Alzheimer’s Disease Related Biomarkers Were Associated with Amnestic Cognitive Impairment in Parkinson’s Disease: A Cross-Sectional Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Clinical Assessments
2.2. Neuropsychological Evaluations
2.3. Sample Collection and Assay of Plasma Biomarkers
2.4. Enrichment of Neuronal EVs and Assay of Biomarkers
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
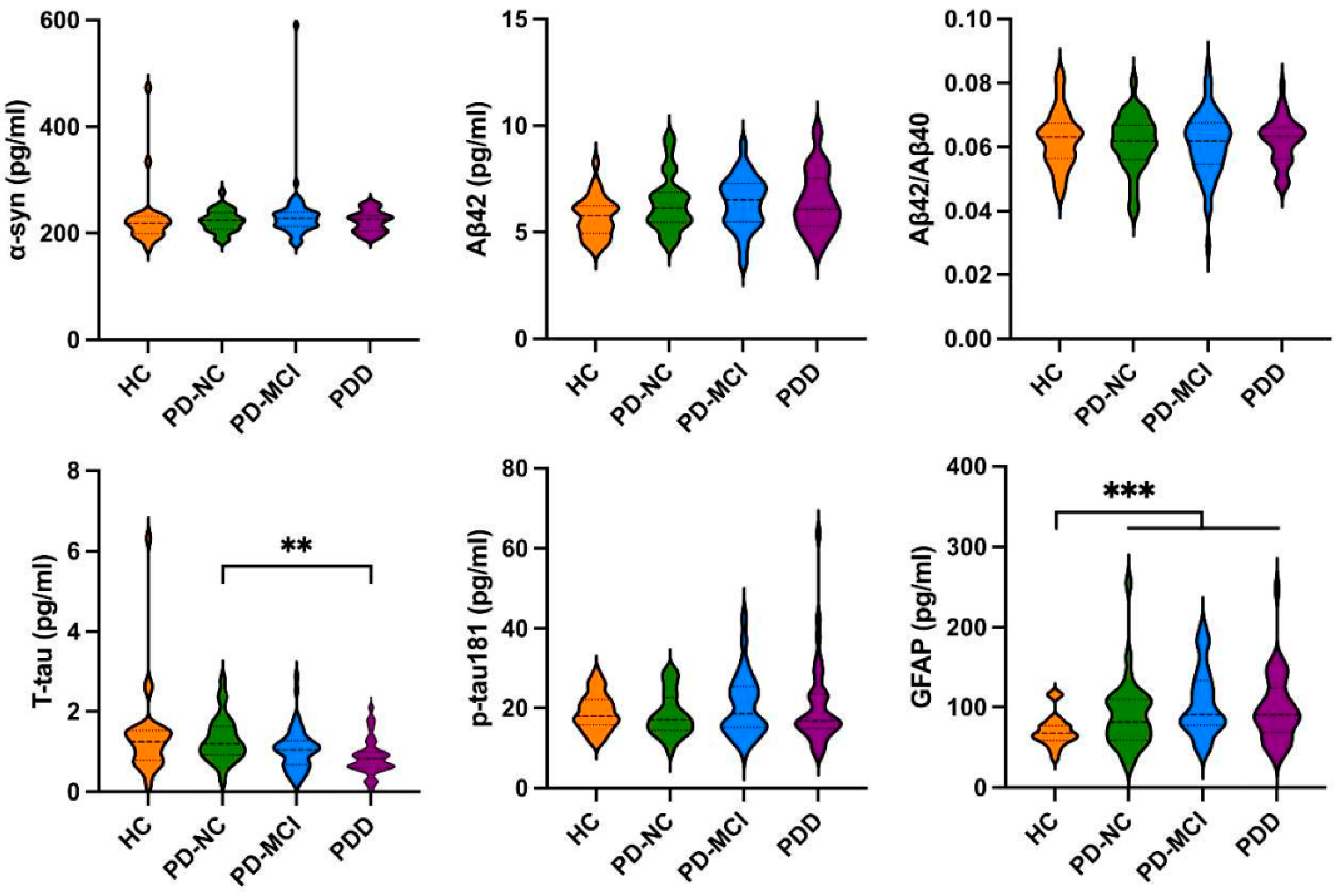
3.2. The Levels of Biomarkers in HCs and PD Patients with Different Cognitive Impairment
3.3. The Levels of Biomarkers in Amnestic and Non-Amnestic PD Patients
3.4. Correlation between Clinical Characteristic and Levels of Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams-Gray, C.H.; Mason, S.L.; Evans, J.R.; Foltynie, T.; Brayne, C.; Robbins, T.W.; Barker, R.A. The Cam-PaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1258–1264. [Google Scholar] [CrossRef]
- Chan, D.K.Y.; Chen, J.; Chen, R.F.; Parikh, J.; Xu, Y.H.; Silburn, P.A.; Mellick, G.D. Plasma biomarkers inclusive of α-synuclein/amyloid-beta40 ratio strongly correlate with Mini-Mental State Examination score in Parkinson’s disease and predict cognitive impairment. J. Neurol. 2022, 269, 6377–6385. [Google Scholar] [CrossRef]
- Irwin, D.J.; White, M.T.; Toledo, J.B.; Xie, S.X.; Robinson, J.L.; Van Deerlin, V.; Lee, V.M.Y.; Leverenz, J.B.; Montine, T.J.; Duda, J.E.; et al. Neuropathologic substrates of Parkinson disease dementia. Ann. Neurol. 2012, 72, 587–598. [Google Scholar] [CrossRef]
- Pagonabarraga, J.; Pérez-González, R.; Bejr-Kasem, H.; Marín-Lahoz, J.; Horta-Barba, A.; Martinez-Horta, S.; Aracil-Bolaños, I.; Sampedro, F.; Campolongo, A.; Rivas, E.; et al. Dissociable contribution of plasma NfL and p-tau181 to cognitive impairment in Parkinson’s disease. Park. Relat. Disord. 2022, 105, 132–138. [Google Scholar] [CrossRef]
- Tang, Y.; Han, L.; Li, S.; Hu, T.; Xu, Z.; Fan, Y.; Liang, X.; Yu, H.; Wu, J.; Wang, J. Plasma GFAP in Parkinson’s disease with cognitive impairment and its potential to predict conversion to dementia. NPJ Park. Dis. 2023, 9, 23. [Google Scholar] [CrossRef]
- Halliday, G.M.; McCann, H. The progression of pathology in Parkinson’s disease. Ann. N. Y. Acad. Sci. 2010, 1184, 188–195. [Google Scholar] [CrossRef]
- Ballard, C.; Ziabreva, I.; Perry, R.; Larsen, J.P.; O′Brien, J.; McKeith, I.; Perry, E.; Aarsland, D. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology 2006, 67, 1931–1934. [Google Scholar] [CrossRef]
- Mao, S.; Teng, X.; Li, Z.; Zu, J.; Zhang, T.; Xu, C.; Cui, G. Association of serum neurofilament light chain and glial fibrillary acidic protein levels with cognitive decline in Parkinson’s disease. Brain Res. 2023, 1805, 148271. [Google Scholar] [CrossRef]
- Shim, Y. Clinical Application of Plasma Neurofilament Light Chain in a Memory Clinic: A Pilot Study. Dement. Neurocogn. Disord. 2022, 21, 59–70. [Google Scholar] [CrossRef]
- Mizutani, Y.; Ohdake, R.; Tatebe, H.; Higashi, A.; Shima, S.; Ueda, A.; Ito, M.; Tokuda, T.; Watanabe, H. Associations of Alzheimer′s-related plasma biomarkers with cognitive decline in Parkinson′s disease. J. Neurol. 2023, 270, 5461–5474. [Google Scholar] [CrossRef]
- Chung, C.-C.; Chan, L.; Chen, J.-H.; Bamodu, O.A.; Hong, C.-T. Neurofilament light chain level in plasma extracellular vesicles and Parkinson’s disease. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420975917. [Google Scholar] [CrossRef]
- Joo, H.S.; Jeon, H.Y.; Hong, E.B.; Kim, H.Y.; Lee, J.M. Exosomes for the diagnosis and treatment of dementia. Curr. Opin. 2023, 36, 119–125. [Google Scholar] [CrossRef]
- Lim, Y.-J.; Lee, S.-J. Are exosomes the vehicle for protein aggregate propagation in neurodegenerative diseases? Acta Neuropathol. Commun. 2017, 5, 64. [Google Scholar] [CrossRef]
- Sharma, V.; Nikolajeff, F.; Kumar, S. Employing nanoparticle tracking analysis of salivary neuronal exosomes for early detection of neurodegenerative diseases. Transl. Neurodegener. 2023, 12, 7. [Google Scholar] [CrossRef]
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Parkinson disease- associated cognitive impairment. Nat. Rev. Dis. Primers 2021, 7, 47. [Google Scholar] [CrossRef]
- Litvan, I.; Goldman, J.G.; Tröster, A.I.; Schmand, B.A.; Weintraub, D.; Petersen, R.C.; Mollenhauer, B.; Adler, C.H.; Marder, K.; Williams-Gray, C.H.; et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 2012, 27, 349–356. [Google Scholar] [CrossRef]
- Emre, M.; Aarsland, D.; Brown, R.; Burn, D.J.; Duyckaerts, C.; Mizuno, Y.; Broe, G.A.; Cummings, J.; Dickson, D.W.; Gauthier, S.; et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 2007, 22, 1689–1707. [Google Scholar] [CrossRef]
- Sabbagh, M.N.; Adler, C.H.; Lahti, T.J.; Connor, D.J.; Vedders, L.; Peterson, L.K.; Caviness, J.N.; Shill, H.A.; Sue, L.I.; Ziabreva, I.; et al. Parkinson disease with dementia: Comparing patients with and without Alzheimer pathology. Alzheimer Dis. Assoc. Disord. 2009, 23, 295–297. [Google Scholar] [CrossRef]
- Compta, Y.; Parkkinen, L.; O’Sullivan, S.S.; Vandrovcova, J.; Holton, J.L.; Collins, C.; Lashley, T.; Kallis, C.; Williams, D.R.; de Silva, R.; et al. Lewy- and Alzheimer-type pathologies in Parkinson′s disease dementia: Which is more important? Brain 2011, 134, 1493–1505. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Dalrymple-Alford, J.C.; MacAskill, M.R.; Nakas, C.T.; Livingston, L.; Graham, C.; Crucian, G.P.; Melzer, T.R.; Kirwan, J.; Keenan, R.; Wells, S.; et al. The MoCA: Well-suited screen for cognitive impairment in Parkinson’s disease. Neurology 2010, 75, 1717–1725. [Google Scholar] [CrossRef]
- Wu, Z.; Xue, X.; Huang, A.; An, J.; Liu, G.; Zhang, L.; Xu, B.; Feng, H.; Chan, P.; Xu, E. Cut-off values of cognitive domain assessment scales for Parkinson disease in Beijing. China J. Apoplexy Nerv. Dis. 2024, 41, 408–412. [Google Scholar]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Sollinger, A.B.; Goldstein, F.C.; Lah, J.J.; Levey, A.I.; Factor, S.A. Mild cognitive impairment in Parkinson’s disease: Subtypes and motor characteristics. Park. Relat. Disord. 2010, 16, 177–180. [Google Scholar] [CrossRef]
- Poletti, M.; Frosini, D.; Pagni, C.; Baldacci, F.; Nicoletti, V.; Tognoni, G.; Lucetti, C.; Del Dotto, P.; Ceravolo, R.; Bonuccelli, U. Mild cognitive impairment and cognitive-motor relationships in newly diagnosed drug-naive patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2012, 83, 601–606. [Google Scholar] [CrossRef]
- Goldman, J.G.; Weis, H.; Stebbins, G.; Bernard, B.; Goetz, C.G. Clinical differences among mild cognitive impairment subtypes in Parkinson’s disease. Mov. Disord. 2012, 27, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Domellöf, M.E.; Ekman, U.; Forsgren, L.; Elgh, E. Cognitive function in the early phase of Parkinson’s disease, a five-year follow-up. Acta Neurol. Scand. 2015, 132, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kehagia, A.A.; Barker, R.A.; Robbins, T.W. Cognitive impairment in Parkinson’s disease: The dual syndrome hypothesis. Neurodegener. Dis. 2013, 11, 79–92. [Google Scholar] [CrossRef]
- Fatemeh, N. Emamzadeh and Andrei Surguchov. Parkinson’s disease: Biomarkers, treatment, and risk factors. Front. Neurol. 2018, 12, 612. [Google Scholar]
- Siderowf, A.; Xie, S.X.; Hurtig, H.; Weintraub, D.; Duda, J.; Chen-Plotkin, A.; Shaw, L.M.; Van Deerlin, V.; Trojanowski, J.Q.; Clark, C. CSF amyloid β 1-42 predicts cognitive decline in Parkinson disease. Neurology 2010, 75, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xie, Z.; Zhang, X.; Mao, J.; Wang, M.; Wei, S.; Fu, Y.; Zheng, H.; He, Y.; Chen, H.; et al. Investigation of alpha-synuclein species in plasma exosomes and the oligomeric and phosphorylated alpha-synuclein as potential peripheral biomarker of Parkinson’s disease. Neuroscience 2021, 469, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.J.; Lee, V.M.Y.; Trojanowski, J.Q. Parkinson’s disease dementia: Convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat. Rev. Neurosci. 2013, 14, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.; Raycheva, M.; Traykov, L. Cognitive profile of the earliest stage of dementia in Parkinson′s disease. Am. J. Alzheimers Dis. Other Demen. 2012, 27, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Papez, J.W. A proposed mechanism of emotion. J. Neuropsychiatry Clin. Neurosci. 1937, 7, 103–112. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer’s Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Kotzbauer, P.T.; Cairns, N.J.; Campbell, M.C.; Wills, A.W.; Racette, B.A.; Tabbal, S.D.; Perlmutter, J.S. Pathologic accumulation of alphasynuclein and Abeta in Parkinson disease patients with dementia. Arch. Neurol. 2012, 69, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Alves, G.; Brønnick, K.; Aarsland, D.; Blennow, K.; Zetterberg, H.; Ballard, C.; Kurz, M.W.; Andreasson, U.; Tysnes, O.B.; Larsen, J.P.; et al. CSF amyloid-beta and tau proteins, and cognitive performance, in early and untreated Parkinson’s disease: The Norwegian ParkWest study. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Ejlerskov, P.; Rasmussen, I.; Nielsen, T.T.; Bergström, A.-L.; Tohyama, Y.; Jensen, P.H.; Vilhardt, F. Tubulin polymerization-promoting protein (TPPP/p25 alpha) promotes unconventional secretion of alpha-Synuclein through Exophagy by impairing Autophagosome-lysosome fusion. J. Biol. Chem. 2013, 288, 17313–17335. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Honsho, M.; Zahn, T.R.; Keller, P.; Geiger, K.D.; Verkade, P.; Simons, K. Alzheimer′s disease beta-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. USA 2006, 103, 11172–11177. [Google Scholar] [CrossRef]
- Zhang, Z.; Zoltewicz, J.S.; Mondello, S.; Newsom, K.J.; Yang, Z.; Yang, B.; Kobeissy, F.; Guingab, J.; Glushakova, O.; Robicsek, S.; et al. Human traumatic brain injury induces autoantibody response against glial fibrillary acidic protein and its breakdown products. PLoS ONE 2014, 9, e92698. [Google Scholar] [CrossRef]
- Hansson, O.; Janelidze, S.; Hall, S.; Magdalinou, N.; Lees, A.J.; Andreasson, U.; Norgren, N.; Linder, J.; Forsgren, L.; Constantinescu, R.; et al. Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology 2017, 88, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Elahi, F.M.; Casaletto, K.B.; La Joie, R.; Walters, S.M.; Harvey, D.; Wolf, A.; Edwards, L.; Rivera-Contreras, W.; Karydas, A.; Cobigo, Y.; et al. Plasma biomarkers of astrocytic and neuronal dysfunction in early- and late-onset Alzheimer′s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2020, 16, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Li, C.H.; Yang, K.C.; Lin, F.J.; Wu, C.C.; Chieh, J.J.; Chiu, M.J. Blood NfL: A biomarker for disease severity and progression in Parkinson disease. Neurology 2019, 93, e1104–e1111. [Google Scholar] [CrossRef] [PubMed]
- Oeckl, P.; Halbgebauer, S.; Anderl-Straub, S.; Steinacker, P.; Huss, A.M.; Neugebauer, H.; von Arnim, C.A.; Diehl-Schmid, J.; Grimmer, T.; Kornhuber, J.; et al. Glial fibrillary acidic protein in serum is in- creased in Alzheimer’s disease and correlates with cognitive im- pairment. J. Alzheimer’s Dis. 2019, 67, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Imarisio, A.; Conforti, F.; Scalvini, A.; Masciocchi, S.; Nocivelli, S.; Turrone, R.; Gipponi, S.; Cottini, E.; Borroni, B.; et al. Plasma NfL clinical subtypes and motor progression in Parkinson′s disease. Park. Relat. Disord. 2021, 87, 41–47. [Google Scholar] [CrossRef]
- Schulz, I.; Kruse, N.; Gera, R.G.; Kremer, T.; Cedarbaum, J.; Barbour, R.; Zago, W.; Schade, S.; Otte, B.; Bartl, M.; et al. Systematic Assessment of 10 Biomarker Candidates Focusing on alpha-Synuclein-Related Disorders. Mov. Disord. 2021, 36, 2874–2887. [Google Scholar] [CrossRef]

| HC (n = 30) | PD (n = 122) | χ2/p | PD-NC (n = 31) | PD-MCI (n = 56) | PDD (n = 35) | χ2/p | |
|---|---|---|---|---|---|---|---|
| Age, years | 61.670 ± 5.585 | 64.010 ± 8.505 | 0.071 | 59.390 ± 10.026 | 64.910 ± 7.655 b | 66.660 ± 6.778 a,h | 0.001 |
| Sex, male (%) | 9 (30.0%) | 59 (48.4%) | 0.101 | 10 (32.3%) | 33 (58.9%) a,b | 16 (45.7%) | 0.029 |
| Education, years | 13.570 ± 2.555 | 12.480 ± 2.814 | 0.055 | 14.230 ± 1.961 | 12.640 ± 2.888 | 10.660 ± 2.248 g,h,f | <0.001 |
| Disease duration, years | NA | 5.050 ± 3.836 | NA | 4.900 ± 3.922 | 4.690 ± 3.780 | 5.840 ± 3.865 | 0.402 |
| Hoehn and Yahr score | NA | 3.040 ± 1.366 | NA | 2.460 ± 1.029 | 2.790 ± 1.109 | 4.000 ± 1.581 h,i | <0.001 |
| MDS-UPDRSII score | NA | 9.790 ± 7.511 | NA | 7.310 ± 5.204 | 9.200 ± 6.350 | 13.130 ± 9.895 b | 0.020 |
| MDS-UPDRSIII score | NA | 30.160 ± 15.222 | NA | 22.440 ± 11.140 | 28.340 ± 12.430 | 40.660 ± 17.715 h,i | <0.001 |
| HAMD score | NA | 8.190 ± 5.985 | NA | 6.300 ± 5.664 | 8.670 ± 5.796 | 9.160 ± 6.393 | 0.124 |
| ESS score | NA | 4.000 ± 3.622 | NA | 3.170 ± 2.791 | 3.960 ± 3.692 | 4.840 ± 3.348 | 0.279 |
| Amnestic patient (%) | NA | 47 (38.5%) | NA | 9 (29.0%) | 38(67.9%) e | NA | 0.001 |
| Plasma/Neuronal EV Biomarkers (pg/mL) | Non-Amnestic PD-NC (n = 21) | Amnestic PD-NC (n = 10) | p | Non-Amnestic PD-MCI (n = 14) | Amnestic PD-MCI (n = 42) | p |
|---|---|---|---|---|---|---|
| α-syn | 223.997 ± 21.282 | 222.519 ± 1.366 | 0.857 | 219.032 ± 27.335 | 235.714 ± 58.721 | 0.327 |
| Aβ42 | 6.690 ± 1.452 | 5.579 ± 0.761 | 0.031 | 6.360 ± 1.382 | 6.403 ± 1.208 | 0.911 |
| Aβ42/Aβ40 | 0.061 ± 0.010 | 0.062 ± 0.008 | 0.770 | 0.061 ± 0.010 | 0.061 ± 0.010 | 0.883 |
| T-tau | 1.250 ± 0.628 | 1.183 ± 0.550 | 0.775 | 0.985 ± 0.419 | 1.038 ± 0.556 | 0.749 |
| p-tau181 | 17.941 ± 6.794 | 17.645 ± 5.519 | 0.905 | 18.147 ± 5.855 | 20.860 ± 8.853 | 0.289 |
| GFAP | 93.653 ± 49.504 | 84.204 ± 25.117 | 0.576 | 91.517 ± 36.880 | 110.067 ± 42.261 | 0.576 |
| p-tau181/T-tau | 16.852 ± 9.147 | 15.777 ± 7.767 | 0.753 | 23.325 ± 18.282 | 27.088 ± 19.250 | 0.654 |
| α-syn/CD81 | 129.264 ± 106.804 | 153.352 ± 84.035 | 0.556 | 113.011 ± 89.129 | 118.277 ± 75.006 | 0.830 |
| Aβ42/CD81 | 1.151 ± 0.882 | 1.046 ± 0.283 | 0.732 | 1.053 ± 0.450 | 1.441 ± 1.380 | 0.310 |
| Aβ42/Aβ40 | 0.277 ± 0.052 | 0.304 ± 0.065 | 0.245 | 0.339 ± 0.090 | 0.343 ± 0.163 | 0.933 |
| T-tau/CD81 | 0.247 ± 0.176 | 0.368 ± 0.319 | 0.194 | 0.203 ± 0.095 | 0.337 ± 0.037 | 0.046 |
| p-tau181/CD81 | 7.795 ± 4.901 | 3.960 ± 1.257 | 0.452 | 4.803 ± 2.616 | 5.955 ± 5.132 | 0.428 |
| GFAP/CD81 | 18.318 ± 6.680 | 3.576 ± 1.765 | 0.498 | 4.730 ± 3.805 | 5.638 ± 5.236 | 0.558 |
| p-tau181/T-tau | 38.829 ± 16.747 | 18.976 ± 13.807 | 0.389 | 31.596 ± 16.075 | 36.075 ± 9.641 | 0.995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, X.; Mei, S.; Huang, A.; Wu, Z.; Zeng, J.; Song, H.; An, J.; Zhang, L.; Liu, G.; Zhou, L.; et al. Alzheimer’s Disease Related Biomarkers Were Associated with Amnestic Cognitive Impairment in Parkinson’s Disease: A Cross-Sectional Cohort Study. Brain Sci. 2024, 14, 787. https://doi.org/10.3390/brainsci14080787
Xue X, Mei S, Huang A, Wu Z, Zeng J, Song H, An J, Zhang L, Liu G, Zhou L, et al. Alzheimer’s Disease Related Biomarkers Were Associated with Amnestic Cognitive Impairment in Parkinson’s Disease: A Cross-Sectional Cohort Study. Brain Sciences. 2024; 14(8):787. https://doi.org/10.3390/brainsci14080787
Chicago/Turabian StyleXue, Xiaofan, Shanshan Mei, Anqi Huang, Zhiyue Wu, Jingrong Zeng, Haixia Song, Jing An, Lijuan Zhang, Guozhen Liu, Lichun Zhou, and et al. 2024. "Alzheimer’s Disease Related Biomarkers Were Associated with Amnestic Cognitive Impairment in Parkinson’s Disease: A Cross-Sectional Cohort Study" Brain Sciences 14, no. 8: 787. https://doi.org/10.3390/brainsci14080787





