Abnormal Dynamic Reconstruction of Overlapping Communities in Schizophrenia Patients
Abstract
:1. Introduction
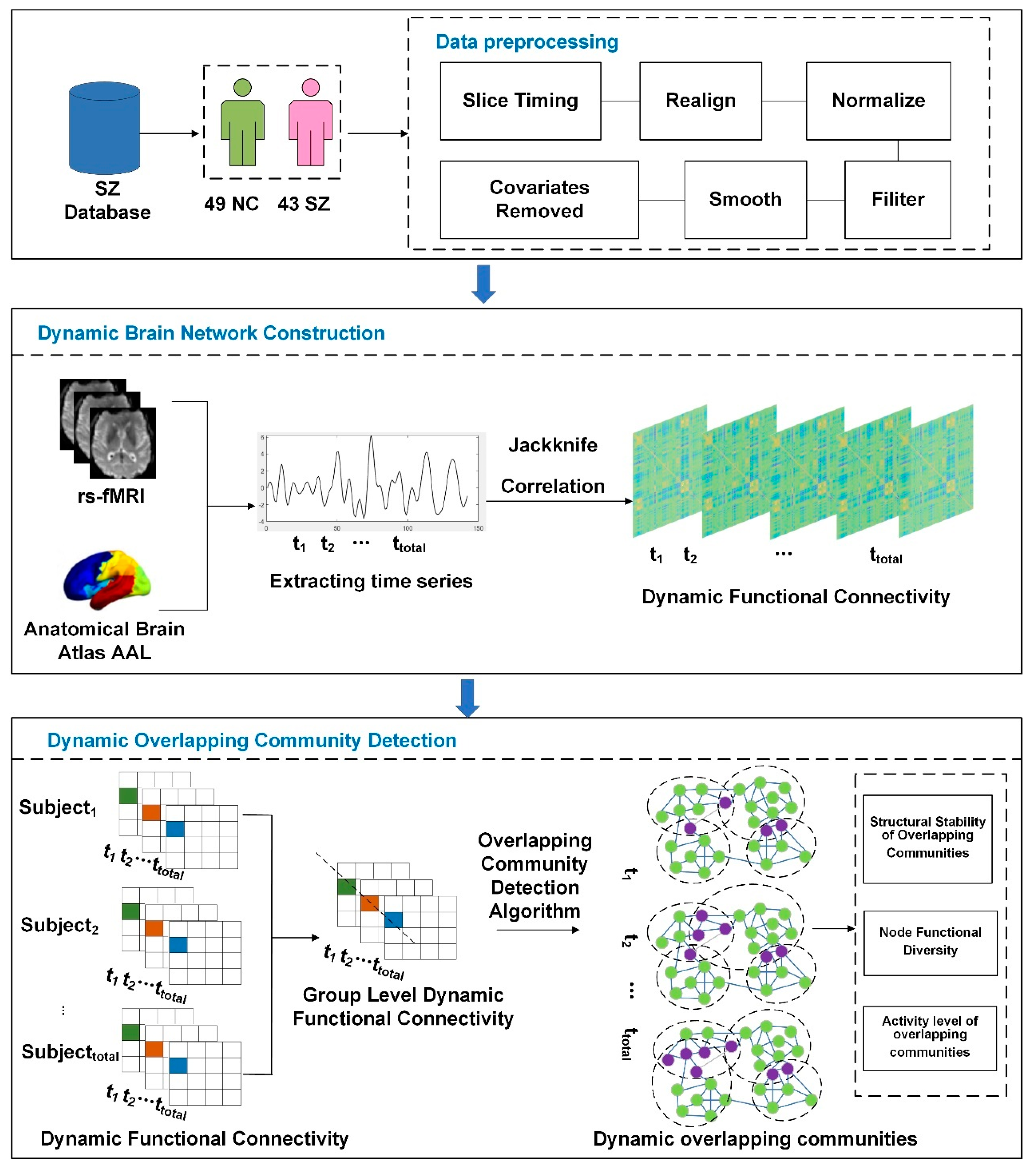
2. Materials and Methods
2.1. Participants
2.2. Imaging Acquisition and Preprocessing
2.3. Construction of Dynamic Brain Networks
2.4. Dynamic Functional Connectivity at the Group Level
| Algorithm 1. Group-level dynamic brain network construction algorithm |
| Input: |
| Functionally connected network of a total of subjects with a four-dimensional matrix of size |
| Process: |
| begin for i = 1 to N do for j = 1 to N do Initialization Vector ds = []; for s1 = 1 to S do Initializing Variables d = 0; for s2 = 1 to S do d = d +; end ds = [ds, d]; end Initialize the variable; index= Index of the smallest value in vector ds; G[:,i,j] = W[index,:,i,j]; end |
| end end Output: Group-level dynamic brain network with a three-dimensional matrix of size |
2.5. Overlapping Community Detection in Dynamic Brain Networks
| Algorithm 2. Iterative process of dynamic overlapping community algorithm for brain networks based on matrix decomposition |
| Iutput: Matrix Parameter value selection: |
| The values of sparsity and number of communities are selected by grid search using a cross-validation algorithm |
| Process: |
| (1) Initialization: For each time point, randomly initialize as a non-negative diagonal moment of size ; Randomly initialize to a non-negative matrix of size ; Normalize the column vector of ; |
| (2) Iterate until convergence: Fixed ; Update in each time point using the multiplicative update rule; |
| Fixed ; Update by using projected gradient descent; |
| Set all negative values in to zero; |
| Renormalize the column vector of ; Output: Matrix and matrix |
2.6. Overlapping Community Metrics for Dynamic Brain Networks
- (1)
- Structural Stability of Overlapping Communities
- (2)
- Nodes’ Functional Diversity
- (3)
- Activity level of overlapping communities
3. Results
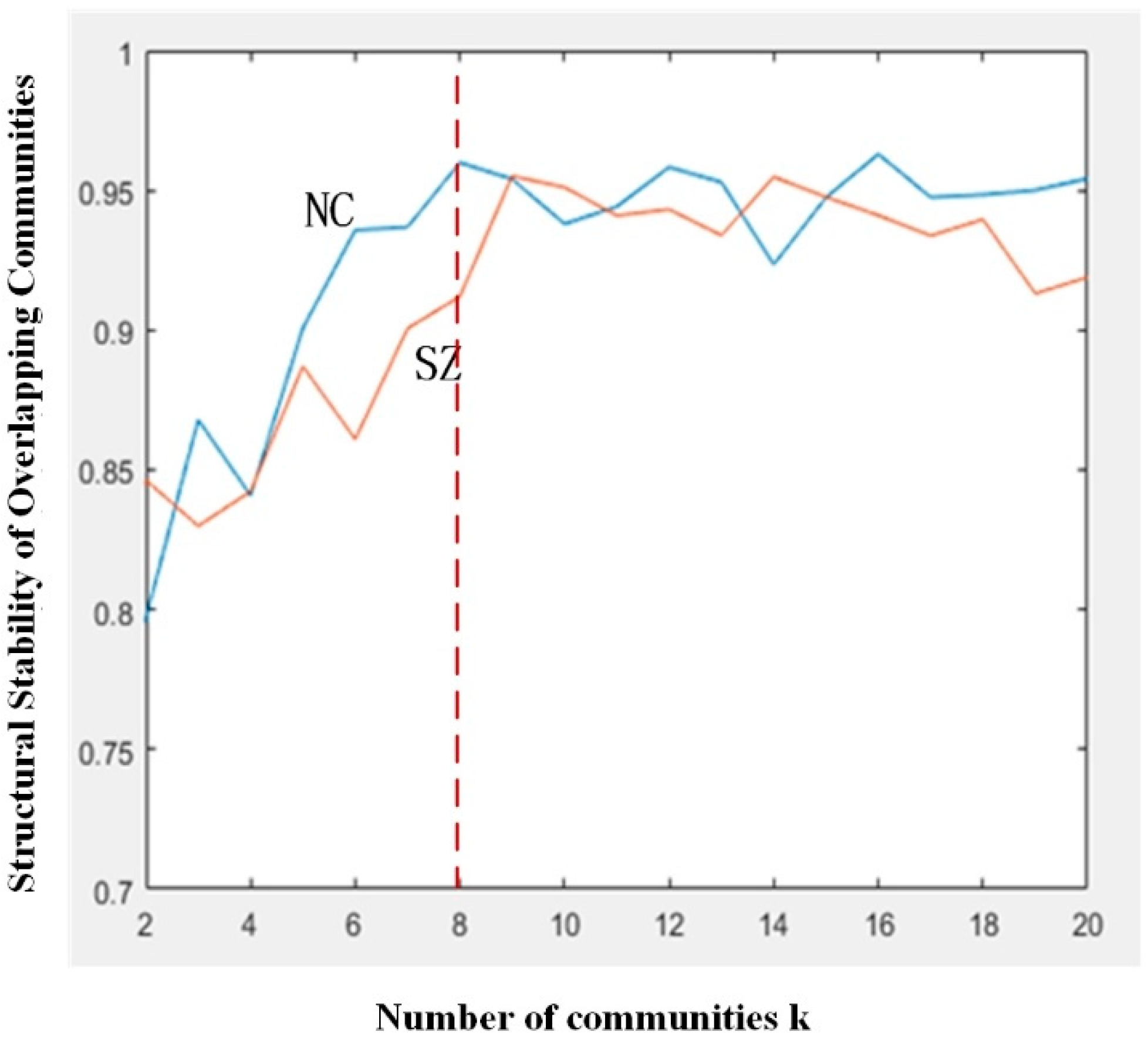
3.1. Structural Stability of Overlapping Communities
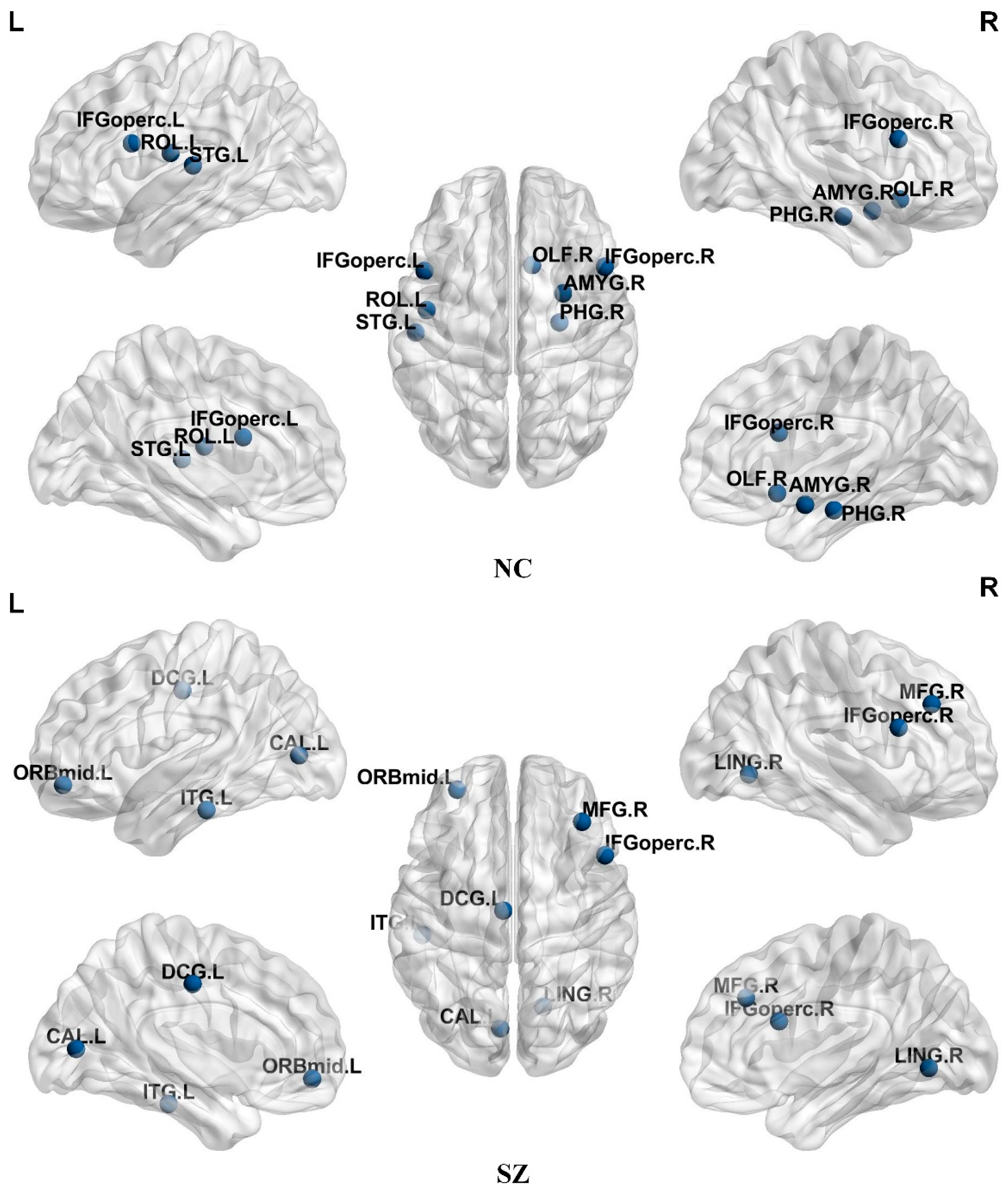
3.2. Discovery of Overlapping Nodes
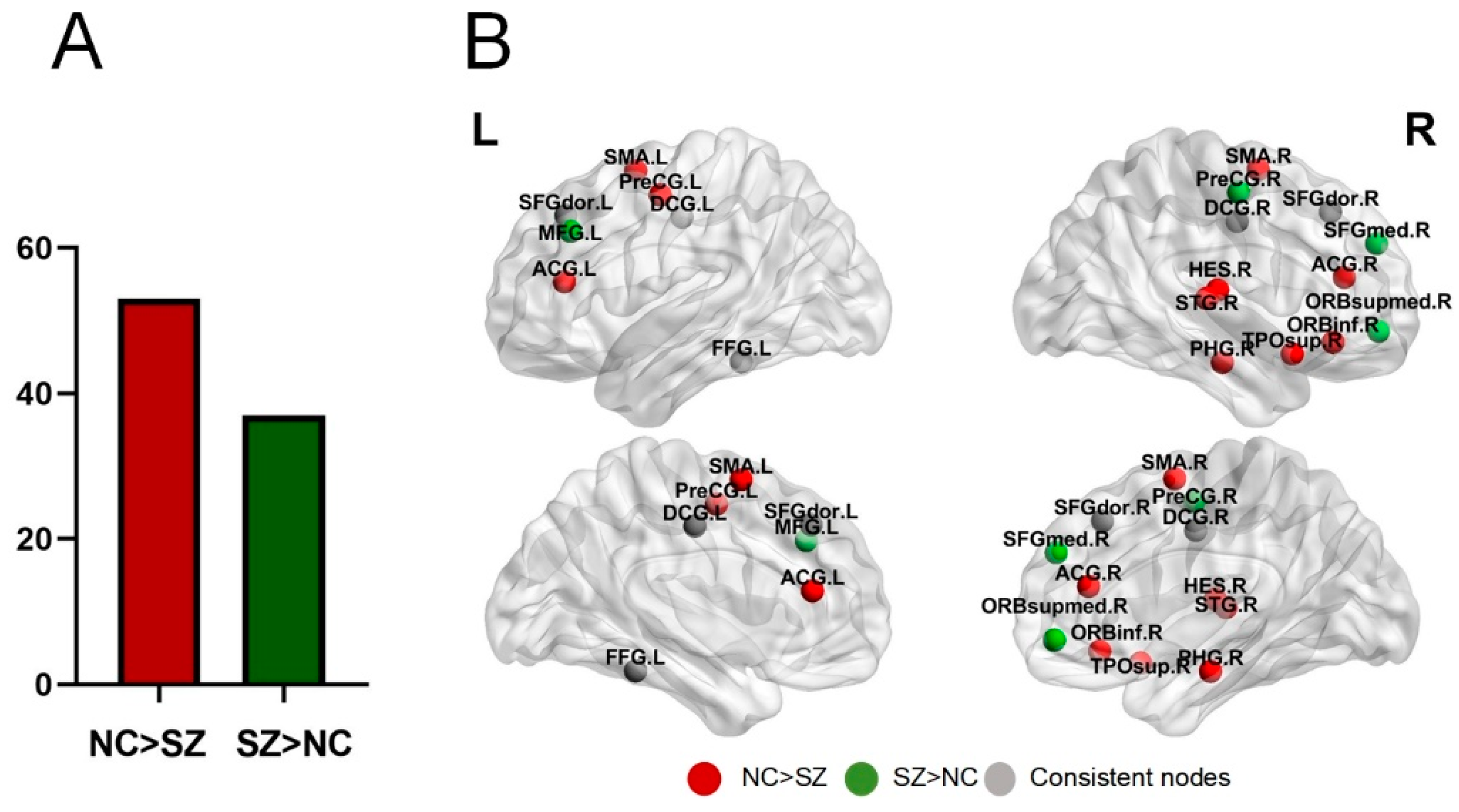
3.3. Nodes Functional Diversity
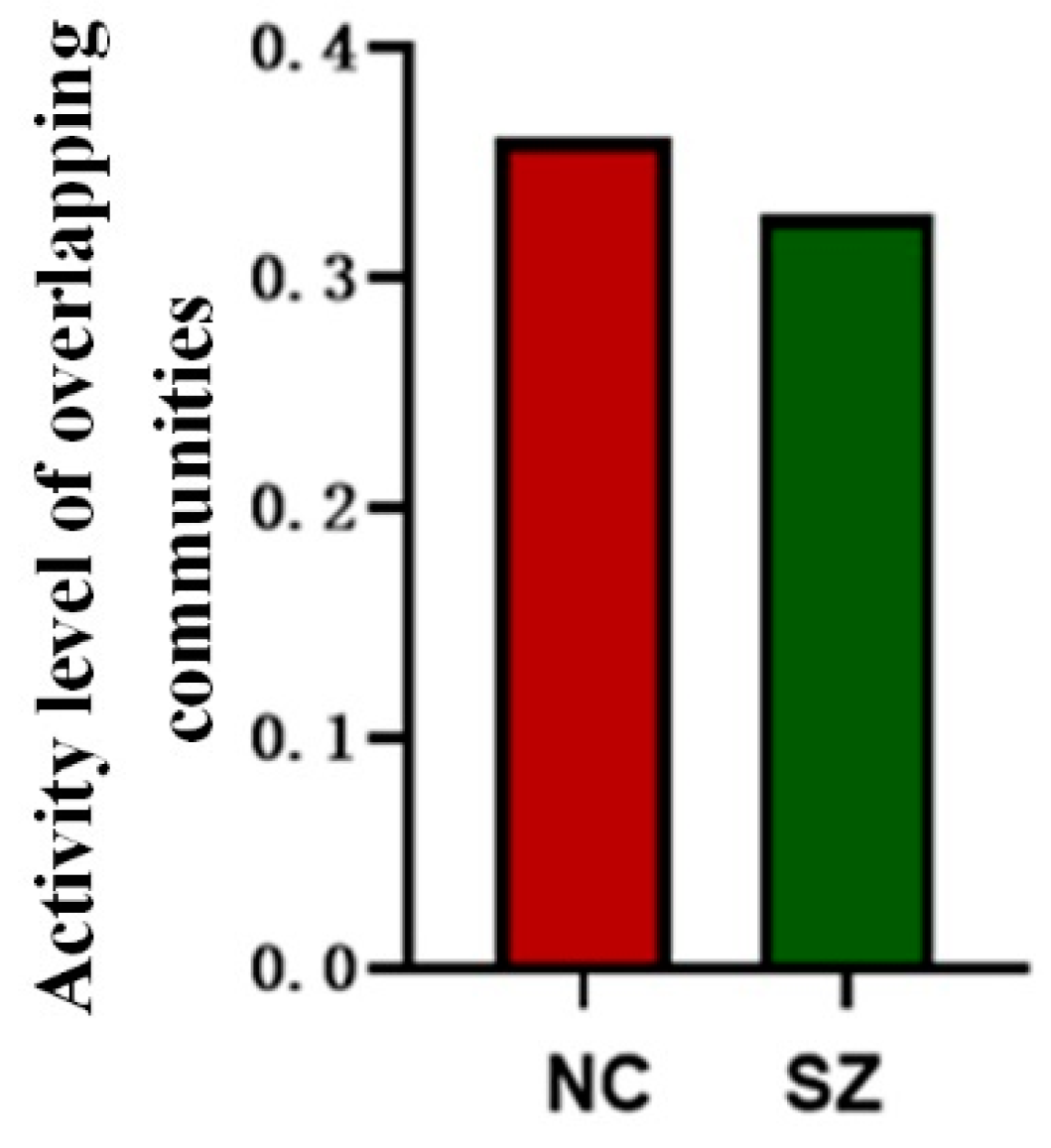
3.4. Activity Level of Overlapping Communities
4. Discussion
4.1. Overlapping and Interweaving of Brain Functional Networks
4.2. Instability of Community Structures for SZ Patients
4.3. Reduction in Community Activity Level and Functional Diversity of Nodes in SZ Patients
5. Limitations and Directions for Future Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| ROI | Regions | Abbreviated | RSN |
|---|---|---|---|
| 1 | Precentral_L | PreCG.L | DMN |
| 2 | Precentral_R | PreCG.R | DMN |
| 3 | Frontal_Sup_L | SFGdor.L | DMN |
| 4 | Frontal_Sup_R | SFGdor.R | DMN |
| 5 | Frontal_Sup_Orb_L | ORBsup.L | DMN |
| 6 | Frontal_Sup_Orb_R | ORBsup.R | DMN |
| 7 | Frontal_Mid_L | MFG.L | Attention |
| 8 | Frontal_Mid_R | MFG.R | Attention |
| 9 | Frontal_Mid_Orb_L | ORBmid.L | Attention |
| 10 | Frontal_Mid_Orb_R | ORBmid.R | Attention |
| 11 | Frontal_Inf_Oper_L | IFGoperc.L | Attention |
| 12 | Frontal_Inf_Oper_R | IFGoperc.R | Attention |
| 13 | Frontal_Inf_Tri_L | IFGtriang.L | Attention |
| 14 | Frontal_Inf_Tri_R | IFGtriang.R | Attention |
| 15 | Frontal_Inf_Orb_L | ORBinf.L | Attention |
| 16 | Frontal_Inf_Orb_R | ORBinf.R | Attention |
| 17 | Rolandic_Oper_L | ROL.L | Sensorimotor |
| 18 | Rolandic_Oper_R | ROL.R | Sensorimotor |
| 19 | Supp_Motor_Area_L | SMA.L | Attention |
| 20 | Supp_Motor_Area_R | SMA.R | Sensorimotor |
| 21 | Olfactory_L | OLF.L | Subcortical |
| 22 | Olfactory_R | OLF.R | DMN |
| 23 | Frontal_Sup_Medial_L | SFGmed.L | DMN |
| 24 | Frontal_Sup_Medial_R | SFGmed.R | DMN |
| 25 | Frontal_Mid_Orb_L | ORBsupmed.L | DMN |
| 26 | Frontal_Mid_Orb_R | ORBsupmed.R | DMN |
| 27 | Rectus_L | REC.L | DMN |
| 28 | Rectus_R | REC.R | DMN |
| 29 | Insula_L | INS.L | Sensorimotor |
| 30 | Insula_R | INS.R | Sensorimotor |
| 31 | Cingulum_Ant_L | ACG.L | DMN |
| 32 | Cingulum_Ant_R | ACG.R | DMN |
| 33 | Cingulum_Mid_L | DCG.L | Subcortical |
| 34 | Cingulum_Mid_R | DCG.R | Subcortical |
| 35 | Cingulum_Post_L | PCG.L | DMN |
| 36 | Cingulum_Post_R | PCG.R | DMN |
| 37 | Hippocampus_L | HIP.L | Subcortical |
| 38 | Hippocampus_R | HIP.R | Subcortical |
| 39 | ParaHippocampal_L | PHG.L | Subcortical |
| 40 | ParaHippocampal_R | PHG.R | Subcortical |
| 41 | Amygdala_L | AMYG.L | Subcortical |
| 42 | Amygdala_R | AMYG.R | Subcortical |
| 43 | Calcarine_L | CAL.L | Visual |
| 44 | Calcarine_R | CAL.R | Visual |
| 45 | Cuneus_L | CUN.L | Visual |
| 46 | Cuneus_R | CUN.R | Visual |
| 47 | Lingual_L | LING.L | Visual |
| 48 | Lingual_R | LING.R | Visual |
| 49 | Occipital_Sup_L | SOG.L | Visual |
| 50 | Occipital_Sup_R | SOG.R | Visual |
| 51 | Occipital_Mid_L | MOG.L | Visual |
| 52 | Occipital_Mid_R | MOG.R | Visual |
| 53 | Occipital_Inf_L | IOG.L | Visual |
| 54 | Occipital_Inf_R | IOG.R | Visual |
| 55 | Fusiform_L | FFG.L | Visual |
| 56 | Fusiform_R | FFG.R | Visual |
| 57 | Postcentral_L | PoCG.L | Sensorimotor |
| 58 | Postcentral_R | PoCG.R | Sensorimotor |
| 59 | Parietal_Sup_L | SPG.L | Sensorimotor |
| 60 | Parietal_Sup_R | SPG.R | Sensorimotor |
| 61 | Parietal_Inf_L | IPL.L | Attention |
| 62 | Parietal_Inf_R | IPL.R | Attention |
| 63 | SupraMarginal_L | SMG.L | Sensorimotor |
| 64 | SupraMarginal_R | SMG.R | Sensorimotor |
| 65 | Angular_L | ANG.L | Attention |
| 66 | Angular_R | ANG.R | Attention |
| 67 | Precuneus_L | PCUN.L | DMN |
| 68 | Precuneus_R | PCUN.R | DMN |
| 69 | Paracentral_Lobule_L | PCL.L | Sensorimotor |
| 70 | Paracentral_Lobule_R | PCL.R | Sensorimotor |
| 71 | Caudate_L | CAU.L | Subcortical |
| 72 | Caudate_R | CAU.R | Subcortical |
| 73 | Putamen_L | PUT.L | Subcortical |
| 74 | Putamen_R | PUT.R | Subcortical |
| 75 | Pallidum_L | PAL.L | Subcortical |
| 76 | Pallidum_R | PAL.R | Subcortical |
| 77 | Thalamus_L | THA.L | Subcortical |
| 78 | Thalamus_R | THA.R | Subcortical |
| 79 | Heschl_L | HES.L | Sensorimotor |
| 80 | Heschl_R | HES.R | Sensorimotor |
| 81 | Temporal_Sup_L | STG.L | Sensorimotor |
| 82 | Temporal_Sup_R | STG.R | Sensorimotor |
| 83 | Temporal_Pole_Sup_L | TPOsup.L | Attention |
| 84 | Temporal_Pole_Sup_R | TPOsup.R | Sensorimotor |
| 85 | Temporal_Mid_L | MTG.L | DMN |
| 86 | Temporal_Mid_R | MTG.R | DMN |
| 87 | Temporal_Pole_Mid_L | TPOmid.L | Subcortical |
| 88 | Temporal_Pole_Mid_R | TPOmid.R | Subcortical |
| 89 | Temporal_Inf_L | ITG.L | Attention |
| 90 | Temporal_Inf_R | ITG.R | DMN |
References
- Metzner, C.; Dimulescu, C.; Kamp, F.; Fromm, S.; Uhlhaas, P.J.; Obermayer, K. Exploring global and local processes underlying alterations in resting-state functional connectivity and dynamics in schizophrenia. Front. Psychiatry 2024, 15, 1352641. [Google Scholar] [CrossRef]
- Luo, Y.; He, H.; Duan, M.; Huang, H.; Hu, Z.; Wang, H.; Yao, G.; Yao, D.; Li, J.; Luo, C. Dynamic Functional Connectivity Strength Within Different Frequency-Band in Schizophrenia. Front. Psychiatry 2019, 10, 995. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Doucet, G.E.; Leibu, E.; Frangou, S. Resting-state network connectivity and metastability predict clinical symptoms in schizophrenia. Schizophr. Res. 2018, 201, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Farinha, M.; Amado, C.; Morgado, P.; Cabral, J. Increased Excursions to Functional Networks in Schizophrenia in the Absence of Task. Front. Neurosci. 2022, 16, 821179. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Xiang, J.; Wang, B.; Li, D.; Cheng, X.; Liu, T.; Cui, X. Frequency-Specific Analysis of the Dynamic Reconfiguration of the Brain in Patients with Schizophrenia. Brain Sci. 2022, 12, 727. [Google Scholar] [CrossRef] [PubMed]
- Zamani Esfahlani, F.; Faskowitz, J.; Slack, J.; Mišić, B.; Betzel, R.F. Local structure-function relationships in human brain networks across the lifespan. Nat. Commun. 2022, 13, 2053. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.M.; Pezzella, P.; Giuliani, L.; Fazio, L.; Mucci, A.; Perrottelli, A.; Blasi, G.; Amore, M.; Rocca, P.; Rossi, A.; et al. Resting-State Brain Activity Dysfunctions in Schizophrenia and Their Associations with Negative Symptom Domains: An fMRI Study. Brain Sci. 2023, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.; Lauriello, J. Schizophrenia: An Overview. Focus 2016, 14, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Lyne, J.; Kelly, B.D.; O’Connor, W.T. Schizophrenia: A review of neuropharmacology. Ir. J. Med. Sci. 2004, 173, 155–159. [Google Scholar] [CrossRef]
- Zhu, C.; Tan, Y.; Yang, S.; Miao, J.; Zhu, J.; Huang, H.; Yao, D.; Luo, C. Temporal Dynamic Synchronous Functional Brain Network for Schizophrenia Classification and Lateralization Analysis. IEEE Trans. Med. Imaging 2024. [Google Scholar] [CrossRef]
- Gifford, G.; Crossley, N.; Kempton, M.J.; Morgan, S.; Dazzan, P.; Young, J.; McGuire, P. Resting state fMRI based multilayer network configuration in patients with schizophrenia. Neuroimage Clin. 2020, 25, 102169. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cui, X.; Ding, C.; Li, D.; Cheng, C.; Wang, B.; Xiang, J. Deficit of Cross-Frequency Integration in Mild Cognitive Impairment and Alzheimer’s Disease: A Multilayer Network Approach. J. Magn. Reson. Imaging 2021, 53, 1387–1398. [Google Scholar] [CrossRef]
- Ding, C.; Xiang, J.; Cui, X.; Wang, X.; Li, D.; Cheng, C.; Wang, B. Abnormal Dynamic Community Structure of Patients with Attention-Deficit/Hyperactivity Disorder in the Resting State. J. Atten. Disord. 2022, 26, 34–47. [Google Scholar] [CrossRef]
- Lerman-Sinkoff, D.B.; Barch, D.M. Network community structure alterations in adult schizophrenia: Identification and localization of alterations. Neuroimage Clin. 2016, 10, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Wisnowski, J.L.; Leahy, R.M. Identification of overlapping and interacting networks reveals intrinsic spatiotemporal organization of the human brain. Neuroimage 2023, 270, 119944. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Potenza, M.N.; Calhoun, V.D.; Zhang, R.; Yip, S.W.; Wall, J.T.; Pearlson, G.D.; Worhunsky, P.D.; Garrison, K.A.; Moran, J.M. Large-scale functional network overlap is a general property of brain functional organization: Reconciling inconsistent fMRI findings from general-linear-model-based analyses. Neurosci. Biobehav. Rev. 2016, 71, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kang, U.; Chang, H.; Cho, K.H. The hidden community architecture of human brain networks. Sci. Rep. 2022, 12, 3540. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, L.; Zhang, Y.; Ma, J.; Yang, C.; Xiao, Y.; Shu, N.; Can, C.; Lin, Y.; Dai, Z. The overlapping modular organization of human brain functional networks across the adult lifespan. Neuroimage 2022, 253, 119125. [Google Scholar] [CrossRef] [PubMed]
- Yeo, B.T.; Krienen, F.M.; Chee, M.W.; Buckner, R.L. Estimates of segregation and overlap of functional connectivity networks in the human cerebral cortex. Neuroimage 2014, 88, 212–227. [Google Scholar] [CrossRef]
- Najafi, M.; McMenamin, B.W.; Simon, J.Z.; Pessoa, L. Overlapping communities reveal rich structure in large-scale brain networks during rest and task conditions. Neuroimage 2016, 135, 92–106. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, J.; Gu, Y.; Yang, S.; Li, L.M.W.; Dai, Z. Intrinsic overlapping modular organization of human brain functional networks revealed by a multiobjective evolutionary algorithm. Neuroimage 2018, 181, 430–445. [Google Scholar] [CrossRef]
- Vafaii, H.; Mandino, F.; Desrosiers-Grégoire, G.; O’Connor, D.; Shen, X.; Ge, X.; Herman, P.; Hyder, F.; Papademetris, X.; Chakravarty, M.; et al. Multimodal measures of spontaneous brain activity reveal both common and divergent patterns of cortical functional organization. Res. Sq. 2023, 15, 229. [Google Scholar] [CrossRef]
- Li, X.; Gan, J.Q.; Wang, H. Collective sparse symmetric non-negative matrix factorization for identifying overlapping communities in resting-state brain functional networks. Neuroimage 2018, 166, 259–275. [Google Scholar] [CrossRef]
- Mirzaei, S.; Soltanian-Zadeh, H. Overlapping brain Community detection using Bayesian tensor decomposition. J. Neurosci. Methods 2019, 318, 47–55. [Google Scholar] [CrossRef]
- Chen, N.; Shi, J.; Li, Y.; Ji, S.; Zou, Y.; Yang, L.; Yao, Z.; Hu, B. Decreased dynamism of overlapping brain sub-networks in Major Depressive Disorder. J. Psychiatr. Res. 2021, 133, 197–204. [Google Scholar] [CrossRef]
- Chao-Gan, Y.; Yu-Feng, Z. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Front. Syst. Neurosci. 2010, 4, 13. [Google Scholar] [CrossRef]
- Cordes, D.; Haughton, V.M.; Arfanakis, K.; Wendt, G.J.; Turski, P.A.; Moritz, C.H.; Quigley, M.A.; Meyerand, M.E. Mapping functionally related regions of brain with functional connectivity MR imaging. Am. J. Neuroradiol. 2000, 21, 1636–1644. [Google Scholar] [PubMed]
- Zuo, X.N.; Di Martino, A.; Kelly, C.; Shehzad, Z.E.; Gee, D.G.; Klein, D.F.; Castellanos, F.X.; Biswal, B.B.; Milham, M.P. The oscillating brain: Complex and reliable. Neuroimage 2010, 49, 1432–1445. [Google Scholar] [CrossRef] [PubMed]
- Stam, C.J. Modern network science of neurological disorders. Nat. Rev. Neurosci. 2014, 15, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, J.; Wang, L.; Chen, Z.J.; Yan, C.; Yang, H.; Tang, H.; Zhu, C.; Gong, Q.; Zang, Y.; et al. Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS ONE 2009, 4, e5226. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Wang, J.; He, Y. BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.H.; Fransson, P. A common framework for the problem of deriving estimates of dynamic functional brain connectivity. Neuroimage 2018, 172, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Fransson, P.; Schiffler, B.C.; Thompson, W.H. Brain network segregation and integration during an epoch-related working memory fMRI experiment. Neuroimage 2018, 178, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Skau, S.; Johansson, B.; Kuhn, H.G.; Thompson, W.H. Segregation over time in functional networks in prefrontal cortex for individuals suffering from pathological fatigue after traumatic brain injury. Front Neurosci 2022, 16, 972720. [Google Scholar] [CrossRef]
- Thompson, W.H.; Fransson, P. The frequency dimension of fMRI dynamic connectivity: Network connectivity, functional hubs and integration in the resting brain. Neuroimage 2015, 121, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Lathauwer, L.D.; Moor, B.D.; Vandewalle, J. A Multilinear Singular Value Decomposition. SIAM J. Matrix Anal. Appl. 2000, 21, 1253–1278. [Google Scholar] [CrossRef]
- Brunet, J.P.; Tamayo, P.; Golub, T.R.; Mesirov, J.P. Metagenes and molecular pattern discovery using matrix factorization. Proc. Natl. Acad. Sci. USA 2004, 101, 4164–4169. [Google Scholar] [CrossRef]
- Viol, A.; Palhano-Fontes, F.; Onias, H.; de Araujo, D.B.; Viswanathan, G.M. Shannon entropy of brain functional complex networks under the influence of the psychedelic Ayahuasca. Sci. Rep. 2017, 7, 7388. [Google Scholar] [CrossRef]
- Yuan, L.; Ma, X.; Li, D.; Ouyang, L.; Fan, L.; Li, C.; He, Y.; Chen, X. Alteration of a brain network with stable and strong functional connections in subjects with schizophrenia. Schizophrenia 2022, 8, 91. [Google Scholar] [CrossRef]
- Zhang, G.; Cai, B.; Zhang, A.; Tu, Z.; Xiao, L.; Stephen, J.M.; Wilson, T.W.; Calhoun, V.D.; Wang, Y.P. Detecting abnormal connectivity in schizophrenia via a joint directed acyclic graph estimation model. Neuroimage 2022, 260, 119451. [Google Scholar] [CrossRef]
- Li, J.; Xu, F.; Gao, N.; Zhu, Y.; Hao, Y.; Qiao, C. Sparse non-convex regularization based explainable DBN in the analysis of brain abnormalities in schizophrenia. Comput. Biol. Med. 2023, 155, 106664. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Suzumura, T.; Ji, H.; Hu, G. Finding overlapping communities in multilayer networks. PLoS ONE 2018, 13, e0188747. [Google Scholar] [CrossRef]
- Dai, W.; Qiu, E.; Lin, X.; Zhang, S.; Zhang, M.; Han, X.; Jia, Z.; Su, H.; Bian, X.; Zang, X.; et al. Abnormal Thalamo-Cortical Interactions in Overlapping Communities of Migraine: An Edge Functional Connectivity Study. Ann. Neurol. 2023, 94, 1168–1181. [Google Scholar] [CrossRef]
- Faskowitz, J.; Esfahlani, F.Z.; Jo, Y.; Sporns, O.; Betzel, R.F. Edge-centric functional network representations of human cerebral cortex reveal overlapping system-level architecture. Nat. Neurosci. 2020, 23, 1644–1654. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Zamani Esfahlani, F.; Faskowitz, J.; Chumin, E.J.; Sporns, O.; Betzel, R.F. The diversity and multiplexity of edge communities within and between brain systems. Cell Rep. 2021, 37, 110032. [Google Scholar] [CrossRef]
- Adámek, P.; Langová, V.; Horáček, J. Early-stage visual perception impairment in schizophrenia, bottom-up and back again. Schizophrenia 2022, 8, 27. [Google Scholar] [CrossRef]
- Teng, X.; Guo, C.; Lei, X.; Yang, F.; Wu, Z.; Yu, L.; Ren, J.; Zhang, C. Comparison of brain network between schizophrenia and bipolar disorder: A multimodal MRI analysis of comparative studies. J. Affect. Disord. 2023, 327, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Nachev, P.; Kennard, C.; Husain, M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008, 9, 856–869. [Google Scholar] [CrossRef]
- Okada, N.; Fukunaga, M.; Yamashita, F.; Koshiyama, D.; Yamamori, H.; Ohi, K.; Yasuda, Y.; Fujimoto, M.; Watanabe, Y.; Yahata, N.; et al. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol. Psychiatry 2016, 21, 1460–1466. [Google Scholar] [CrossRef]
| Characteristic | SZ | NC | Statistical Test |
|---|---|---|---|
| Number of subjects | 43 | 49 | – |
| Age (years) | 34.84 ± 8.60 | 33.12 ± 8.22 | p = 0.331 |
| Sex (male/female) | 30/13 | 30/19 | p = 0.112 |
| Full Name of the Brain Region | Brain Area Abbreviations | ROI | RSN |
|---|---|---|---|
| Frontal_Inf_Oper_L | IFGoperc.L | 11 | Attention Network |
| Frontal_Inf_Oper_R | IFGoperc.R | 12 | Attention Network |
| Rolandic_Oper_L | ROL.L | 17 | Sensorimotor Network |
| Olfactory_R | OLF.R | 22 | Default Network |
| ParaHippocampal_R | PHG.R | 40 | Limbic/paralimbic and Subcortical Networks |
| Amygdala_R | AMYG.R | 42 | Limbic/paralimbic and Subcortical Networks |
| Temporal_Sup_L | STG.L | 81 | Sensorimotor Network |
| Full Name of the Brain Region | Brain Area Abbreviations | ROI | RSN |
|---|---|---|---|
| Frontal_Mid_R | MFG.R | 8 | Attention Network |
| Frontal_Mid_Orb_L | ORBmid.L | 9 | Attention Network |
| Frontal_Inf_Oper_R | IFGoperc.R | 12 | Attention Network |
| Cingulum_Mid_L | DCG.L | 33 | Limbic/paralimbic and Subcortical Networks |
| Calcarine_L | CAL.L | 43 | Visual Network |
| Lingual_R | LING.R | 48 | Visual Network |
| Temporal_Inf_L | ITG.L | 89 | Attention Network |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Wu, X.; Sun, Y.; Dong, Y.; Sun, J.; Song, Z.; Xiang, J.; Cui, X. Abnormal Dynamic Reconstruction of Overlapping Communities in Schizophrenia Patients. Brain Sci. 2024, 14, 783. https://doi.org/10.3390/brainsci14080783
Guo Y, Wu X, Sun Y, Dong Y, Sun J, Song Z, Xiang J, Cui X. Abnormal Dynamic Reconstruction of Overlapping Communities in Schizophrenia Patients. Brain Sciences. 2024; 14(8):783. https://doi.org/10.3390/brainsci14080783
Chicago/Turabian StyleGuo, Yuxiang, Xubin Wu, Yumeng Sun, Yanqing Dong, Jie Sun, Zize Song, Jie Xiang, and Xiaohong Cui. 2024. "Abnormal Dynamic Reconstruction of Overlapping Communities in Schizophrenia Patients" Brain Sciences 14, no. 8: 783. https://doi.org/10.3390/brainsci14080783





