Frequency Breathing (%) | Overall RF—87.50 Optimal—50.00 Individual—37.50 Paced/preset—12.50 Unclear—0.00 | Overall RF—57.58 Optimal—39.40 Individual—18.19 Paced/preset—24.24 Unclear—18.19 | Overall RF—80.00 Optimal—60.00 Individual—12.5 Paced/preset—12.50 Unclear—0.00 |
| Risk of Bias (Mean) | 14.50 | 14.52 | 15.00 |
| Features of Included Studies | Effect of Intervention (n = 19) | No Effect of Intervention (n = 18) | Other Effect (n = 4) (i.e., Time or Effect of Comparator) |
|---|---|---|---|
| Clinical population (%) | 47.37 | 72.22 | 75.00 |
| Comparator Group (%) a | NI—36.36 TAU—22.73 AC—22.73 S/P—18.18 | NI—47.37 TAU—21.05 AC—21.05 S/P—10.53 | NI—60.00 TAU—20.00 AC—20.00 S/P—0.00 |
| Intervention Intensity (%) | Mild—31.58 Moderate—36.84 Intense—31.58 | Mild—33.33 Moderate—22.22 Intense—44.44 | Mild—0.00 Moderate—50.00 Intense—50.00 |
| Resonance Frequency Breathing (%) | Overall RF–63.16 Optimal—52.63 Individual—10.53 Paced/preset—31.58 Unclear—5.26 | Overall RF—50.00 Optimal—38.39 Individual—16.17 Paced/preset—22.22 Unclear—22.22 | Overall RF—100.00 Optimal—25.00 Individual—75.00 Paced/preset—0.00 Unclear—0.00 |
| Risk of Bias (Mean) | 15.05 | 14.28 | 13.50 |
| Features of Included Studies | Effect of Intervention (n = 26) | No Effect of Intervention (n = 21) | Other Effect (n = 4) (i.e., Time or Effect of Comparator) |
|---|---|---|---|
| Clinical population (%) | 65.38 | 61.90 | 0.00 |
| Comparator Group (%) a | NI—34.48 TAU—24.14 AC—34.48 S/P—6.90 | NI—39.13 TAU—21.74 AC—26.09 S/P—13.04 | NI—80.00 TAU—0.00 AC—20.00 S/P—0.00 |
| Intervention Intensity (%) | Mild—26.92 Moderate—34.62 Intense—38.46 | Mild—38.10 Moderate—33.33 Intense—28.57 | Mild—50.00 Moderate—25.00 Intense—25.00 |
| Resonance Frequency Breathing (%) | Overall RF—73.08 Optimal—50.00 Individual—23.08 Paced/preset—19.23 Unclear—7.69 | Overall RF—66.67 Optimal—42.86 Individual—23.80 Paced/preset—23.81 Unclear—9.52 | Overall RF—50.00 Optimal—25.00 Individual—25.00 Paced/preset—50.00 Unclear—0.00 |
| Risk of Bias (M) | 14.85 | 15.05 | 15.00 |
| Features of Included Studies | Effect of Intervention (n = 3) | No Effect of Intervention (n = 8) | Other Effect (n = 1) (i.e., Time or Effect of Comparator) |
|---|---|---|---|
| Clinical population (%) | 33.33 | 62.50 | 0.00 |
| Comparator Group (%) a | NI—33.33 TAU—33.33 AC—33.33 S/P—0.00 | NI—37.50 TAU—12.50 AC—37.50 S/P—12.50 | NI—0.00 TAU—0.00 AC—100.00 S/P—0.00 |
| Intervention Intensity (%) | Mild—66.67 Moderate—0.00 Intense—33.33 | Mild—25.00 Moderate—37.50 Intense—37.50 | Mild—100.00 Moderate—0.00 Intense—0.00 |
| Resonance Frequency Breathing (%) | Overall RF—66.67 Optimal—33.33 Individual—33.33 Paced/preset—0.00 Unclear—33.33 | Overall RF—87.50 Optimal—50.00 Individual—37.50 Paced/preset—12.50 Unclear—0.00 | Overall RF—0.00 Optimal—0.00 Individual—0.00 Paced/preset—0.00 Unclear—0.00 |
| Risk of Bias (Mean) | 14.67 | 14.63 | 16.00 |
| Features of Included Studies | Effect of Intervention (n = 8) | No Effect of Intervention (n = 7) | Other Effect (n = 0) (i.e., Time or Effect of Comparator) |
|---|---|---|---|
| Clinical population (%) | 75.00 | 57.14 | - |
| Comparator Group (%) a | NI—20.00 TAU—30.00 AC—20.00 S/P—30.00 | NI—37.50 TAU—25.00 AC—25.00 S/P—12.50 | - |
| Intervention Intensity (%) | Mild—12.50 Moderate—25.00 Intense—62.50 | Mild—14.29 Moderate—42.86 Intense—42.86 | - |
| Resonance Frequency Breathing (%) | Overall RF—50.00 Optimal—37.50 Individual—12.50 Paced/preset—37.50 Unclear—12.50 | Overall RF—42.86 Optimal—28.57 Individual—14.29 Paced/preset—42.86 Unclear—14.29 | - |
| Risk of Bias (Mean) | 15.38 | 13.86 | - |
| Features of Included Studies | Effect of Intervention (n = 3) | No Effect of Intervention (n = 2) | Other Effect (n = 0) (i.e., Time or Effect of Comparator) |
|---|---|---|---|
| Clinical population (%) | 66.67 | 50.00 | - |
| Comparator Group (%) a | NI—75.00 TAU—0.00 AC—25.00 S/P—0.00 | NI—0.00 TAU—50.00 AC—50.00 S/P—0.00 | - |
| Intervention Intensity (%) | Mild—33.33 Moderate—33.33 Intense—33.33 | Mild—100.00 Moderate—0.00 Intense—0.00 | - |
| Resonance Frequency Breathing (%) | Overall RF—66.67 Optimal—0.00 Individual—66.67 Paced/preset—33.33 Unclear—0.00 | Overall RF—0.00 Optimal—0.00 Individual—0.00 Paced/preset—100.00 Unclear—0.00 | - |
| Risk of Bias | 14.33 | 16.00 | - |
| Features of Included Studies | Effect of Intervention (n = 3) | No Effect of Intervention (n = 2) | Other Effect (n = 0) (i.e., Time or Effect of Comparator) |
|---|---|---|---|
| Clinical population (%) | 33.33 | 50.00 | - |
| Comparator Group (%) a | NI—50.00 TAU—0.00 AC—50.00 S/P—0.00 | NI—0.00 TAU—0.00 AC—550.00 S/P—0.00 | - |
| Intervention Intensity (%) | Mild—0.00 Moderate—75.00 Intense—25.00 | Mild—0.00 Moderate—50.00 Intense—50.00 | - |
| Resonance Frequency Breathing (%) | Overall RF—100.00 Optimal—100.00 Individual—0.00 Paced/preset—0.00 Unclear—0.00 | Overall RF—50.00 Optimal—50.00 Individual—0.00 Paced/preset—50.00 Unclear—0.00 | - |
| Risk of Bias | 12.33 | 14.00 b | - |
| Features of Included Studies | Effect of Intervention (n = 2) | No Effect of Intervention (n = 3) | Other Effect (n = 0) (i.e., Time or Effect of Comparator) |
|---|---|---|---|
| Clinical population (%) | 50.00 | 33.33 | - |
| Comparator Group (%) a | NI—0.00 TAU—50.00 AC—50.00 S/P—0.00 | NI—66.67 TAU—0.00 AC—33.33 S/P—0.00 | - - - - |
| Intervention Intensity (%) | Mild—0.00 Moderate—50.00 Intense—50.00 | Mild—66.67 Moderate—33.33 Intense—0.00 | - - - |
| Resonance Frequency Breathing (%) | Overall RF—100.00 Optimal—0.00 Individual—100.00 Paced/preset—0.00 Unclear—0.00 | Overall RF—33.33 Optimal—33.33 Individual—0.00 Paced/preset—66.67 Unclear—0.00 | - - - - - |
| Risk of Bias | 15.50 | 14.33 | - |
| Features of Included Studies | Effect of Intervention (n = 3) | No Effect of Intervention (n = 1) | Other Effect (n = 0) (i.e., Time or Effect of Comparator) |
|---|---|---|---|
| Clinical population (%) | 0.00 | 100.00 | - |
| Comparator Group (%) a | NI—33.33 TAU—0.00 AC—33.33 S/P—33.33 | NI—0.00 TAU—100.00 AC—0.00 S/P—0.00 | - |
| Intervention Intensity (%) | Mild—33.33 Moderate—33.33 Intense—33.33 | Mild—0.00 Moderate—100.00 Intense—0.00 | - |
| Resonance Frequency Breathing (%) | Overall RF—66.67 Optimal—66.67 Individual—0.00 Paced/preset—0.00 Unclear—33.33 | Overall RF—100.00 Optimal—100.00 Individual—0.00 Paced/preset—0.00 Unclear—0.00 | - |
| Risk of Bias | 16.33 | 13.00 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wareing, L.; Readman, M.R.; Longo, M.R.; Linkenauger, S.A.; Crawford, T.J. The Utility of Heartrate and Heartrate Variability Biofeedback for the Improvement of Interoception across Behavioural, Physiological and Neural Outcome Measures: A Systematic Review. Brain Sci. 2024, 14, 579. https://doi.org/10.3390/brainsci14060579
Wareing L, Readman MR, Longo MR, Linkenauger SA, Crawford TJ. The Utility of Heartrate and Heartrate Variability Biofeedback for the Improvement of Interoception across Behavioural, Physiological and Neural Outcome Measures: A Systematic Review. Brain Sciences. 2024; 14(6):579. https://doi.org/10.3390/brainsci14060579
Chicago/Turabian StyleWareing, Lettie, Megan Rose Readman, Matthew R. Longo, Sally A. Linkenauger, and Trevor J. Crawford. 2024. "The Utility of Heartrate and Heartrate Variability Biofeedback for the Improvement of Interoception across Behavioural, Physiological and Neural Outcome Measures: A Systematic Review" Brain Sciences 14, no. 6: 579. https://doi.org/10.3390/brainsci14060579





