Transcranial Direct Current Stimulation to Ameliorate Post-Stroke Cognitive Impairment
Abstract
:1. Introduction
2. Current State of Cognitive Neurorehabilitation in Post-Stroke Recovery
2.1. Cognitive Profile
2.2. Impact of Cognitive Impairment on Other Modalities of Recovery
2.3. Interventions to Improve Post-Stroke Cognitive Impairment
3. tDCS Mechanism, Considerations and Other Uses
3.1. Mechanism of Action
3.2. Practical Features of tDCS
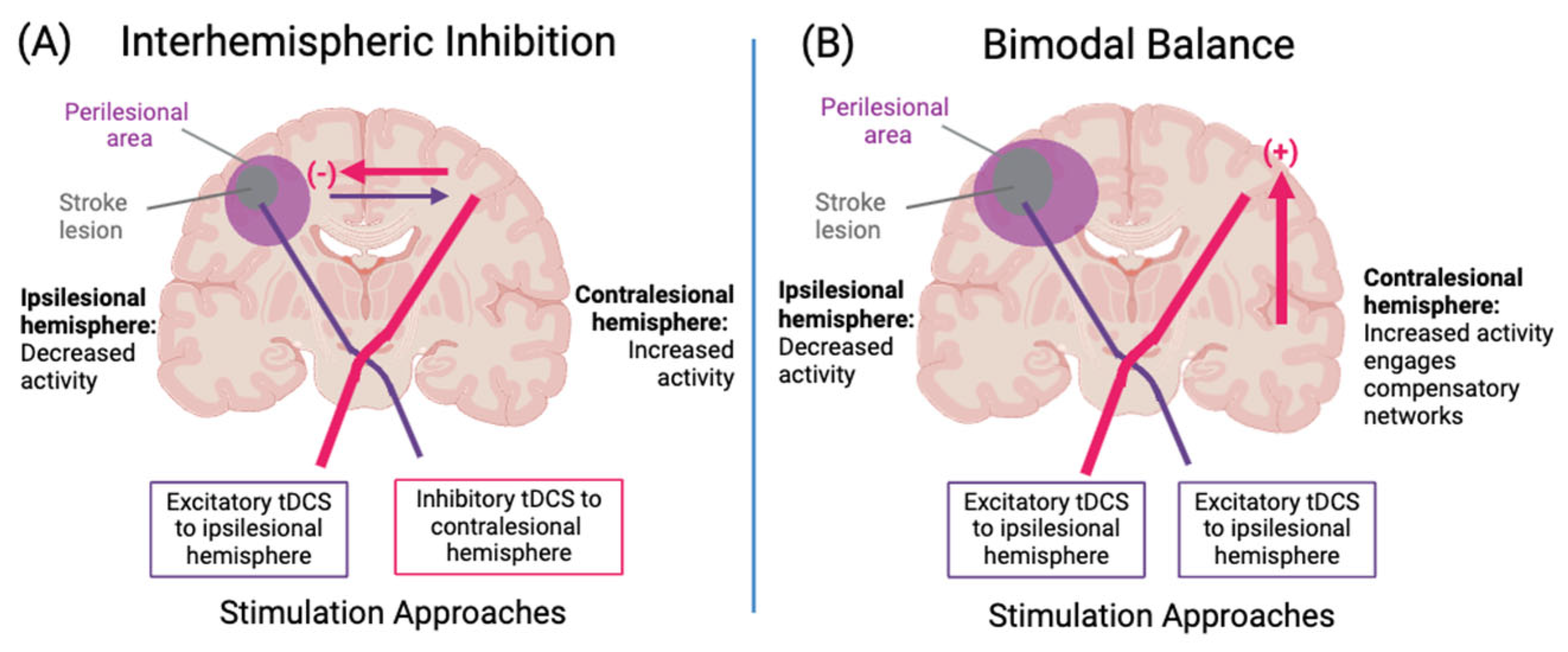
3.3. Models of Neuroplasticity to Guide Stimulation Targets
3.4. Task-Dependency of tDCS and Approaches to Therapy
3.5. Brain State-Dependency for tDCS and Priming
3.6. Limitations of tDCS
3.7. Comparison of tDCS to Other Neuromodulatory Techniques Used in Post-Stroke Cognitive Impairment
4. tDCS Therapy in Stroke Patient Populations
4.1. tDCS for Cognitive Performance in Stroke: Language
4.2. tDCS for Cognitive Performance in Stroke: Attention
4.3. tDCS for Cognitive Performance in Stroke: Other Domains of Cognition
4.3.1. Global Cognition
4.3.2. Memory and Verbal Learning
4.3.3. Executive Function
4.3.4. Perceptual Motor Function
4.3.5. Social Cognition
4.4. Considerations Unique to Post-Stroke Patient Populations
4.4.1. Medications Acting on the Central Nervous System
4.4.2. Chronicity of Intervention
4.4.3. Neurovascular Effects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics’ 2017 Update: A Report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Nys, G.M.S.; van Zandvoort, M.J.E.; de Kort, P.L.M.; Jansen, B.P.W.; de Haan, E.H.F.; Kappelle, L.J. Cognitive Disorders in Acute Stroke: Prevalence and Clinical Determinants. Cerebrovasc. Dis. 2007, 23, 408–416. [Google Scholar] [CrossRef]
- Jaillard, A.; Naegele, B.; Trabucco-Miguel, S.; LeBas, J.F.; Hommel, M. Hidden Dysfunctioning in Subacute Stroke. Stroke 2009, 40, 2473–2479. [Google Scholar] [CrossRef]
- Allan, L.M.; Rowan, E.N.; Firbank, M.J.; Thomas, A.J.; Parry, S.W.; Polvikoski, T.M.; O’Brien, J.T.; Kalaria, R.N. Long Term Incidence of Dementia, Predictors of Mortality and Pathological Diagnosis in Older Stroke Survivors. Brain 2011, 134, 3716–3727. [Google Scholar] [CrossRef]
- Levine, D.A.; Wadley, V.G.; Langa, K.M.; Unverzagt, F.W.; Kabeto, M.U.; Giordani, B.; Howard, G.; Howard, V.J.; Cushman, M.; Judd, S.E.; et al. Risk Factors for Poststroke Cognitive Decline: The REGARDS Study (Reasons for Geographic and Racial Differences in Stroke). Stroke 2018, 49, 987–994. [Google Scholar]
- Gottesman, R.F.; Hillis, A.E. Predictors and Assessment of Cognitive Dysfunction Resulting from Ischaemic Stroke. Lancet Neurol. 2010, 9, 895–905. [Google Scholar] [CrossRef]
- Zietemann, V.; Georgakis, M.K.; Dondaine, T.; Muller, C.; Mendyk, A.M.; Kopczak, A.; Hénon, H.; Bombois, S.; Wollenweber, F.A.; Bordet, R.; et al. Early MoCA Predicts Long-Term Cognitive and Functional Outcome and Mortality after Stroke. Neurology 2018, 91, E1838–E1850. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Skidmore, E.R.; Rodakowski, J. Relationship Consensus and Caregiver Burden in Adults with Cognitive Impairments 6 Months Following Stroke. PM&R 2019, 11, 597–603. [Google Scholar] [CrossRef]
- Jokinen, H.; Melkas, S.; Ylikoski, R.; Pohjasvaara, T.; Kaste, M.; Erkinjuntti, T.; Hietanen, M. Post-Stroke Cognitive Impairment Is Common Even after Successful Clinical Recovery. Eur. J. Neurol. 2015, 22, 1288–1294. [Google Scholar] [CrossRef]
- Lo, J.W.; Crawford, J.D.; Desmond, D.W.; Godefroy, O.; Jokinen, H.; Mahinrad, S.; Bae, H.J.; Lim, J.S.; Köhler, S.; Douven, E.; et al. Profile of and Risk Factors for Poststroke Cognitive Impairment in Diverse Ethnoregional Groups. Neurology 2019, 93, E2257–E2271. [Google Scholar] [CrossRef]
- Hyndman, D.; Ashburn, A. People with Stroke Living in the Community: Attention Deficits, Balance, ADL Ability and Falls. Disabil. Rehabil. 2003, 25, 817–822. [Google Scholar] [CrossRef]
- Hyndman, D.; Ashburn, A.; Stack, E. Fall Events among People with Stroke Living in the Community: Circumstances of Falls and Characteristics of Fallers. Arch. Phys. Med. Rehabil. 2002, 83, 165–170. [Google Scholar] [CrossRef]
- Elgh, E.; Hu, X. Dynamic Trajectory of Long-Term Cognitive Improvement up to 10 Years in Young Community-Dwelling Stroke Survivors: A Cohort Study. Front. Neurol. 2019, 10, 97. [Google Scholar] [CrossRef]
- McDowd, J.M.; Filion, D.L.; Pohl, P.S.; Richards, L.G.; Stiers, W. Attentional Abilities and Functional Outcomes Following Stroke. J. Gerontol.—Ser. B Psychol. Sci. Soc. Sci. 2003, 58, 45–53. [Google Scholar] [CrossRef]
- das Nair, R.; Cogger, H.; Worthington, E.; Lincoln, N. Cognitive Rehabilitation for Memory Deficits after Stroke. Cochrane Database Syst. Rev. 2016, 2016, CD002293. [Google Scholar] [CrossRef]
- Bhogal, S.K.; Teasell, R.; Speechley, M. Intensity of Aphasia Therapy, Impact on Recovery. Stroke 2003, 34, 987–992. [Google Scholar] [CrossRef]
- Narasimhalu, K.; Effendy, S.; Sim, C.H.; Lee, J.M.; Chen, I.; Hia, S.B.; Xue, H.L.; Corrales, M.P.; Chang, H.M.; Wong, M.C.; et al. A Randomized Controlled Trial of Rivastigmine in Patients with Cognitive Impairment No Dementia Because of Cerebrovascular Disease. Acta Neurol. Scand. 2010, 121, 217–224. [Google Scholar] [CrossRef]
- Iso-Markku, P.; Kujala, U.M.; Knittle, K.; Polet, J.; Vuoksimaa, E.; Waller, K. Physical Activity as a Protective Factor for Dementia and Alzheimer’s Disease: Systematic Review, Meta-Analysis and Quality Assessment of Cohort and Case–Control Studies. Br. J. Sports Med. 2022, 56, 701–709. [Google Scholar] [CrossRef]
- Dao, E.; Barha, C.K.; Zou, J.; Wei, N.; Liu-Ambrose, T. Prevention of Vascular Contributions to Cognitive Impairment and Dementia: The Role of Physical Activity and Exercise. Stroke 2024, 55, 812–821. [Google Scholar] [CrossRef]
- Moore, S.A.; Hallsworth, K.; Jakovljevic, D.G.; Blamire, A.M.; He, J.; Ford, G.A.; Rochester, L.; Trenell, M.I. Effects of Community Exercise Therapy on Metabolic, Brain, Physical, and Cognitive Function Following Stroke: A Randomized Controlled Pilot Trial. Neurorehabil. Neural Repair 2015, 29, 623–635. [Google Scholar] [CrossRef]
- Cicerone, K.D.; Langenbahn, D.M.; Braden, C.; Malec, J.F.; Kalmar, K.; Fraas, M.; Felicetti, T.; Laatsch, L.; Harley, J.P.; Bergquist, T.; et al. Evidence-Based Cognitive Rehabilitation: Updated Review of the Literature from 2003 through 2008. Arch. Phys. Med. Rehabil. 2011, 92, 519–530. [Google Scholar] [CrossRef]
- Cicerone, K.D.; Dahlberg, C.; Kalmar, K.; Langenbahn, D.M.; Malec, J.F.; Bergquist, T.F.; Felicetti, T.; Giacino, J.T.; Harley, J.P.; Harrington, D.E.; et al. Evidence-Based Cognitive Rehabilitation: Recommendations for Clinical Practice. Arch. Phys. Med. Rehabil. 2000, 81, 1596–1615. [Google Scholar] [CrossRef]
- Yan, R.B.; Zhang, X.L.; Li, Y.H.; Hou, J.M.; Chen, H.; Liu, H.L. Effect of Transcranial Direct-Current Stimulation on Cognitive Function in Stroke Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0233903. [Google Scholar] [CrossRef]
- Biou, E.; Cassoudesalle, H.; Cogné, M.; Sibon, I.; De Gabory, I.; Dehail, P.; Aupy, J.; Glize, B. Transcranial Direct Current Stimulation in Post-Stroke Aphasia Rehabilitation: A Systematic Review. Ann. Phys. Rehabil. Med. 2019, 62, 104–121. [Google Scholar] [CrossRef]
- Ferrucci, R.; Mameli, F.; Guidi, I.; Mrakic-Sposta, S.; Vergari, M.; Marceglia, S.; Cogiamanian, F.; Barbieri, S.; Scarpini, E.; Priori, A. Transcranial Direct Current Stimulation Improves Recognition Memory in Alzheimer Disease. Neurology 2008, 71, 493–498. [Google Scholar] [CrossRef]
- Norise, C.; Sacchetti, D.; Hamilton, R. Transcranial Direct Current Stimulation in Post-Stroke Chronic Aphasia: The Impact of Baseline Severity and Task Specificity in a Pilot Sample. Front. Hum. Neurosci. 2017, 11, 260. [Google Scholar] [CrossRef]
- Dubreuil-Vall, L.; Chau, P.; Ruffini, G.; Widge, A.S.; Camprodon, J.A. TDCS to the Left DLPFC Modulates Cognitive and Physiological Correlates of Executive Function in a State-Dependent Manner. Brain Stimul. 2019, 12, 1456–1463. [Google Scholar] [CrossRef]
- Mesulam, M.-M. Large-Scale Neurocognitive Networks and Distributed Processing for Attention, Language, and Memory. Ann. Neurol. 1990, 28, 597–613. [Google Scholar] [CrossRef]
- Stolwyk, R.J.; Mihaljcic, T.; Wong, D.K.; Chapman, J.E.; Rogers, J.M. Poststroke Cognitive Impairment Negatively Impacts Activity and Participation Outcomes. Stroke 2021, 52, 748–760. [Google Scholar] [CrossRef]
- Blackburn, D.J.; Bafadhel, L.; Randall, M.; Harkness, K.A. Cognitive Screening in the Acute Stroke Setting. Age Ageing 2013, 42, 113–116. [Google Scholar] [CrossRef]
- Aam, S.; Einstad, M.S.; Munthe-Kaas, R.; Lydersen, S.; Ihle-Hansen, H.; Knapskog, A.B.; Ellekjær, H.; Seljeseth, Y.; Saltvedt, I. Post-Stroke Cognitive Impairment—Impact of Follow-Up Time and Stroke Subtype on Severity and Cognitive Profile: The Nor-COAST Study. Front. Neurol. 2020, 11, 551524. [Google Scholar]
- El Husseini, N.; Katzan, I.L.; Rost, N.S.; Blake, M.L.; Byun, E.; Pendlebury, S.T.; Aparicio, H.J.; Marquine, M.J.; Gottesman, R.F.; Smith, E.E. Cognitive Impairment After Ischemic and Hemorrhagic Stroke: A Scientific Statement from the American Heart Association/American Stroke Association. Stroke 2023, 54, E272–E291. [Google Scholar] [CrossRef]
- Stephens, S.; Kenny, R.A.; Rowan, E.; Allan, L.; Kalaria, R.N.; Bradbury, M.; Ballard, C.G. Neuropsychological Characteristics of Mild Vascular Cognitive Impairment and Dementia after Stroke. Int. J. Geriatr. Psychiatry 2004, 19, 1053–1057. [Google Scholar] [CrossRef]
- Lees, R.; Fearon, P.; Harrison, J.K.; Broomfield, N.M.; Quinn, T.J. Cognitive and Mood Assessment in Stroke Research: Focused Review of Contemporary Studies. Stroke 2012, 43, 1678–1680. [Google Scholar] [CrossRef]
- Pendlebury, S.T.; Rothwell, P.M. Prevalence, Incidence, and Factors Associated with Pre-Stroke and Post-Stroke Dementia: A Systematic Review and Meta-Analysis. Lancet Neurol. 2009, 8, 1006–1018. [Google Scholar] [CrossRef]
- Douiri, A.; Rudd, A.G.; Wolfe, C.D.A. Prevalence of Poststroke Cognitive Impairment: South London Stroke Register 1995–2010. Stroke 2013, 44, 138–145. [Google Scholar] [CrossRef]
- Corbetta, M.; Ramsey, L.; Callejas, A.; Baldassarre, A.; Hacker, C.D.; Siegel, J.S.; Astafiev, S.V.; Rengachary, J.; Zinn, K.; Lang, C.E.; et al. Common Behavioral Clusters and Subcortical Anatomy in Stroke. Neuron 2015, 85, 927–941. [Google Scholar] [CrossRef]
- Puy, L.; Barbay, M.; Roussel, M.; Canaple, S.; Lamy, C.; Arnoux, A.; Leclercq, C.; Mas, J.L.; Tasseel-Ponche, S.; Constans, J.M.; et al. Neuroimaging Determinants of Poststroke Cognitive Performance: The GRECogVASC Study. Stroke 2018, 49, 2666–2673. [Google Scholar] [CrossRef]
- Mullick, A.A.; Subramanian, S.K.; Levin, M.F. Emerging Evidence of the Association between Cognitive Deficits and Arm Motor Recovery after Stroke: A Meta-Analysis. Restor. Neurol. Neurosci. 2015, 33, 389–403. [Google Scholar] [CrossRef]
- Dignam, J.; Copland, D.; O’Brien, K.; Burfein, P.; Khan, A.; Rodriguez, A.D. Influence of Cognitive Ability on Therapy Outcomes for Anomia in Adults with Chronic Poststroke Aphasia. J. Speech Lang. Hear. Res. 2017, 60, 406–421. [Google Scholar]
- Barker-Collo, S.; Feigin, V.; Feigin, V. The Impact of Neuropsychological Deficits on Functional Stroke Outcomes. Neuropsychol. Rev. 2006, 16, 53–64. [Google Scholar] [CrossRef]
- Gilmore, N.; Meier, E.L.; Johnson, J.P.; Kiran, S. Nonlinguistic Cognitive Factors Predict Treatment-Induced Recovery in Chronic Poststroke Aphasia. Arch. Phys. Med. Rehabil. 2019, 100, 1251–1258. [Google Scholar] [CrossRef]
- Hyndman, D.; Pickering, R.M.; Ashburn, A. The Influence of Attention Deficits on Functional Recovery Post Stroke during the First 12 Months after Discharge from Hospital. J. Neurol. Neurosurg. Psychiatry 2008, 79, 656–663. [Google Scholar] [CrossRef]
- Cirstea, M.C.; Levin, M.F. Improvement of Arm Movement Patterns and Endpoint Control Depends on Type of Feedback during Practice in Stroke Survivors. Neurorehabil. Neural Repair 2007, 21, 398–411. [Google Scholar] [CrossRef]
- Chen, C.; Leys, D.; Esquenazi, A. The Interaction between Neuropsychological and Motor Deficits in Patients after Stroke. Neurology 2013, 80, S27–S34. [Google Scholar] [CrossRef]
- Einstad, M.S.; Saltvedt, I.; Lydersen, S.; Ursin, M.H.; Munthe-Kaas, R.; Ihle-Hansen, H.; Knapskog, A.B.; Askim, T.; Beyer, M.K.; Næss, H.; et al. Associations between Post-Stroke Motor and Cognitive Function: A Cross-Sectional Study. BMC Geriatr. 2021, 21, 1–10. [Google Scholar] [CrossRef]
- Cramer, S.C. Improving Outcomes after Stroke by LEAPS (Locomotor Experience Applied Post-Stroke) and Bounds. Stroke 2011, 42, 3659–3660. [Google Scholar] [CrossRef]
- Rice, J.; Corp, D.T.; Swarowsky, A.; Cahalin, L.P.; Cabral, D.F.; Nunez, C.; Koch, S.; Rundek, T.; Gomes-Osman, J. Greater Cognitive-Motor Interference in Individuals Post-Stroke during More Complex Motor Tasks. J. Neurol. Phys. Ther. 2022, 46, 26–33. [Google Scholar] [CrossRef]
- Yagi, M.; Yasunaga, H.; Matsui, H.; Morita, K.; Fushimi, K.; Fujimoto, M.; Koyama, T.; Fujitani, J. Impact of Rehabilitation on Outcomes in Patients with Ischemic Stroke: A Nationwide Retrospective Cohort Study in Japan. Stroke 2017, 48, 740–746. [Google Scholar] [CrossRef]
- Cogan, A.M.; Weaver, J.A.; Davidson, L.F.; Khromouchkine, N.; Mallinson, T. Association of Therapy Time and Cognitive Recovery in Stroke Patients in Post-Acute Rehabilitation. J. Am. Med. Dir. Assoc. 2021, 22, 453–458.e3. [Google Scholar] [CrossRef]
- West, T.; Bernhardt, J. Physical Activity in Hospitalised Stroke Patients. Stroke Res. Treat. 2012, 2012, 13. [Google Scholar] [CrossRef]
- Dorsch, A.; Thomas, S.; Xu, X.; Kaiser, W.; Dobkin, B.H. SIRRACT: An International Randomized Clinical Trial of Activity Feedback During Inpatient Stroke Rehabilitation Enabled by Wireless Sensing. Neurorehabil. Neural Repair 2015, 29, 407–415. [Google Scholar] [CrossRef]
- Skolarus, L.E.; Burke, J.F.; Morgenstern, L.B.; Meurer, W.J.; Adelman, E.E.; Kerber, K.A.; Callaghan, B.C.; Lisabeth, L.D. Impact of State Medicaid Coverage on Utilization of Inpatient Rehabilitation Facilities among Patients with Stroke. Stroke 2014, 45, 2472–2474. [Google Scholar] [CrossRef]
- Chan, L.; Wang, H.; Terdiman, J.; Hoffman, J.; Ciol, M.A.; Lattimore, B.F.; Sidney, S.; Quesenberry, C.; Lu, Q.; Sandel, M.E. Disparities in Outpatient and Home Health Service Utilization Following Stroke: Results of a 9-Year Cohort Study in Northern California. PM&R 2009, 1, 997–1003. [Google Scholar] [CrossRef]
- Young, B.M.; Holman, E.A.; Cramer, S.C.; Shah, S.; Griessenauer, C.J.; Patel, N.; Lin, D.J.; Gee, J.; Moon, J.; Schwertfeger, J.; et al. Rehabilitation Therapy Doses Are Low After Stroke and Predicted by Clinical Factors. Stroke 2023, 54, 831–839. [Google Scholar] [CrossRef]
- Nicholson, S.; Sniehotta, F.F.; Van Wijck, F.; Greig, C.A.; Johnston, M.; Mcmurdo, M.E.T.; Dennis, M.; Mead, G.E. A Systematic Review of Perceived Barriers and Motivators to Physical Activity after Stroke. Int. J. Stroke 2013, 8, 357–364. [Google Scholar] [CrossRef]
- Black, S.; Román, G.C.; Geldmacher, D.S.; Salloway, S.; Hecker, J.; Burns, A.; Perdomo, C.; Kumar, D.; Pratt, R. Efficacy and Tolerability of Donepezil in Vascular Dementia Positive Results of a 24-Week, Multicenter, International, Randomized, Placebo-Controlled Clinical Trial. Stroke 2003, 34, 2323–2330. [Google Scholar] [CrossRef]
- Jorge, R.E.; Acion, L.; Moser, D.; Adams, H.P.; Robinson, R.G. Escitalopram and Enhancement of Cognitive Recovery Following Stroke. Arch. Gen. Psychiatry 2010, 67, 187–196. [Google Scholar] [CrossRef]
- Gagnon, D.J.; Leclerc, A.M.; Riker, R.R.; Brown, C.S.; May, T.; Nocella, K.; Cote, J.; Eldridge, A.; Seder, D.B. Amantadine and Modafinil as Neurostimulants During Post-Stroke Care: A Systematic Review. Neurocrit. Care 2020, 33, 283–297. [Google Scholar]
- Kim, B.R.; Chun, M.H.; Kim, L.S.; Park, J.Y. Effect of Virtual Reality on Cognition in Stroke Patients. Ann. Rehabil. Med. 2011, 35, 450. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, H.; Li, G.; Xu, C.; Wu, Y.; Li, H. Efficacy of Computerized Cognitive Training on Improving Cognitive Functions of Stroke Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Nurs. Pract. 2022, 28, e12966. [Google Scholar] [CrossRef]
- Zheng, G.; Xia, R.; Zhou, W.; Tao, J.; Chen, L. Aerobic Exercise Ameliorates Cognitive Function in Older Adults with Mild Cognitive Impairment: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Br. J. Sports Med. 2016, 50, 1443–1450. [Google Scholar] [CrossRef]
- Alonso-Alonso, M.; Fregni, F.; Pascual-Leone, A. Brain Stimulation in Poststroke Rehabilitation. Cerebrovasc. Dis. 2007, 24, 157–166. [Google Scholar] [CrossRef]
- Yamada, Y.; Sumiyoshi, T. Neurobiological Mechanisms of Transcranial Direct Current Stimulation for Psychiatric Disorders; Neurophysiological, Chemical, and Anatomical Considerations. Front. Hum. Neurosci. 2021, 15, 631838. [Google Scholar]
- Iyer, P.C.; Madhavan, S. Non-Invasive Brain Stimulation in the Modulation of Cerebral Blood Flow after Stroke: A Systematic Review of Transcranial Doppler Studies. Clin. Neurophysiol. 2018, 129, 2544–2551. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained Excitability Elevations Induced by Transcranial DC Motor Cortex Stimulation in Humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.F.; Nitsche, M.A. Partially Non-Linear Stimulation Intensity-Dependent Effects of Direct Current Stimulation on Motor Cortex Excitability in Humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability Changes Induced in the Human Motor Cortex by Weak Transcranial Direct Current Stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Baeken, C.; Remue, J.; Vanderhasselt, M.A.; Brunoni, A.R.; De Witte, S.; Duprat, R.; Koster, E.H.W.; De Raedt, R.; Wu, G.R. Increased Left Prefrontal Brain Perfusion after MRI Compatible TDCS Attenuates Momentary Ruminative Self-Referential Thoughts. Brain Stimul. 2017, 10, 1088–1095. [Google Scholar] [CrossRef]
- Bikson, M.; Grossman, P.; Thomas, C.; Zannou, A.L.; Jiang, J.; Adnan, T.; Mourdoukoutas, A.P.; Kronberg, G.; Truong, D.; Boggio, P.; et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul. 2016, 9, 641–661. [Google Scholar] [CrossRef]
- Knotkova, H.; Riggs, A.; Berisha, D.; Borges, H.; Bernstein, H.; Patel, V.; Truong, D.Q.; Unal, G.; Arce, D.; Datta, A.; et al. Automatic M1-SO Montage Headgear for Transcranial Direct Current Stimulation (TDCS) Suitable for Home and High-Throughput In-Clinic Applications. Neuromodul. Technol. Neural Interface 2018, 22, 904–910. [Google Scholar] [CrossRef]
- Charvet, L.E.; Kasschau, M.; Datta, A.; Knotkova, H.; Stevens, M.C.; Alonzo, A.; Loo, C.; Krull, K.R.; Bikson, M. Remotely-Supervised Transcranial Direct Current Stimulation (TDCS) for Clinical Trials: Guidelines for Technology and Protocols. Front. Syst. Neurosci. 2015, 9, 26. [Google Scholar] [CrossRef]
- Shaw, M.; Pilloni, G.; Charvet, L. Delivering Transcranial Direct Current Stimulation Away from Clinic: Remotely Supervised TDCS. Mil. Med. 2020, 185, 319–325. [Google Scholar] [CrossRef]
- Dobbs, B.; Pawlak, N.; Biagioni, M.; Agarwal, S.; Shaw, M.; Pilloni, G.; Bikson, M.; Datta, A.; Charvet, L. Generalizing Remotely Supervised Transcranial Direct Current Stimulation (TDCS): Feasibility and Benefit in Parkinson’s Disease. J. Neuroeng. Rehabil. 2018, 15, 114. [Google Scholar] [CrossRef]
- Pilloni, G.; Vogel-Eyny, A.; Lustberg, M.; Best, P.; Malik, M.; Walton-Masters, L.; George, A.; Mirza, I.; Zhovtis, L.; Datta, A.; et al. Tolerability and Feasibility of At-Home Remotely Supervised Transcranial Direct Current Stimulation (RS-TDCS): Single-Center Evidence from 6779 Sessions. Brain Stimul. 2022, 15, 707–716. [Google Scholar] [CrossRef]
- Koutsomitros, T.; Schwarz, S.A.; van der Zee, K.T.; Schuhmann, T.; Sack, A.T. Home-Administered Transcranial Direct Current Stimulation with Asynchronous Remote Supervision in the Treatment of Depression: Feasibility, Tolerability, and Clinical Effectiveness. Front. Psychiatry 2023, 14, 1206805. [Google Scholar] [CrossRef]
- Alonzo, A.; Fong, J.; Ball, N.; Martin, D.; Chand, N.; Loo, C. Pilot Trial of Home-Administered Transcranial Direct Current Stimulation for the Treatment of Depression. J. Affect. Disord. 2019, 252, 475–483. [Google Scholar] [CrossRef]
- Ko, M.H.; Yoon, J.Y.; Jo, Y.J.; Son, M.N.; Kim, D.S.; Kim, G.W.; Won, Y.H.; Park, S.H.; Seo, J.H.; Kim, Y.H. Home-Based Transcranial Direct Current Stimulation to Enhance Cognition in Stroke: Randomized Controlled Trial. Stroke 2022, 53, 2992–3001. [Google Scholar] [CrossRef]
- Villamar, M.F.; Volz, M.S.; Bikson, M.; Datta, A.; Dasilva, A.F.; Fregni, F. Technique and Considerations in the Use of 4x1 Ring High-Definition Transcranial Direct Current Stimulation (HD-TDCS). J. Vis. Exp. 2013, e50309. [Google Scholar] [CrossRef]
- Murase, N.; Duque, J.; Mazzocchio, R.; Cohen, L.G. Influence of Interhemispheric Interactions on Motor Function in Chronic Stroke. Ann. Neurol. 2004, 55, 400–409. [Google Scholar] [CrossRef]
- Feng, W.; Kautz, S.A.; Schlaug, G.; Meinzer, C.; George, M.S.; Chhatbar, P.Y. Transcranial Direct Current Stimulation for Post-Stroke Motor Recovery: Challenges and Opportunities. PM&R 2018, 10, S157–S164. [Google Scholar] [CrossRef]
- Schlaug, G.; Renga, V.; Nair, D. Transcranial Direct Current Stimulation in Stroke Recovery. Arch. Neurol. 2008, 65, 1571–1576. [Google Scholar] [CrossRef]
- González-Rodriguez, B.; Serradell-Ribé, N.; Viejo-Sobera, R.; Romero-Muñoz, J.P.; Marron, E.M. Transcranial Direct Current Stimulation in Neglect Rehabilitation after Stroke: A Systematic Review. J. Neurol. 2022, 269, 6310–6329. [Google Scholar] [CrossRef]
- Cappon, D.; Jahanshahi, M.; Bisiacchi, P. Value and Efficacy of Transcranial Direct Current Stimulation in the Cognitive Rehabilitation: A Critical Review since 2000. Front. Neurosci. 2016, 10, 157. [Google Scholar] [CrossRef]
- You, D.S.; Kim, D.Y.; Chun, M.H.; Jung, S.E.; Park, S.J. Cathodal Transcranial Direct Current Stimulation of the Right Wernicke’s Area Improves Comprehension in Subacute Stroke Patients. Brain Lang. 2011, 119, 1–5. [Google Scholar] [CrossRef]
- Elsner, B.; Kugler, J.; Mehrholz, J. Transcranial Direct Current Stimulation (TDCS) for Improving Aphasia after Stroke: A Systematic Review with Network Meta-Analysis of Randomized Controlled Trials. J. Neuroeng. Rehabil. 2020, 17, 88. [Google Scholar]
- Fridriksson, J.; Rorden, C.; Elm, J.; Sen, S.; George, M.S.; Bonilha, L. Transcranial Direct Current Stimulation vs Sham Stimulation to Treat Aphasia After Stroke: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 1470–1476. [Google Scholar] [CrossRef]
- Di Pino, G.; Pellegrino, G.; Assenza, G.; Capone, F.; Ferreri, F.; Formica, D.; Ranieri, F.; Tombini, M.; Ziemann, U.; Rothwell, J.C.; et al. Modulation of Brain Plasticity in Stroke: A Novel Model for Neurorehabilitation. Nat. Rev. Neurol. 2014, 10, 597–608. [Google Scholar] [CrossRef]
- Fox, M.D. Mapping Symptoms to Brain Networks with the Human Connectome. N. Engl. J. Med. 2018, 379, 2237–2245. [Google Scholar] [CrossRef]
- Bahmani, Z.; Clark, K.; Merrikhi, Y.; Mueller, A.; Pettine, W.; Isabel Vanegas, M.; Moore, T.; Noudoost, B. Prefrontal Contributions to Attention and Working Memory. Curr. Top. Behav. Neurosci. 2019, 41, 129–153. [Google Scholar] [CrossRef]
- Curtis, C.E.; D’Esposito, M. Persistent Activity in the Prefrontal Cortex during Working Memory. Trends Cogn. Sci. 2003, 7, 415–423. [Google Scholar] [CrossRef]
- Shulman, G.L.; Tansy, A.P.; Kincade, M.; Petersen, S.E.; McAvoy, M.P.; Corbetta, M. Reactivation of Networks Involved in Preparatory States. Cereb. Cortex 2002, 12, 590–600. [Google Scholar] [CrossRef]
- Chao, L.L.; Knight, R.T. Contribution of Human Prefrontal Cortex to Delay Performance. J. Cogn. Neurosci. 1998, 10, 161–177. [Google Scholar] [CrossRef]
- Stagg, C.J.; Lin, R.L.; Mezue, M.; Segerdahl, A.; Kong, Y.; Xie, J.; Tracey, I. Widespread Modulation of Cerebral Perfusion Induced during and after Transcranial Direct Current Stimulation Applied to the Left Dorsolateral Prefrontal Cortex. J. Neurosci. 2013, 33, 11425–11431. [Google Scholar] [CrossRef]
- Jamil, A.; Batsikadze, G.; Kuo, H.I.; Labruna, L.; Hasan, A.; Paulus, W.; Nitsche, M.A. Systematic Evaluation of the Impact of Stimulation Intensity on Neuroplastic After-Effects Induced by Transcranial Direct Current Stimulation. J. Physiol. 2017, 595, 1273–1288. [Google Scholar] [CrossRef]
- Artola, A.; Bröcher, S.; Singer, W. Different Voltage-Dependent Thresholds for Inducing Long-Term Depression and Long-Term Potentiation in Slices of Rat Visual Cortex. Nature 1990, 347, 69–72. [Google Scholar] [CrossRef]
- Sjöström, P.J.; Häusser, M. A Cooperative Switch Determines the Sign of Synaptic Plasticity in Distal Dendrites of Neocortical Pyramidal Neurons. Neuron 2006, 51, 227–238. [Google Scholar] [CrossRef]
- Kronberg, G.; Bridi, M.; Abel, T.; Bikson, M.; Parra, L.C. Direct Current Stimulation Modulates LTP and LTD: Activity Dependence and Dendritic Effects. Brain Stimul. 2017, 10, e23–e24. [Google Scholar] [CrossRef]
- Li, L.M.; Violante, I.R.; Leech, R.; Ross, E.; Hampshire, A.; Opitz, A.; Rothwell, J.C.; Carmichael, D.W.; Sharp, D.J. Brain State and Polarity Dependent Modulation of Brain Networks by Transcranial Direct Current Stimulation. Hum. Brain Mapp. 2019, 40, 904. [Google Scholar] [CrossRef]
- Bergmann, T.O. Brain State-Dependent Brain Stimulation. Front. Psychol. 2018, 9, 422698. [Google Scholar]
- Hulme, S.R.; Jones, O.D.; Abraham, W.C. Emerging Roles of Metaplasticity in Behaviour and Disease. Trends Neurosci. 2013, 36, 353–362. [Google Scholar] [CrossRef]
- Saleem, M.; Herrmann, N.; Dinoff, A.; Mielke, M.M.; Oh, P.I.; Shammi, P.; Cao, X.; Venkata, S.L.V.; Haughey, N.J.; Lanctôt, K.L. A Lipidomics Approach to Assess the Association between Plasma Sphingolipids and Verbal Memory Performance in Coronary Artery Disease Patients Undertaking Cardiac Rehabilitation: A C18:0 Signature for Cognitive Response to Exercise. J. Alzheimers Dis. 2017, 60, 829. [Google Scholar] [CrossRef]
- Singh, A.M.; Neva, J.L.; Staines, W.R. Acute Exercise Enhances the Response to Paired Associative Stimulation-Induced Plasticity in the Primary Motor Cortex. Exp. Brain Res. 2014, 232, 3675–3685. [Google Scholar] [CrossRef]
- Steinberg, F.; Pixa, N.H.; Fregni, F. A Review of Acute Aerobic Exercise and Transcranial Direct Current Stimulation Effects on Cognitive Functions and Their Potential Synergies. Front. Hum. Neurosci. 2018, 12, 534. [Google Scholar] [CrossRef]
- Liu, C.S.; Herrmann, N.; Song, B.X.; Ba, J.; Gallagher, D.; Oh, P.I.; Marzolini, S.; Rajji, T.K.; Charles, J.; Papneja, P.; et al. Exercise Priming with Transcranial Direct Current Stimulation: A Study Protocol for a Randomized, Parallel-Design, Sham-Controlled Trial in Mild Cognitive Impairment and Alzheimer’s Disease. BMC Geriatr. 2021, 21, 677. [Google Scholar] [CrossRef]
- Sathappan, A.V.; Luber, B.M.; Lisanby, S.H. The Dynamic Duo: Combining Noninvasive Brain Stimulation with Cognitive Interventions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 89, 347–360. [Google Scholar] [CrossRef]
- Hermans, E.J.; Van Marle, H.J.F.; Ossewaarde, L.; Henckens, M.J.A.G.; Qin, S.; Van Kesteren, M.T.R.; Schoots, V.C.; Cousijn, H.; Rijpkema, M.; Oostenveld, R.; et al. Stress-Related Noradrenergic Activity Prompts Large-Scale Neural Network Reconfiguration. Science 2011, 334, 1151–1153. [Google Scholar] [CrossRef]
- De Smet, S.; Razza, L.B.; Pulopulos, M.M.; De Raedt, R.; Baeken, C.; Brunoni, A.R.; Vanderhasselt, M.A. Stress Priming Transcranial Direct Current Stimulation (TDCS) Enhances Updating of Emotional Content in Working Memory. Brain Stimul. 2024, 17, 434–443. [Google Scholar] [CrossRef]
- Christova, M.; Rafolt, D.; Gallasch, E. Cumulative Effects of Anodal and Priming Cathodal TDCS on Pegboard Test Performance and Motor Cortical Excitability. Behav. Brain Res. 2015, 287, 27–33. [Google Scholar] [CrossRef]
- Müller-Dahlhaus, F.; Ziemann, U. Metaplasticity in Human Cortex. Neuroscientist 2015, 21, 185–202. [Google Scholar] [CrossRef]
- Zhu, F.; Xu, X.; Jin, M.; Chen, J.; Feng, X.; Wang, J.; Yu, D.; Wang, R.; Lian, Y.; Huai, B.; et al. Priming Transcranial Direct Current Stimulation for Improving Hemiparetic Upper Limb in Patients with Subacute Stroke: Study Protocol for a Randomised Controlled Trial. BMJ Open 2024, 14, e079372. [Google Scholar] [CrossRef]
- Wagner, T.; Valero-Cabre, A.; Pascual-Leone, A. Noninvasive Human Brain Stimulation. Annu. Rev. Biomed. Eng. 2007, 9, 527–565. [Google Scholar] [CrossRef]
- Datta, A.; Bansal, V.; Diaz, J.; Patel, J.; Reato, D.; Bikson, M. Gyri–Precise Head Model of Transcranial DC Stimulation: Improved Spatial Focality Using a Ring Electrode versus Conventional Rectangular Pad. Brain Stimul. 2009, 2, 201. [Google Scholar] [CrossRef]
- Fregni, F.; El-Hagrassy, M.M.; Pacheco-Barrios, K.; Carvalho, S.; Leite, J.; Simis, M.; Brunelin, J.; Nakamura-Palacios, E.M.; Marangolo, P.; Venkatasubramanian, G.; et al. Evidence-Based Guidelines and Secondary Meta-Analysis for the Use of Transcranial Direct Current Stimulation in Neurological and Psychiatric Disorders. Int. J. Neuropsychopharmacol. 2021, 24, 256–313. [Google Scholar] [CrossRef]
- Pascual-leone, A.; Valls-solé, J.; Wassermann, E.M.; Hallett, M. Responses to Rapid-Rate Transcranial Magnetic Stimulation of the Human Motor Cortex. Brain 1994, 117 Pt 4, 847–858. [Google Scholar] [CrossRef]
- Schrader, L.M.; Stern, J.M.; Koski, L.; Nuwer, M.R.; Engel, J. Seizure Incidence during Single- and Paired-Pulse Transcranial Magnetic Stimulation (TMS) in Individuals with Epilepsy. Clin. Neurophysiol. 2004, 115, 2728–2737. [Google Scholar] [CrossRef]
- Lerner, A.J.; Wassermann, E.M.; Tamir, D.I. Seizures from Transcranial Magnetic Stimulation 2012–2016: Results of a Survey of Active Laboratories and Clinics. Clin. Neurophysiol. 2019, 130, 1409–1416. [Google Scholar] [CrossRef]
- Chou, Y.-H.; Ton That, V.; Chen, A.Y.C.; Sundman, M.; Huang, Y.Z. TMS-Induced Seizure Cases Stratified by Population, Stimulation Protocol, and Stimulation Site: A Systematic Literature Search. Clin. Neurophysiol. 2020, 131, 1019–1020. [Google Scholar] [CrossRef]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and Recommendations for TMS Use in Healthy Subjects and Patient Populations, with Updates on Training, Ethical and Regulatory Issues: Expert Guidelines. Clin. Neurophysiol. 2021, 132, 269–306. [Google Scholar] [CrossRef]
- Passera, B.; Chauvin, A.; Raffin, E.; Bougerol, T.; David, O.; Harquel, S. Exploring the Spatial Resolution of TMS-EEG Coupling on the Sensorimotor Region. NeuroImage 2022, 259, 119419. [Google Scholar] [CrossRef]
- Cattaneo, L. Fancies and Fallacies of Spatial Sampling with Transcranial Magnetic Stimulation (TMS). Front. Psychol. 2018, 9, 373783. [Google Scholar]
- Gao, Y.; Qiu, Y.; Yang, Q.; Tang, S.; Gong, J.; Fan, H.; Wu, Y.; Lu, X. Repetitive Transcranial Magnetic Stimulation Combined with Cognitive Training for Cognitive Function and Activities of Daily Living in Patients with Post-Stroke Cognitive Impairment: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2023, 87, 101919. [Google Scholar] [CrossRef]
- Hofmeijer, J.; Ham, F.; Kwakkel, G. Evidence of RTMS for Motor or Cognitive Stroke Recovery: Hype or Hope? Stroke 2023, 54, 2500–2511. [Google Scholar] [CrossRef]
- O’Brien, W.D. Ultrasound—Biophysics Mechanisms. Prog. Biophys. Mol. Biol. 2007, 93, 212. [Google Scholar] [CrossRef]
- Kim, H.; Taghados, S.J.; Fischer, K.; Maeng, L.S.; Park, S.; Yoo, S.S. Noninvasive Transcranial Stimulation of Rat Abducens Nerve by Focused Ultrasound. Ultrasound Med. Biol. 2012, 38, 1568–1575. [Google Scholar] [CrossRef]
- Tufail, Y.; Yoshihiro, A.; Pati, S.; Li, M.M.; Tyler, W.J. Ultrasonic Neuromodulation by Brain Stimulation with Transcranial Ultrasound. Nat. Protoc. 2011, 6, 1453–1470. [Google Scholar] [CrossRef]
- Shin, J.; Kong, C.; Cho, J.S.; Lee, J.; Koh, C.S.; Yoon, M.S.; Na, Y.C.; Chang, W.S.; Chang, J.W. Focused Ultrasound-Mediated Noninvasive Blood-Brain Barrier Modulation: Preclinical Examination of Efficacy and Safety in Various Sonication Parameters. Neurosurg. Focus. 2018, 44, E15. [Google Scholar] [CrossRef]
- Yoo, S.S.; Bystritsky, A.; Lee, J.H.; Zhang, Y.; Fischer, K.; Min, B.K.; McDannold, N.J.; Pascual-Leone, A.; Jolesz, F.A. Focused Ultrasound Modulates Region-Specific Brain Activity. NeuroImage 2011, 56, 1267–1275. [Google Scholar] [CrossRef]
- Guo, J.; Lo, W.L.A.; Hu, H.; Yan, L.; Li, L. Transcranial Ultrasound Stimulation Applied in Ischemic Stroke Rehabilitation: A Review. Front. Neurosci. 2022, 16, 964060. [Google Scholar] [CrossRef]
- Ciullo, V.; Spalletta, G.; Caltagirone, C.; Banaj, N.; Vecchio, D.; Piras, F.; Piras, F. Transcranial Direct Current Stimulation and Cognition in Neuropsychiatric Disorders: Systematic Review of the Evidence and Future Directions. Neuroscientist 2021, 27, 285–309. [Google Scholar] [CrossRef]
- Zaninotto, A.L.; El-Hagrassy, M.M.; Green, J.R.; Babo, M.; Paglioni, V.M.; Benute, G.G.; Paiva, W.S. Transcranial Direct Current Stimulation (TDCS) Effects on Traumatic Injury (TBI) Recovery: A Systematic Review. Dement. Neuropsychol. 2019, 13, 172. [Google Scholar] [CrossRef]
- Gill, J.; Shah-Basak, P.P.; Hamilton, R. It’s the Thought That Counts: Examining the Task-Dependent Effects of Transcranial Direct Current Stimulation on Executive Function. Brain Stimul. 2015, 8, 253–259. [Google Scholar] [CrossRef]
- Nissim, N.R.; O’Shea, A.; Indahlastari, A.; Kraft, J.N.; von Mering, O.; Aksu, S.; Porges, E.; Cohen, R.; Woods, A.J. Effects of Transcranial Direct Current Stimulation Paired with Cognitive Training on Functional Connectivity of the Working Memory Network in Older Adults. Front. Aging Neurosci. 2019, 11, 340. [Google Scholar] [CrossRef]
- Hsu, W.-Y.; Ku, Y.; Zanto, T.P.; Gazzaley, A. Effects of Non-Invasive Brain Stimulation on Cognitive Function in Healthy Aging and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neurobiol. Aging 2015, 36, 2348–2359. [Google Scholar]
- Mattioli, F.; Bellomi, F.; Stampatori, C.; Capra, R.; Miniussi, C. Neuroenhancement through Cognitive Training and Anodal TDCS in Multiple Sclerosis. Mult. Scler. J. 2016, 22, 222–230. [Google Scholar] [CrossRef]
- Ashrafi, A.; Mohseni-Bandpei, M.A.; Seydi, M. The Effect of TDCS on the Fatigue in Patients with Multiple Sclerosis: A Systematic Review of Randomized Controlled Clinical Trials. J. Clin. Neurosci. 2020, 78, 277–283. [Google Scholar] [CrossRef]
- Ahorsu, D.K.; Adjaottor, E.S.; Yin, B.; Lam, H.; Borgomaneri, S.; Sellaro, R. Intervention Effect of Non-Invasive Brain Stimulation on Cognitive Functions among People with Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Brain Sci. 2021, 11, 840. [Google Scholar] [CrossRef]
- Dhaliwal, S.K.; Meek, B.P.; Modirrousta, M.M. Non-Invasive Brain Stimulation for the Treatment of Symptoms Following Traumatic Brain Injury. Front. Psychiatry 2015, 6, 119. [Google Scholar] [CrossRef]
- Monti, A.; Ferrucci, R.; Fumagalli, M.; Mameli, F.; Cogiamanian, F.; Ardolino, G.; Priori, A. Transcranial Direct Current Stimulation (TDCS) and Language. J. Neurol. Neurosurg. Psychiatry 2013, 84, 832–842. [Google Scholar] [CrossRef]
- Baker, J.M.; Rorden, C.; Fridriksson, J. Using Transcranial Direct-Current Stimulation to Treat Stroke Patients with Aphasia. Stroke 2010, 41, 1229–1236. [Google Scholar] [CrossRef]
- Fiori, V.; Nitsche, M.A.; Cucuzza, G.; Caltagirone, C.; Marangolo, P. High-Definition Transcranial Direct Current Stimulation Improves Verb Recovery in Aphasic Patients Depending on Current Intensity. Neuroscience 2019, 406, 159–166. [Google Scholar] [CrossRef]
- Fridriksson, J.; Richardson, J.D.; Baker, J.M.; Rorden, C. Transcranial Direct Current Stimulation Improves Naming Reaction Time in Fluent Aphasia: A Double-Blind, Sham-Controlled Study. Stroke 2011, 42, 819–821. [Google Scholar] [CrossRef]
- Stockbridge, M.D.; Faria, A.V.; Fridriksson, J.; Rorden, C.; Bonilha, L.; Hillis, A.E. Subacute Aphasia Recovery Is Associated with Resting-State Connectivity within and beyond the Language Network. Ann. Clin. Transl. Neurol. 2023, 10, 1525–1532. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Coslett, H.B.; Thomas, A.L.; Faseyitan, O.; Benson, J.; Norise, C.; Hamilton, R.H. The Right Hemisphere Is Not Unitary in Its Role in Aphasia Recovery. Cortex 2012, 48, 1179–1186. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Messing, S.; Norise, C.; Hamilton, R.H. Are Networks for Residual Language Function and Recovery Consistent across Aphasic Patients? Neurology 2011, 76, 1726–1734. [Google Scholar] [CrossRef]
- Fridriksson, J.; Elm, J.; Stark, B.C.; Basilakos, A.; Rorden, C.; Sen, S.; George, M.S.; Gottfried, M.; Bonilha, L. BDNF Genotype and TDCS Interaction in Aphasia Treatment. Brain Stimul. 2018, 11, 1276–1281. [Google Scholar] [CrossRef]
- Vestito, L.; Rosellini, S.; Mantero, M.; Bandini, F. Long-Term Effects of Transcranial Direct-Current Stimulation in Chronic Post-Stroke Aphasia: A Pilot Study. Front. Hum. Neurosci. 2014, 8, 785. [Google Scholar] [CrossRef]
- Campana, S.; Caltagirone, C.; Marangolo, P. Combining Voxel-Based Lesion-Symptom Mapping (VLSM) with A-TDCS Language Treatment: Predicting Outcome of Recovery in Nonfluent Chronic Aphasia. Brain Stimul. 2015, 8, 769–776. [Google Scholar] [CrossRef]
- Fiori, V.; Coccia, M.; Marinelli, C.V.; Vecchi, V.; Bonifazi, S.; Gabriella Ceravolo, M.; Provinciali, L.; Tomaiuolo, F.; Marangolo, P. Transcranial Direct Current Stimulation Improves Word Retrieval in Healthy and Nonfluent Aphasic Subjects. J. Cogn. Neurosci. 2011, 23, 2309–2323. [Google Scholar] [CrossRef]
- Marangolo, P.; Fiori, V.; Calpagnano, M.A.; Campana, S.; Razzano, C.; Caltagirone, C.; Marini, A. TDCS over the Left Inferior Frontal Cortex Improves Speech Production in Aphasia. Front. Hum. Neurosci. 2013, 7, 61793. [Google Scholar] [CrossRef]
- Woodhead, Z.V.J.; Kerry, S.J.; Aguilar, O.M.; Ong, Y.H.; Hogan, J.S.; Pappa, K.; Leff, A.P.; Crinion, J.T. Randomized Trial of IReadMore Word Reading Training and Brain Stimulation in Central Alexia. Brain 2018, 141, 2127–2141. [Google Scholar] [CrossRef]
- Kang, E.K.; Kim, Y.K.; Sohn, H.M.; Cohen, L.G.; Paik, N.J. Improved Picture Naming in Aphasia Patients Treated with Cathodal TDCS to Inhibit the Right Broca’s Homologue Area. Restor. Neurol. Neurosci. 2011, 29, 141. [Google Scholar] [CrossRef]
- Cheng, W.; Li, Y.; Cheng, B.; Chen, Y.; Chen, Z.; Cui, L.; Chen, X.; Chen, Z. Effects of Transcranial Direct Current Stimulation over the Right Hemisphere on Naming Ability in Patients with Poststroke Aphasia: A Meta-Analysis. J. Neurolinguist. 2021, 58, 100986. [Google Scholar] [CrossRef]
- Mariën, P.; Ackermann, H.; Adamaszek, M.; Barwood, C.H.S.; Beaton, A.; Desmond, J.; De Witte, E.; Fawcett, A.J.; Hertrich, I.; Küper, M.; et al. Consensus Paper: Language and the Cerebellum: An Ongoing Enigma. Cerebellum 2014, 13, 386. [Google Scholar] [CrossRef]
- Wessel, M.J.; Hummel, F.C. Non-Invasive Cerebellar Stimulation: A Promising Approach for Stroke Recovery? Cerebellum 2018, 17, 359–371. [Google Scholar] [CrossRef]
- Sebastian, R.; Kim, J.H.; Brenowitz, R.; Tippett, D.C.; Desmond, J.E.; Celnik, P.A.; Hillis, A.E. Cerebellar Neuromodulation Improves Naming in Post-Stroke Aphasia. Brain Commun. 2020, 2, fcaa179. [Google Scholar] [CrossRef]
- van Dun, K.; Bodranghien, F.C.A.A.; Mariën, P.; Manto, M.U. TDCS of the Cerebellum: Where Do We Stand in 2016? Technical Issues and Critical Review of the Literature. Front. Hum. Neurosci. 2016, 10, 199. [Google Scholar] [CrossRef]
- Vallar, G.; Perani, D. The Anatomy of Unilateral Neglect after Right-Hemisphere Stroke Lesions. A Clinical/CT-Scan Correlation Study in Man. Neuropsychologia 1986, 24, 609–622. [Google Scholar] [CrossRef]
- Malhotra, P.; Coulthard, E.J.; Husain, M. Role of Right Posterior Parietal Cortex in Maintaining Attention to Spatial Locations over Time. Brain 2009, 132, 645. [Google Scholar] [CrossRef]
- Yi, Y.G.; Chun, M.H.; Do, K.H.; Sung, E.J.; Kwon, Y.G.; Kim, D.Y. The Effect of Transcranial Direct Current Stimulation on Neglect Syndrome in Stroke Patients. Ann. Rehabil. Med. 2016, 40, 223–229. [Google Scholar] [CrossRef]
- da Silva, T.R.; de Carvalho Nunes, H.R.; Martins, L.G.; da Costa, R.D.M.; de Souza, J.T.; Winckler, F.C.; Sartor, L.C.A.; Modolo, G.P.; Ferreira, N.C.; da Silva Rodrigues, J.C.; et al. Non-Invasive Brain Stimulation Can Reduce Unilateral Spatial Neglect after Stroke: ELETRON Trial. Ann. Neurol. 2022, 92, 400–410. [Google Scholar] [CrossRef]
- Sunwoo, H.; Kim, Y.H.; Chang, W.H.; Noh, S.; Kim, E.J.; Ko, M.H. Effects of Dual Transcranial Direct Current Stimulation on Post-Stroke Unilateral Visuospatial Neglect. Neurosci. Lett. 2013, 554, 94–98. [Google Scholar] [CrossRef]
- Richardson, A.G.; Overduin, S.A.; Valero-Cabré, A.; Padoa-Schioppa, C.; Pascual-Leone, A.; Bizzi, E.; Press, D.Z. Disruption of Primary Motor Cortex before Learning Impairs Memory of Movement Dynamics. J. Neurosci. 2006, 26, 12466. [Google Scholar] [CrossRef]
- O’Shea, J.; Revol, P.; Cousijn, H.; Near, J.; Petitet, P.; Jacquin-Courtois, S.; Berg, H.J.; Rode, G.; Rossetti, Y. Induced Sensorimotor Cortex Plasticity Remediates Chronic Treatment-Resistant Visual Neglect. eLife 2017, 6, e26602. [Google Scholar] [CrossRef]
- Kazuta, T.; Takeda, K.; Osu, R.; Tanaka, S.; Oishi, A.; Kondo, K.; Liu, M. Transcranial Direct Current Stimulation Improves Audioverbal Memory in Stroke Patients. Am. J. Phys. Med. Rehabil. 2017, 96, 565–571. [Google Scholar] [CrossRef]
- Yun, G.J.; Chun, M.H.; Kim, B.R. The Effects of Transcranial Direct-Current Stimulation on Cognition in Stroke Patients. J. Stroke 2015, 17, 354–358. [Google Scholar] [CrossRef]
- Emch, M.; von Bastian, C.C.; Koch, K. Neural Correlates of Verbal Working Memory: An FMRI Meta-Analysis. Front. Hum. Neurosci. 2019, 13, 447322. [Google Scholar]
- Jo, J.M.; Kim, Y.H.; Ko, M.H.; Ohn, S.H.; Joen, B.; Lee, K.H. Enhancing the Working Memory of Stroke Patients Using TDCS. Am. J. Phys. Med. Rehabil. 2009, 88, 404–409. [Google Scholar] [CrossRef]
- Park, S.-H.; Koh, E.-J.; Choi, H.-Y.; Ko, M.-H. A Double-Blind, Sham-Controlled, Pilot Study to Assess the Effects of the Concomitant Use of Transcranial Direct Current Stimulation with the Computer Assisted Cognitive Rehabilitation to the Prefrontal Cortex on Cognitive Functions in Patients with Strok. J. Korean Neurosurg. Soc. 2013, 54, 484–488. [Google Scholar] [CrossRef]
- Chiou, R.; Lambon Ralph, M.A. The Anterior-Ventrolateral Temporal Lobe Contributes to Boosting Visual Working Memory Capacity for Items Carrying Semantic Information. NeuroImage 2018, 169, 453–461. [Google Scholar] [CrossRef]
- Takeuchi, H.; Taki, Y.; Sassa, Y.; Hashizume, H.; Sekiguchi, A.; Fukushima, A.; Kawashima, R. Brain Structures Associated with Executive Functions during Everyday Events in a Non-Clinical Sample. Brain Struct. Funct. 2013, 218, 1017. [Google Scholar] [CrossRef]
- Rusnáková, Š.; Daniel, P.; Chládek, J.; Jurák, P.; Rektor, I. The Executive Functions in Frontal and Temporal Lobes: A Flanker Task Intracerebral Recording Study. J. Clin. Neurophysiol. 2011, 28, 30–35. [Google Scholar] [CrossRef]
- Imburgio, M.J.; Orr, J.M. Effects of Prefrontal TDCS on Executive Function: Methodological Considerations Revealed by Meta-Analysis. Neuropsychologia 2018, 117, 156–166. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.A.; Mazhari, S.; Najafi, K.; Ahmadi, M.; Aghaei, I.; Khaksarian, M. Anodal Transcranial Direct Current Stimulation Enhances Positive Changes in Movement Functions, Visual Attention and Depression of Patients with Chronic Ischemic Stroke: A Clinical Trial. Biomed. Res. Ther. 2018, 5, 2841–2849. [Google Scholar] [CrossRef]
- Kleynen, M.; Braun, S.M.; Bleijlevens, M.H.; Lexis, M.A.; Rasquin, S.M.; Halfens, J.; Wilson, M.R.; Beurskens, A.J.; Masters, R.S.W. Using a Delphi Technique to Seek Consensus Regarding Definitions, Descriptions and Classification of Terms Related to Implicit and Explicit Forms of Motor Learning. PLoS ONE 2014, 9, e100227. [Google Scholar] [CrossRef]
- Meyer, S.; De Bruyn, N.; Krumlinde-Sundholm, L.; Peeters, A.; Feys, H.; Thijs, V.; Verheyden, G. Associations between Sensorimotor Impairments in the Upper Limb at 1 Week and 6 Months after Stroke. J. Neurol. Phys. Ther. 2016, 40, 186–195. [Google Scholar] [CrossRef]
- Buch, E.R.; Santarnecchi, E.; Antal, A.; Born, J.; Celnik, P.A.; Classen, J.; Gerloff, C.; Hallett, M.; Hummel, F.C.; Nitsche, M.A.; et al. Effects of TDCS on Motor Learning and Memory Formation: A Consensus and Critical Position Paper. Clin. Neurophysiol. 2017, 128, 589–603. [Google Scholar] [CrossRef]
- Reis, J.; Schambra, H.M.; Cohen, L.G.; Buch, E.R.; Fritsch, B.; Zarahn, E.; Celnik, P.A.; Krakauer, J.W. Noninvasive Cortical Stimulation Enhances Motor Skill Acquisition over Multiple Days through an Effect on Consolidation. Proc. Natl. Acad. Sci. USA 2009, 106, 1590. [Google Scholar] [CrossRef]
- Arioli, M.; Crespi, C.; Canessa, N. Social Cognition through the Lens of Cognitive and Clinical Neuroscience. BioMed Res. Int. 2018, 2018, 4283427. [Google Scholar] [CrossRef]
- Mars, R.B.; Sallet, J.; Schüffelgen, U.; Jbabdi, S.; Toni, I.; Rushworth, M.F.S. Connectivity-Based Subdivisions of the Human Right “Temporoparietal Junction Area”: Evidence for Different Areas Participating in Different Cortical Networks. Cereb. Cortex 2012, 22, 1894–1903. [Google Scholar] [CrossRef]
- Santiesteban, I.; Banissy, M.J.; Catmur, C.; Bird, G. Enhancing Social Ability by Stimulating Right Temporoparietal Junction. Curr. Biol. 2012, 22, 2274–2277. [Google Scholar] [CrossRef]
- Mai, X.; Zhang, W.; Hu, X.; Zhen, Z.; Xu, Z.; Zhang, J.; Liu, C. Using TDCS to Explore the Role of the Right Temporo-Parietal Junction in Theory of Mind and Cognitive Empathy. Front. Psychol. 2016, 7, 177459. [Google Scholar]
- Thirugnanasambandam, N.; Grundey, J.; Adam, K.; Drees, A.; Skwirba, A.C.; Lang, N.; Paulus, W.; Nitsche, M.A. Nicotinergic Impact on Focal and Non-Focal Neuroplasticity Induced by Non-Invasive Brain Stimulation in Non-Smoking Humans. Neuropsychopharmacology 2011, 36, 879. [Google Scholar] [CrossRef]
- Di Marcello Valladão Lugon, M.; Batsikadze, G.; Fresnoza, S.; Grundey, J.; Kuo, M.F.; Paulus, W.; Nakamura-Palacios, E.M.; Nitsche, M.A. Mechanisms of Nicotinic Modulation of Glutamatergic Neuroplasticity in Humans. Cereb. Cortex 2017, 27, 544–553. [Google Scholar] [CrossRef]
- Turner, T.J. Nicotine Enhancement of Dopamine Release by a Calcium-Dependent Increase in the Size of the Readily Releasable Pool of Synaptic Vesicles. J. Neurosci. 2004, 24, 11328–11336. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.; Steketee, J.D.; Sharp, B.M. Upregulation of Ionotropic Glutamate Receptor Subunits within Specific Mesocorticolimbic Regions during Chronic Nicotine Self-Administration. Neuropsychopharmacology 2006, 32, 103–109. [Google Scholar] [CrossRef]
- Chollet, F.; Tardy, J.; Albucher, J.F.; Thalamas, C.; Berard, E.; Lamy, C.; Bejot, Y.; Deltour, S.; Jaillard, A.; Niclot, P.; et al. Fluoxetine for Motor Recovery after Acute Ischaemic Stroke (FLAME): A Randomised Placebo-Controlled Trial. Lancet Neurol. 2011, 10, 123–130. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Kuo, M.F.; Karrasch, R.; Wächter, B.; Liebetanz, D.; Paulus, W. Serotonin Affects Transcranial Direct Current-Induced Neuroplasticity in Humans. Biol. Psychiatry 2009, 66, 503–508. [Google Scholar] [CrossRef]
- Kuo, H.I.; Paulus, W.; Batsikadze, G.; Jamil, A.; Kuo, M.F.; Nitsche, M.A. Chronic Enhancement of Serotonin Facilitates Excitatory Transcranial Direct Current Stimulation-Induced Neuroplasticity. Neuropsychopharmacology 2016, 41, 1223–1230. [Google Scholar] [CrossRef]
- Kuo, H.I.; Paulus, W.; Batsikadze, G.; Jamil, A.; Kuo, M.F.; Nitsche, M.A. Acute and Chronic Effects of Noradrenergic Enhancement on Transcranial Direct Current Stimulation-induced Neuroplasticity in Humans. J. Physiol. 2017, 595, 1305. [Google Scholar] [CrossRef]
- Normann, C.; Clark, K. Selective Modulation of Ca2+ Influx Pathways by 5-HT Regulates Synaptic Long-Term Plasticity in the Hippocampus. Brain Res. 2005, 1037, 187–193. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Grundey, J.; Liebetanz, D.; Lang, N.; Tergau, F.; Paulus, W. Catecholaminergic Consolidation of Motor Cortical Neuroplasticity in Humans. Cereb. Cortex 2004, 14, 1240–1245. [Google Scholar] [CrossRef]
- Monte-Silva, K.; Liebetanz, D.; Grundey, J.; Paulus, W.; Nitsche, M.A. Dosage-Dependent Non-Linear Effect of l-Dopa on Human Motor Cortex Plasticity. J. Physiol. 2010, 588 Pt 18, 3415. [Google Scholar] [CrossRef]
- Fresnoza, S.; Stiksrud, E.; Klinker, F.; Liebetanz, D.; Paulus, W.; Kuo, M.F.; Nitsche, M.A. Dosage-Dependent Effect of Dopamine D2 Receptor Activation on Motor Cortex Plasticity in Humans. J. Neurosci. 2014, 34, 10701. [Google Scholar] [CrossRef]
- Li, S.; Carmichael, S.T. Growth-Associated Gene and Protein Expression in the Region of Axonal Sprouting in the Aged Brain after Stroke. Neurobiol. Dis. 2006, 23, 362–373. [Google Scholar] [CrossRef]
- Murphy, T.H.; Corbett, D. Plasticity during Stroke Recovery: From Synapse to Behaviour. Nat. Rev. Neurosci. 2009, 10, 861–872. [Google Scholar] [CrossRef]
- Wieloch, T.; Nikolich, K. Mechanisms of Neural Plasticity Following Brain Injury. Curr. Opin. Neurobiol. 2006, 16, 258–264. [Google Scholar] [CrossRef]
- Liu, H.; Tian, T.; Qin, W.; Li, K.; Yu, C.; Sousa, N.; Patrick Gilman, C.; Hayashi, T. Contrasting Evolutionary Patterns of Functional Connectivity in Sensorimotor and Cognitive Regions after Stroke. Front. Behav. Neurosci. 2016, 10, 72. [Google Scholar] [CrossRef]
- Lashley, K.S. In Search of the Engram. In Physiological Mechanisms in Animal Behavior (Society’s Symposium IV); Academic Press: Oxford, UK, 1950; pp. 454–482. [Google Scholar]
- Krakauer, J.W.; Carmichael, S.T.; Corbett, D.; Wittenberg, G.F. Getting Neurorehabilitation Right—What Can We Learn from Animal Models? Neurorehabil. Neural Repair 2012, 26, 923. [Google Scholar] [CrossRef]
- Zeiler, S.R.; Krakauer, J.W. The Interaction between Training and Plasticity in the Post-Stroke Brain. Curr. Opin. Neurol. 2013, 26, 609–616. [Google Scholar] [CrossRef]
- Prabhakaran, S.; Zarahn, E.; Riley, C.; Speizer, A.; Chong, J.Y.; Lazar, R.M.; Marshall, R.S.; Krakauer, J.W. Inter-Individual Variability in the Capacity for Motor Recovery after Ischemic Stroke. Neurorehabil. Neural Repair 2008, 22, 64–71. [Google Scholar] [CrossRef]
- Turrigiano, G.G.; Nelson, S.B. Homeostatic Plasticity in the Developing Nervous System. Nat. Rev. Neurosci. 2004, 5, 97–107. [Google Scholar] [CrossRef]
- Butts, D.A.; Kanold, P.O.; Shatz, C.J. A Burst-Based “Hebbian” Learning Rule at Retinogeniculate Synapses Links Retinal Waves to Activity-Dependent Refinement. PLoS Biol. 2007, 5, e61. [Google Scholar] [CrossRef]
- Kwakkel, G.; Kollen, B.; Twisk, J. Impact of Time on Improvement of Outcome after Stroke. Stroke 2006, 37, 2348–2353. [Google Scholar] [CrossRef]
- Bernhardt, J.; Borschmann, K.N.; Kwakkel, G.; Burridge, J.H.; Eng, J.J.; Walker, M.F.; Bird, M.L.; Cramer, S.C.; Hayward, K.S.; O’Sullivan, M.J.; et al. Setting the Scene for the Second Stroke Recovery and Rehabilitation Roundtable. Int. J. Stroke 2019, 14, 450–456. [Google Scholar] [CrossRef]
- Teasell, R.W.; Foley, N.C.; Salter, K.L.; Jutai, J.W. A Blueprint for Transforming Stroke Rehabilitation Care in Canada: The Case for Change. Arch. Phys. Med. Rehabil. 2008, 89, 575–578. [Google Scholar] [CrossRef]
- Zheng, X.; Alsop, D.C.; Schlaug, G. Effects of Transcranial Direct Current Stimulation (TDCS) on Human Regional Cerebral Blood Flow. NeuroImage 2011, 58, 26. [Google Scholar] [CrossRef]
- Bahr-Hosseini, M.; Bikson, M. Neurovascular-Modulation: A Review of Primary Vascular Responses to Transcranial Electrical Stimulation as a Mechanism of Action. Brain Stimul. 2021, 14, 837–847. [Google Scholar] [CrossRef]
- Gozalov, A.; Jansen-Olesen, I.; Klaerke, D.; Olesen, J. Role of K ATP Channels in Cephalic Vasodilatation Induced by Calcitonin Gene-Related Peptide, Nitric Oxide, and Transcranial Electrical Stimulation in the Rat. Headache 2008, 48, 1202–1213. [Google Scholar] [CrossRef]
- Vernieri, F.; Assenza, G.; Maggio, P.; Tibuzzi, F.; Zappasodi, F.; Altamura, C.; Corbetto, M.; Trotta, L.; Palazzo, P.; Ercolani, M.; et al. Cortical Neuromodulation Modifies Cerebral Vasomotor Reactivity. Stroke 2010, 41, 2087–2090. [Google Scholar] [CrossRef]
- Bahr-Hosseini, M.; Nael, K.; Unal, G.; Iacoboni, M.; Liebeskind, D.S.; Bikson, M.; Saver, J.L.; Group, T.T.; Sanossian, N.; Wu, A.; et al. High-Definition Cathodal Direct Current Stimulation for Treatment of Acute Ischemic Stroke: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2319231. [Google Scholar] [CrossRef]
- Turunen, K.E.A.; Laari, S.P.K.; Kauranen, T.V.; Uimonen, J.; Mustanoja, S.; Tatlisumak, T.; Poutiainen, E. Domain-Specific Cognitive Recovery after First-Ever Stroke: A 2-Year Follow-Up. J. Int. Neuropsychol. Soc. 2018, 24, 117–127. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sloane, K.L.; Hamilton, R.H. Transcranial Direct Current Stimulation to Ameliorate Post-Stroke Cognitive Impairment. Brain Sci. 2024, 14, 614. https://doi.org/10.3390/brainsci14060614
Sloane KL, Hamilton RH. Transcranial Direct Current Stimulation to Ameliorate Post-Stroke Cognitive Impairment. Brain Sciences. 2024; 14(6):614. https://doi.org/10.3390/brainsci14060614
Chicago/Turabian StyleSloane, Kelly L., and Roy H. Hamilton. 2024. "Transcranial Direct Current Stimulation to Ameliorate Post-Stroke Cognitive Impairment" Brain Sciences 14, no. 6: 614. https://doi.org/10.3390/brainsci14060614




