Correlates of Theta and Gamma Activity during Visuospatial Incidental/Intentional Encoding and Retrieval Indicate Differences in Processing in Young and Elderly Healthy Participants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
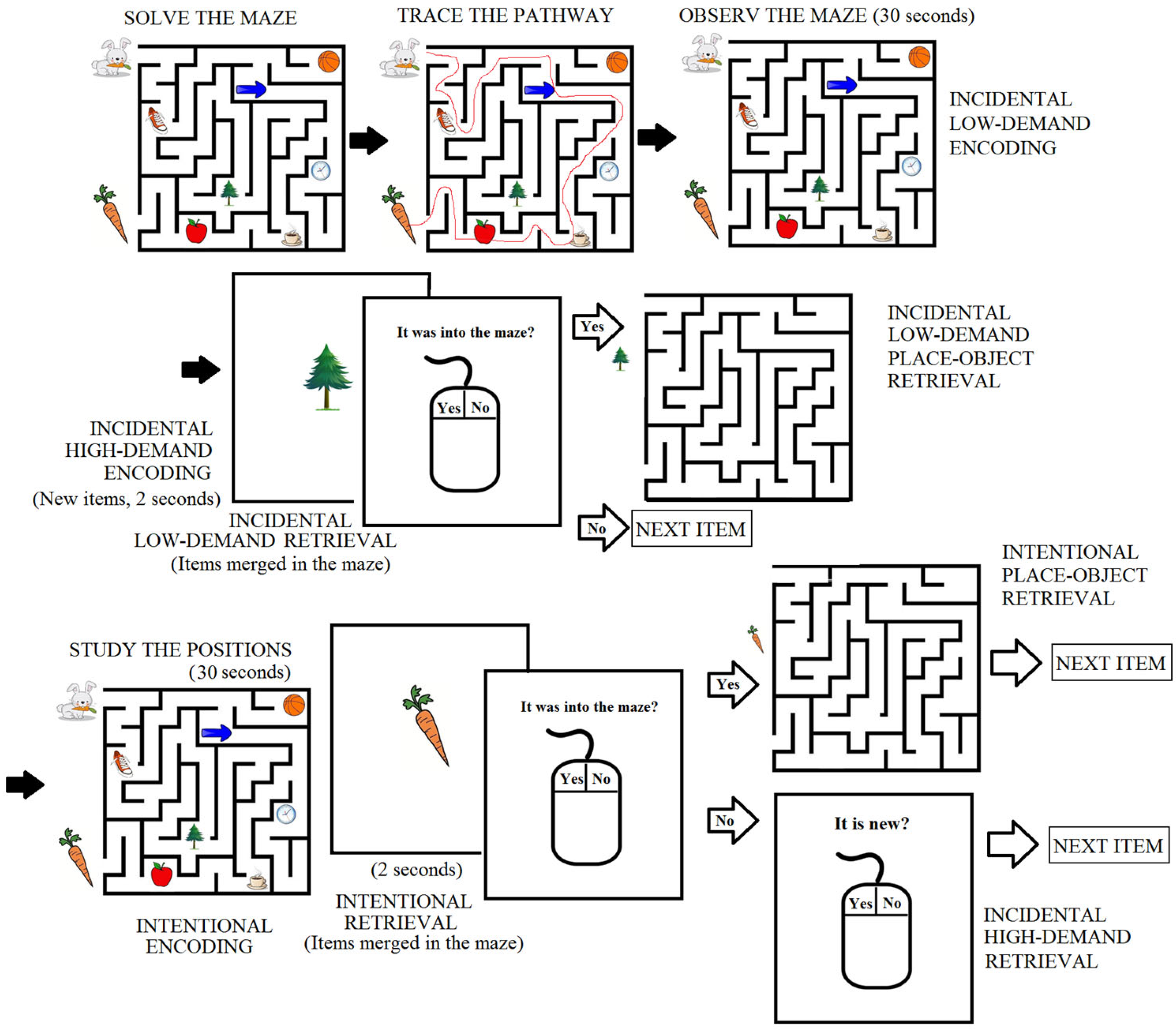
2.3. Incidental/Intentional Visuospatial Task
2.4. EEG Records
3. Results
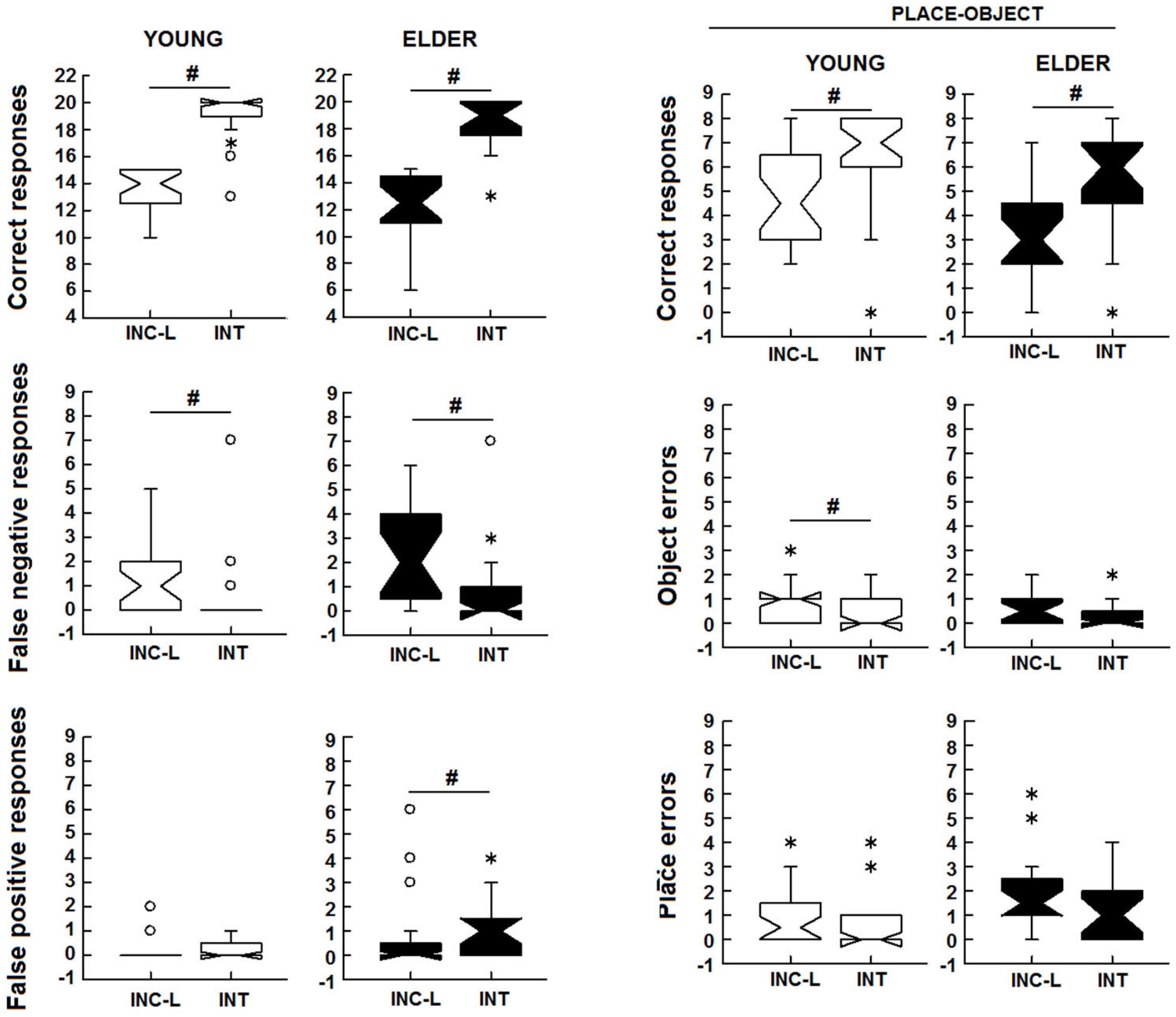
3.1. Incidental/Intentional Visuospatial Test
3.2. EEG Activity
3.2.1. Theta and Gamma Activity during Encoding
3.2.2. Theta and Gamma Activity at Retrieval
Theta and Gamma Power
Power Behavior Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasher, L.; Zacks, R.T. Working Memory, Comprehension, and Aging: A Review and a New View. Psychol. Learn. Motiv. 1988, 22, 193–225. [Google Scholar]
- Naveh-Benjamin, M. Adult age differences in memory performance: Tests of an associative deficit hypothesis. J. Exp. Psychol. Learn. Mem. Cogn. 2000, 26, 1170–1187. [Google Scholar] [CrossRef] [PubMed]
- Naveh-Benjamin, M.; Shing, Y.L.; Kilb, A.; Werkle-Bergner, M.; Lindenberger, U.; Li, S.C. Adult age differences in memory for name-face associations: The effects of intentional and incidental learning. Memory 2009, 17, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Rugg, M.D.; Fletcher, P.C.; Frith, C.D.; Frackowiak, R.S.; Dolan, R.J. Brain regions supporting intentional and incidental memory: A PET study. Neuroreport 1997, 8, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Caine, K.E.; Nichols, T.A.; Fisk, A.D.; Rogers, W.A.; Meyer, B. Age-related differences in learning incidental, environmental information. Exp. Aging Res. 2011, 37, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Loeza, E.; Rangel-Argueta, A.R.; Lopez-Vazquez, M.A.; Cervantes, M.; Olvera-Cortes, M.E. Differences in EEG power in young and mature healthy adults during an incidental/spatial learning task are related to age and execution efficiency. Age 2016, 38, 37. [Google Scholar] [CrossRef] [PubMed]
- Squire, L.R. The organization and neural substrates of human memory. Int. J. Neurol. 1987, 21–22, 218–222. [Google Scholar]
- Wagner, A.D.; Schacter, D.L.; Rotte, M.; Koutstaal, W.; Maril, A.; Dale, A.M.; Rosen, B.R.; Buckner, R.L. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science 1998, 281, 1188–1191. [Google Scholar] [CrossRef] [PubMed]
- Gabrieli, J.D.; Brewer, J.B.; Desmond, J.E.; Glover, G.H. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science 1997, 276, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Schacter, D.L.; Wagner, A.D. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus 1999, 9, 7–24. [Google Scholar] [CrossRef]
- Davachi, L.; Mitchell, J.P.; Wagner, A.D. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proc. Natl. Acad. Sci. USA 2003, 100, 2157–2162. [Google Scholar] [CrossRef] [PubMed]
- Schacter, D.L.; Curran, T.; Reiman, E.M.; Chen, K.; Bandy, D.J.; Frost, J.T. Medial temporal lobe activation during episodic encoding and retrieval: A PET study. Hippocampus 1999, 9, 575–581. [Google Scholar] [CrossRef]
- Dolan, R.J.; Fletcher, P.F. Encoding and retrieval in human medial temporal lobes: An empirical investigation using functional magnetic resonance imaging (fMRI). Hippocampus 1999, 9, 25–34. [Google Scholar] [CrossRef]
- Fuentemilla, L.; Barnes, G.R.; Duzel, E.; Levine, B. Theta oscillations orchestrate medial temporal lobe and neocortex in remembering autobiographical memories. Neuroimage 2014, 85 Pt 2, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Herweg, N.A.; Apitz, T.; Leicht, G.; Mulert, C.; Fuentemilla, L.; Bunzeck, N. Theta-Alpha Oscillations Bind the Hippocampus, Prefrontal Cortex, and Striatum during Recollection: Evidence from Simultaneous EEG-fMRI. J. Neurosci. 2016, 36, 3579–3587. [Google Scholar] [CrossRef] [PubMed]
- Nyhus, E.; Curran, T. Functional role of gamma and theta oscillations in episodic memory. Neurosci. Biobehav. Rev. 2010, 34, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Vogt, F.; Doppelmayr, M. Interindividual differences in alpha and theta power reflect memory performance. Intelligence 1999, 27, 347–362. [Google Scholar] [CrossRef]
- Klimesch, W.; Doppelmayr, M.; Schimke, H.; Ripper, B. Theta synchronization and alpha desynchronization in a memory task. Psychophysiology 1997, 34, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Muller, H.M.; Rappelsberger, P. Theta synchronization predicts efficient memory encoding of concrete and abstract nouns. Neuroreport 2000, 11, 2357–2361. [Google Scholar] [CrossRef] [PubMed]
- Gruber, T.; Muller, M.M. Oscillatory brain activity in the human EEG during indirect and direct memory tasks. Brain Res. 2006, 1097, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Doppelmayr, M.; Yonelinas, A.; Kroll, N.E.; Lazzara, M.; Rohm, D.; Gruber, W. Theta synchronization during episodic retrieval: Neural correlates of conscious awareness. Brain Res. Cogn. Brain Res. 2001, 12, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, C.; Mangels, J.A. Coherent theta-band EEG activity predicts item-context binding during encoding. Neuroimage 2005, 24, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Klass, D.W.; Brenner, R.P. Electroencephalography of the elderly. J. Clin. Neurophysiol. 1995, 12, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef] [PubMed]
- Jurado, S.; Villegas, E.M.; Mendez, L.; Rodríguez, F.; Loperena, V.; Varela, R. La estandarización del inventario de depresión de Beck para los residentes de la ciudad de México. Salud Ment. 1998, 21, 26–31. [Google Scholar]
- Ledesma, L.; García, P.; Salvador, J. Test MOCA, Versión Mexicana 7.2. Volume 2014. 2014. Available online: www.mocatest.org (accessed on 1 January 2014).
- Pons, J.I.; Pabón, L.F.; Matías-Carrelo, L.; Rodríguez, M.; Hernández, E.R.; Rodríguez, J.M.; Herrans, L.L.; Yang, J. Estudios de validez de la Escala de Inteligencia Wechsler para Adultos Versión III, Puerto Rico (EIWA-III). Rev. Puertorriqueña Psicol. 2008, 19, 75–111. [Google Scholar]
- Lara-Ramos, J.A.; Zavala-Santoyo, F.L.; Tellez-Anguiano, A.C.; Diaz-Huerta, J.L.; Olvera-Cortes, M.E. Computational interface to automate cognitive memory tests and synchronize with measured EEG signals. In Proceedings of the 2019 16th International Conference on Electrical Engineering, Computing Science and Automatic Control, CCE 2019, Mexico City, Mexico, 11–13 September 2019; Volume CCE 2019. IEEE: Mexico City, Mexico, 2019. [Google Scholar]
- Cansino, S.; Hernandez-Ramos, E.; Estrada-Manilla, C.; Torres-Trejo, F.; Martinez-Galindo, J.G.; Ayala-Hernandez, M.; Gomez-Fernandez, T.; Osorio, D.; Cedillo-Tinoco, M.; Garces-Flores, L.; et al. The decline of verbal and visuospatial working memory across the adult life span. Age 2013, 35, 2283–2302. [Google Scholar] [CrossRef] [PubMed]
- Old, S.R.; Naveh-Benjamin, M. Differential effects of age on item and associative measures of memory: A meta-analysis. Psychol. Aging 2008, 23, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Koster, M.; Haese, A.; Czernochowski, D. Neuronal oscillations reveal the processes underlying intentional compared to incidental learning in children and young adults. PLoS ONE 2017, 12, e0182540. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.; Bartlett, J.; Rose, R.; Gray, C. The aging eyewitness: Effects of age on face, delay, and source-memory ability. J. Gerontol. B Psychol. Sci. Soc. Sci. 2003, 58, P338–P345. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, H. Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron 2004, 44, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Yonelinas, A.P.; Kroll, N.E.; Quamme, J.R.; Lazzara, M.M.; Sauve, M.J.; Widaman, K.F.; Knight, R.T. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat. Neurosci. 2002, 5, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Yonelinas, A.P.; Levy, B.J. Dissociating familiarity from recollection in human recognition memory: Different rates of forgetting over short retention intervals. Psychon. Bull. Rev. 2002, 9, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Osipova, D.; Takashima, A.; Oostenveld, R.; Fernandez, G.; Maris, E.; Jensen, O. Theta and gamma oscillations predict encoding and retrieval of declarative memory. J. Neurosci. 2006, 26, 7523–7531. [Google Scholar] [CrossRef] [PubMed]
- Lega, B.C.; Jacobs, J.; Kahana, M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus 2012, 22, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, B.; Sala-Padro, J.; Cucurell, D.; Santurino, M.; Falip, M.; Fuentemilla, L. Theta rhythm supports hippocampus-dependent integrative encoding in schematic/semantic memory networks. Neuroimage 2021, 226, 117558. [Google Scholar] [CrossRef] [PubMed]
- Rondina, R., 2nd; Olsen, R.K.; McQuiggan, D.A.; Fatima, Z.; Li, L.; Oziel, E.; Meltzer, J.A.; Ryan, J.D. Age-related changes to oscillatory dynamics in hippocampal and neocortical networks. Neurobiol. Learn. Mem. 2016, 134 Pt A, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol. Aging 2002, 17, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; Anderson, N.D.; Locantore, J.K.; McIntosh, A.R. Aging gracefully: Compensatory brain activity in high-performing older adults. Neuroimage 2002, 17, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.S.; Maraver, M.J.; Hanslmayr, S.; Bajo, T. Theta oscillations show impaired interference detection in older adults during selective memory retrieval. Sci. Rep. 2019, 9, 9977. [Google Scholar] [CrossRef] [PubMed]
- Staudigl, T.; Hanslmayr, S.; Bauml, K.H. Theta oscillations reflect the dynamics of interference in episodic memory retrieval. J. Neurosci. 2010, 30, 11356–11362. [Google Scholar] [CrossRef]
- Amer, T.; Ngo, K.W.J.; Weeks, J.C.; Hasher, L. Spontaneous Distractor Reactivation with Age: Evidence for Bound Target-Distractor Representations in Memory. Psychol. Sci. 2020, 31, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Biss, R.K.; Campbell, K.L.; Hasher, L. Interference from previous distraction disrupts older adults’ memory. J. Gerontol. B Psychol. Sci. Soc. Sci. 2013, 68, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Gruber, T.; Muller, M.M. Oscillatory brain activity dissociates between associative stimulus content in a repetition priming task in the human EEG. Cereb. Cortex 2005, 15, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Zion-Golumbic, E.; Kutas, M.; Bentin, S. Neural dynamics associated with semantic and episodic memory for faces: Evidence from multiple frequency bands. J. Cogn. Neurosci. 2010, 22, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Barr, M.S.; Radhu, N.; Guglietti, C.L.; Zomorrodi, R.; Rajji, T.K.; Ritvo, P.; Daskalakis, Z.J. Age-related differences in working memory evoked gamma oscillations. Brain Res. 2014, 1576, 43–51. [Google Scholar] [CrossRef] [PubMed]



| Variable | Young (n = 28, 17 Women) | Elderly (n = 20, 13 Women) | t-Test/df | p |
|---|---|---|---|---|
| Age | 33.321 (±6.608) | 69.900 (±7.96) | −16.826, 36.1 | <0.001 |
| Years of schooling | 15.500 (±3.097) | 11.20 (±4.538) | 3.671, 31.3 | 0.001 |
| WAIS | 100.143 (±11.906) | 97.600 (±13.240) | 0.684, 1 | 0.498 |
| MoCA | 19.24 (±1.261) | 18.250 (±0.55) | 4.527, 1 | <0.001 |
| U test/df | ||||
| Raven | 2.5 (1–3) | 1.5 (1–3) | 159, 1 | 0.006 |
| Encoding | Retrieval | |||||||
|---|---|---|---|---|---|---|---|---|
| Young | Elderly | F | dCohen | Young | Elderly | F | dCohen | |
| Theta | ||||||||
| PF1 | 7.015 (0.173) | 8.81 (0.139) | 40.427 | 1.137 | 7.750 (0.141) | 8.769 (0.111) | 34.302 | 0.806 |
| F3 | 6.861 (0.170) | 8.643 (0.125) | 40.024 | 1.132 | 7.410 (0.122) | 8.498 (0.091) | 49.943 | 0.973 |
| F7 | 6.513 (0.185) | 8.507 (0.100) | 51.750 | 1.287 | 7.080 (0.123) | 8.57 (0.073) | 74.917 | 1.191 |
| T3 | 6.195 (0.183) | 7.904 (0.089) | 44.854 | 1.198 | 6.895 (0.151) | 7.765 (0.074) | 19.853 | 0.613 |
| T5 | 6.296 (0.199) | 7.882 (0.070) | 35.844 | 1.071 | 6.887 (0.136) | 7.801 (0.089) | 41.748 | 0.889 |
| FP2 | 7.015 (0.180) | 8.833 (0.140) | 38.311 | 1.107 | 7.645 (0.131) | 8.747 (0.098) | 41.508 | 0.887 |
| F4 | 6.853 (0.170) | 8.541 (0.145) | 32.063 | 1.013 | 7.34 (0.120) | 8.326 (0.082) | 37.646 | 0.844 |
| F8 | 6.317 (0.156) | 8.200 (0.108) | 52.827 | 1.300 | 6.787 (0.119) | 8.154 (0.071) | 73.475 | 1.180 |
| T4 | 5.921 (0.184) | 7.887 (0.096) | 45.437 | 1.200 | 6.563 (0.129) | 7.706 (0.071) | 37.163 | 0.839 |
| T6 | 6.356 (0.153) | 7.952 (0.069) | 69.953 | 1.496 | 6.882 (0.115) | 7.825 (0.080) | 61.497 | 1.079 |
| Gamma | ||||||||
| PF1 | 4.773 (0.215) | 7.719 (0.124) | 101.623 | 1.803 | 5.904 (0.183) | 7.747 (0.094) | 75.692 | 1.197 |
| F3 | 4.700 (0.208) | 7.365 (0.117) | 88.201 | 1.650 | 5.708 (0.181) | 7.430 (0.091) | 63.758 | 1.099 |
| F7 | 4.607 (0.213) | 7.148 (0.119) | 65.941 | 1.452 | 5.571 (0.178) | 7.411 (0.100) | 67.350 | 1.130 |
| T3 | 5.013 (0.221) | 7.393 (0.109) | 63.023 | 1.420 | 6.109 (0.183) | 7.512 (0.071) | 37.027 | 0.873 |
| T5 | 4.515 (0.218) | 7.179 (0.113) | 90.588 | 1.702 | 5.634 (0.187) | 7.289 (0.068) | 72.607 | 1.173 |
| FP2 | 4.675 (0.221) | 7.400 (0.122) | 92.778 | 1.723 | 5.817 (0.179) | 7.562 (0.095) | 65.731 | 1.116 |
| F4 | 4.734 (0.212) | 7.091 (0.112) | 52.654 | 1.298 | 5.706 (0.179) | 7.247 (0.086) | 44.488 | 0.918 |
| F8 | 4.532 (0.209) | 6.815 (0.117) | 61.433 | 1.402 | 5.435 (0.170) | 7.110 (0.090) | 58.620 | 1.054 |
| T4 | 4.862 (0.232) | 7.482 (0.130) | 49.279 | 1.256 | 5.724 (0.181) | 7.551 (0.094) | 43.710 | 0.910 |
| T6 | 4.401 (0.203) | 7.499 (0.146) | 124.997 | 2.000 | 5.51 (0.174) | 7.539 (0.080) | 127.36 | 1.553 |
| Encoding | Retrieval | |||||
|---|---|---|---|---|---|---|
| F | p | dCohen | F | p | dCohen | |
| Theta | ||||||
| PF1 | 5.465 | 0.001 | 0.759 | 3.318 | 0.004 | 0.592 |
| F3 | 5.459 | 0.001 | 0.759 | 4.875 | <0.001 | 0.717 |
| F7 | 4.730 | 0.004 | 0.707 | 6.007 | <0.001 | 0.796 |
| T3 | 5.789 | 0.001 | 0.782 | 5.233 | <0.001 | 0.743 |
| T5 | 3.974 | 0.010 | 0.648 | 3.544 | 0.002 | 0.612 |
| FP2 | 4.871 | 0.003 | 0.717 | 3.126 | 0.006 | 0.574 |
| F4 | 5.360 | 0.002 | 0.752 | 3.276 | 0.004 | 0.588 |
| F8 | 5.048 | 0.002 | 0.730 | 8.260 | <0.001 | 0.934 |
| T4 | 4.016 | 0.009 | 0.651 | 4.699 | <0.001 | 0.704 |
| T6 | 8.485 | <0.001 | 0.946 | 6.052 | <0.001 | 0.799 |
| Gamma | ||||||
| PF1 | 15.810 | <0.001 | 1.292 | 13.572 | <0.001 | 1.197 |
| F3 | 22.304 | <0.001 | 1.534 | 19.619 | <0.001 | 1.143 |
| F7 | 17.060 | <0.001 | 1.342 | 15.561 | <0.001 | 1.281 |
| T3 | 16.885 | <0.001 | 1.335 | 13.298 | <0.001 | 1.185 |
| T5 | 17.031 | <0.001 | 1.341 | 14.232 | <0.001 | 1.226 |
| FP2 | 12.216 | <0.001 | 1.135 | 12.059 | <0.001 | 1.128 |
| F4 | 15.423 | <0.001 | 1.276 | 13.847 | <0.001 | 1.209 |
| F8 | 14.950 | <0.001 | 1.256 | 14.677 | <0.001 | 1.245 |
| T4 | 9.512 | <0.001 | 1.002 | 7.524 | <0.001 | 0.891 |
| T6 | 15.445 | <0.001 | 1.277 | 19.599 | <0.001 | 1.438 |
| Theta | Gamma | |||||||
|---|---|---|---|---|---|---|---|---|
| Incidental Low-Demand | ||||||||
| Age-adjusted | Age-adjusted | |||||||
| r, p | r, p | qCohen | r, p | r, p | qCohen | |||
| CR | T3 T4 | −0.373, 0.032 −0.354, 0.034 | −0.257, 0.097 −0.254, 0.099 | 0.129 0.110 | T3 T4 | −0.321, 0.033 −0.309, 0.034 | −0.191, 0.109 −0.213, 0.102 | 0.139 0.103 |
| FNE | Fp1 Fp2 | 0.350, 0.034 0.342, 0.036 | 0.235, 0.123 0.238, 0.119 | 0.126 0.114 | T3 | 0.299, 0.048 | 0.147, 0.222 | 0.160 |
| P-O CR | F4 T3 T4 | −0.289, 0.041 −0.376, 0.011 −0.304, 0.037 | −0.158, 0.182 −0.226, 0.055 −0.191, 0.144 | 0.138 0.165 0.121 | ||||
| P-O E P-O FNE | T3 T4 | 0.338, 0.024 0.294, 0.044 | 0.170, 0.154 0.170, 0.196 | 0.180 0.131 | ||||
| Incidental High-Demand | ||||||||
| CR FPE | T6 | −0.438, 0.017 | 0.003, 0.981 | 0.473 | Fp1 Fp2 F3 F4 T3 T4 Fp1 Fp2 F4 T3 T6 | −0.393,0.027 −0.409, 0.020 −0.364, 0.048 −0.249, 0.160 −0.242, 0.195 −0.158, 0.367 0.369, 0.040 0.404, 0.021 0.126, 0.487 0.070, 0.713 0.371, 0.042 | 0.067, 0.613 0.057, 0.652 0.182, 0.114 0.257, 0.027 0.274, 0.032 0.292, 0.025 0.023, 0.862 0.056, 0.654 −0.264, 0.022 −0.337, 0.006 0.007, 0.954 | 0.482 0.491 0.566 0.517 0.528 0.460 0.410 0.372 0.397 0.421 0.397 |
| Intentional | ||||||||
| PE | T3 T6 | 0.332, 0.027 0.294, 0.047 | 0.163, 0.184 0.105, 0.348 | 0.181 0.198 | ||||
| Behavioral Variable | ||||||||
| Theta | Gamma | |||||||
|---|---|---|---|---|---|---|---|---|
| Incidental Low-Demand | ||||||||
| Age-adjusted | Age-adjusted | |||||||
| r, p | r, p | qCohen | r, p | r, p | qCohen | |||
| FPE | F8 T3 T4 | 0.359, 0.079 0.384, 0.059 0.393, 0.044 | 0.335, 0.026 0.367, 0.042 0.381, 0.042 | 0.027 0.020 0.014 | F7 F8 | 0.257, 0.087 0.252, 0.105 | 0.236, 0.050 0.226, 0.029 | 0.022 0.028 |
| PE | Fp1 F3 F4 F7 F8 T3 T4 | 0.415, 0.046 0.440, 0.023 0.474, 0.015 0.420, 0.042 0.401, 0.046 0.518, 0.007 0.429, 0.025 | 0.355, 0.087 0.354, 0.054 0.403, 0.034 0.300, 0.099 0.255, 0.105 0.419, 0.017 0.362, 0.055 | 0.070 0.102 0.088 0.138 0.164 0.127 0.079 | Fp2 F4 F7 F8 | 0.350, 0.038 0.280, 0.022 0.374, 0.007 0.372, 0.010 | 0.255, 0.072 0.205, 0.036 0.293, 0.010 0.267, 0.007 | 0.105 0.080 0.091 0.117 |
| P-O CR | F3 F4 | −0.295, 0.033 −0.324, 0.025 | −0.273, 0.051 −0.292, 0.046 | 0.024 0.035 | ||||
| Intentional | ||||||||
| P-O CR | Fp2 F3 F7 T3 T5 | −0.338, 0.047 −0.356, 0.037 −0.358, 0.025 −0.396, 0.017 −0.336, 0.047 | −0.111, 0.473 −0.141, 0.370 −0.119, 0.397 −0.237, 0.138 −0.103, 0.500 | 0.240 0.230 0.255 0.177 0.246 | ||||
| Behavioral Variable | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junco-Muñoz, M.L.; Mejía-Rodríguez, O.; Cervantes-Alfaro, J.M.; Téllez-Anguiano, A.d.C.; López-Vázquez, M.Á.; Olvera-Cortés, M.E. Correlates of Theta and Gamma Activity during Visuospatial Incidental/Intentional Encoding and Retrieval Indicate Differences in Processing in Young and Elderly Healthy Participants. Brain Sci. 2024, 14, 786. https://doi.org/10.3390/brainsci14080786
Junco-Muñoz ML, Mejía-Rodríguez O, Cervantes-Alfaro JM, Téllez-Anguiano AdC, López-Vázquez MÁ, Olvera-Cortés ME. Correlates of Theta and Gamma Activity during Visuospatial Incidental/Intentional Encoding and Retrieval Indicate Differences in Processing in Young and Elderly Healthy Participants. Brain Sciences. 2024; 14(8):786. https://doi.org/10.3390/brainsci14080786
Chicago/Turabian StyleJunco-Muñoz, Mariana Lizeth, Oliva Mejía-Rodríguez, José Miguel Cervantes-Alfaro, Adriana del Carmen Téllez-Anguiano, Miguel Ángel López-Vázquez, and María Esther Olvera-Cortés. 2024. "Correlates of Theta and Gamma Activity during Visuospatial Incidental/Intentional Encoding and Retrieval Indicate Differences in Processing in Young and Elderly Healthy Participants" Brain Sciences 14, no. 8: 786. https://doi.org/10.3390/brainsci14080786






