Application of an Auditory-Based Feedback Distortion to Modify Gait Symmetry in Healthy Individuals
Abstract
:1. Introduction
2. Materials and Methods
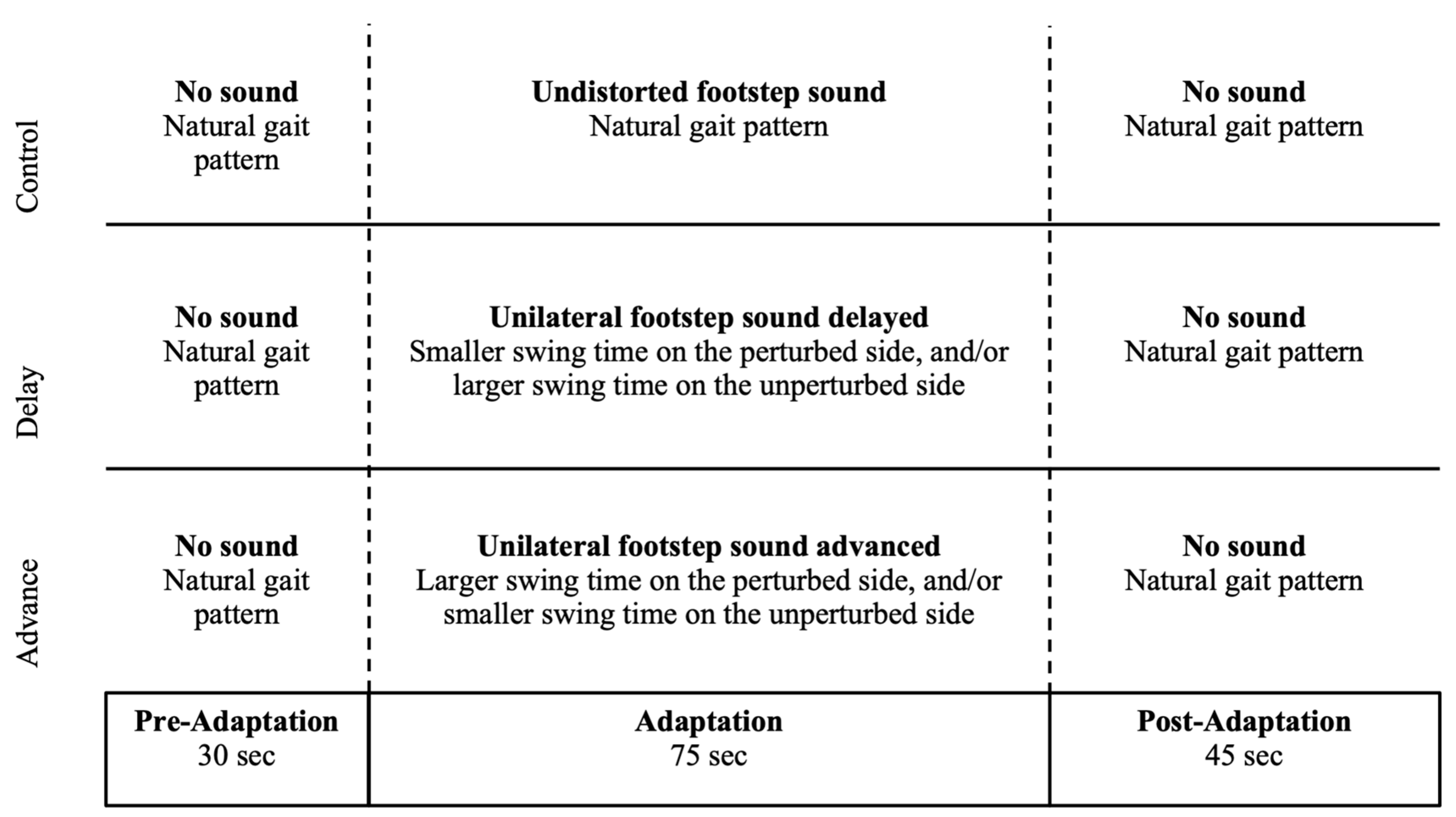
2.1. Experimental Design
2.2. Participants
2.3. Experimental Setup
2.4. Experimental Procedure
2.5. Data Analysis
2.6. Statistical Analysis
3. Results
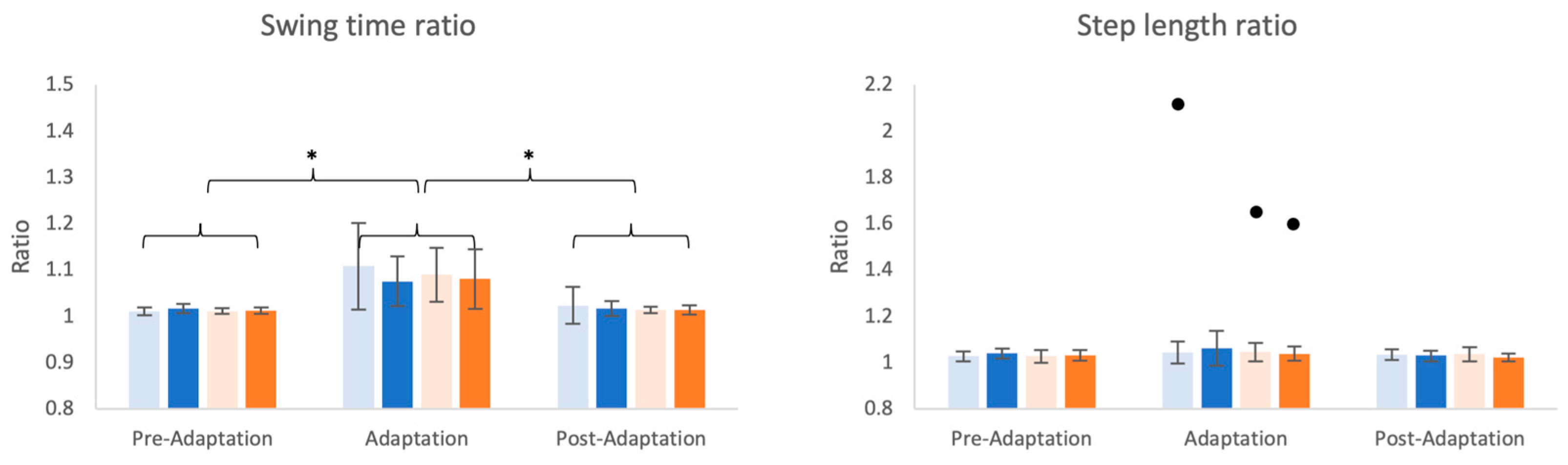
3.1. Changes in Spatiotemporal Parameters
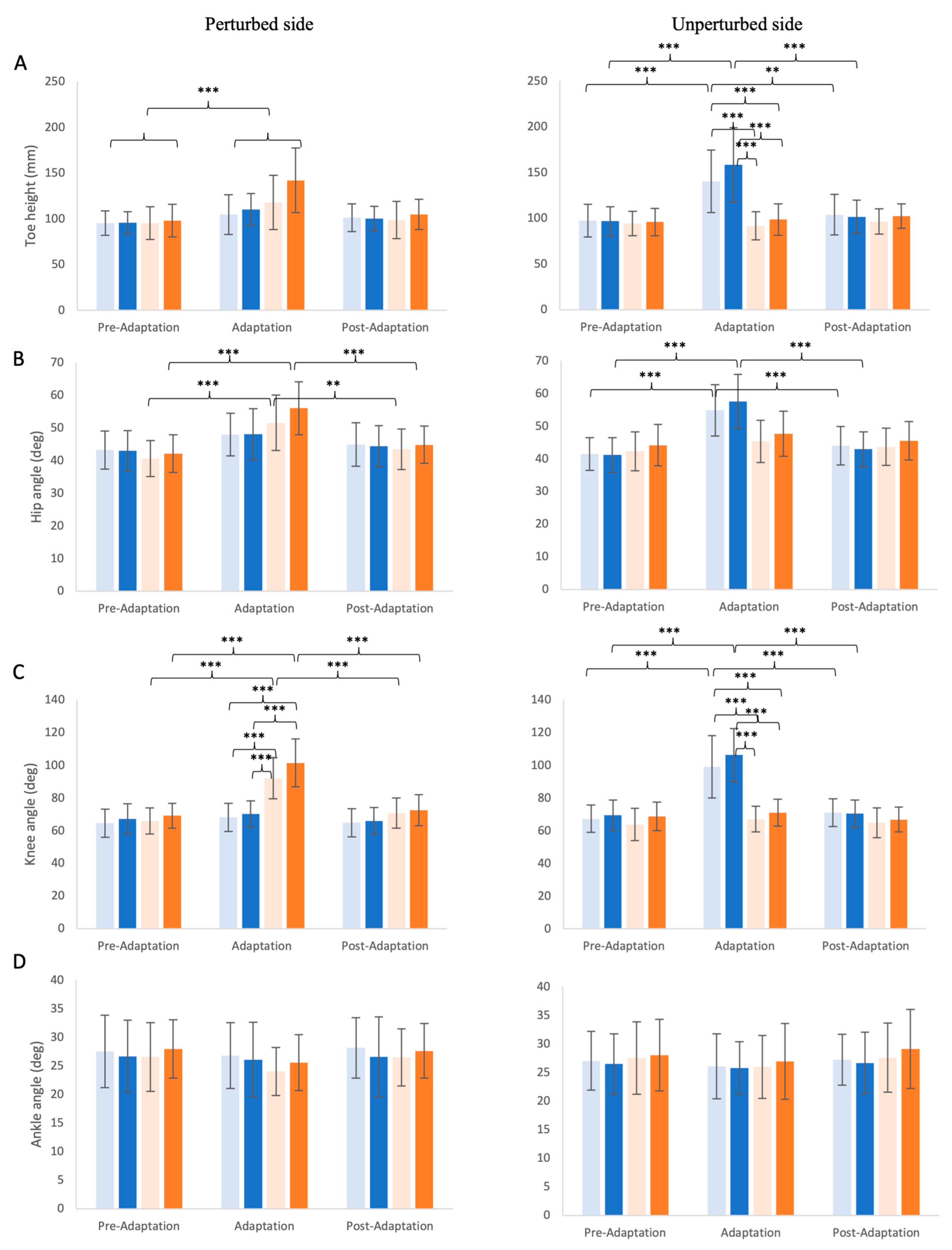
3.2. Changes in Kinematic Outcomes
3.2.1. Maximum Toe Height
3.2.2. Hip, Knee, and Ankle Angle Excursion
4. Discussion
4.1. Temporal vs. Spatial Changes
4.2. Magnitude of Distortion Does Not Matter
4.3. Implications for Future Studies
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krakauer, J.W. Motor learning: Its relevance to stroke recovery and neurorehabilitation. Curr. Opin. Neurol. 2006, 19, 84–90. [Google Scholar] [CrossRef]
- Kleim, J.A.; Hogg, T.M.; VandenBerg, P.M.; Cooper, N.R.; Bruneau, R.; Remple, M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J. Neurosci. 2004, 24, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R. Motor Control and Learning: A Behavioral Emphasis, 2nd ed.; Human Kinetics Publishers: Champaign, IL, USA, 1988. [Google Scholar]
- Schmidt, R.A. Motor Learning & Performance: From Principles to Practice; Human Kinetics Books: Champaign, IL, USA, 1991; p. 310. [Google Scholar]
- Gilmore, P.E.; Spaulding, S.J. Motor control and motor learning: Implications for treatment of individuals post stroke. Phys. Occup. Ther. Geriatr. 2001, 20, 1–15. [Google Scholar] [CrossRef]
- Krasovsky, T.; Levin, M.F. Review: Toward a better understanding of coordination in healthy and poststroke gait. Neurorehabilit. Neural Repair 2010, 24, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Sangani, S.; Patterson, K.K.; Fung, J.; Lamontagne, A. Instantaneous effect of real-time avatar visual feedback on interlimb coordination during walking post-stroke. Clin. Biomech. 2022, 100, 105821. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.K.; Mansfield, A.; Biasin, L.; Brunton, K.; Inness, E.L.; McIlroy, W.E. Longitudinal changes in poststroke spatiotemporal gait asymmetry over inpatient rehabilitation. Neurorehabilit. Neural Repair 2015, 29, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Ghai, I. Effects of (music-based) rhythmic auditory cueing training on gait and posture post-stroke: A systematic review & dose-response meta-analysis. Sci. Rep. 2019, 9, 2183. [Google Scholar] [CrossRef]
- Ghai, S.; Ghai, I.; Schmitz, G.; Effenberg, A.O. Effect of rhythmic auditory cueing on parkinsonian gait: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 506. [Google Scholar] [CrossRef]
- Thaut, M.H. The discovery of human auditory-motor entrainment and its role in the development of neurologic music therapy. Prog. Brain Res. 2015, 217, 253–266. [Google Scholar] [CrossRef]
- Sejdić, E.; Fu, Y.; Pak, A.; Fairley, J.A.; Chau, T. The effects of rhythmic sensory cues on the temporal dynamics of human gait. PLoS ONE 2012, 7, e43104. [Google Scholar] [CrossRef]
- Crosby, L.D.; Wong, J.S.; Chen, J.L.; Grahn, J.; Patterson, K.K. An Initial Investigation of the Responsiveness of Temporal Gait Asymmetry to Rhythmic Auditory Stimulation and the Relationship to Rhythm Ability Following Stroke. Front. Neurol. 2020, 11, 517028. [Google Scholar] [CrossRef] [PubMed]
- Roerdink, M.; Lamoth, C.J.; Kwakkel, G.; van Wieringen, P.C.; Beek, P.J. Gait Coordination after Stroke: Benefits of Acoustically Paced Treadmill Walking. Phys. Ther. 2007, 87, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Thaut, M.; Leins, A.; Rice, R.; Argstatter, H.; Kenyon, G.; McIntosh, G.; Bolay, H.; Fetter, M. Rhythmic auditor y stimulation improves gait more than NDT/Bobath training in near-ambulatory patients early poststroke: A single-blind, randomized trial. Neurorehabilit. Neural Repair 2007, 21, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Baram, Y.; Lenger, R. Gait improvement in patients with cerebral palsy by visual and auditory feedback. Neuromodulation: Technol. Neural Interface 2012, 15, 48–52; discussion 5. [Google Scholar] [CrossRef] [PubMed]
- Brasileiro, A.; Gama, G.; Trigueiro, L.; Ribeiro, T.; Silva, E.; Galvão, É.; Lindquist, A. Influence of visual and auditory biofeedback on partial body weight support treadmill training of individuals with chronic hemiparesis: A randomized controlled clinical trial. Eur. J. Phys. Rehabil. Med. 2015, 51, 49–58. [Google Scholar] [PubMed]
- Ginis, P.; Nieuwboer, A.; Dorfman, M.; Ferrari, A.; Gazit, E.; Canning, C.G.; Rocchi, L.; Chiari, L.; Hausdorff, J.M.; Mirelman, A. Feasibility and effects of home-based smartphone-delivered automated feedback training for gait in people with Parkinson’s disease: A pilot randomized controlled trial. Park. Relat. Disord. 2016, 22, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, S.; Song, C. The Effects of Auditory Feedback Gait Training Using Smart Insole on Stroke Patients. Brain Sci. 2021, 11, 1377. [Google Scholar] [CrossRef]
- Patton, J.L.; Stoykov, M.E.; Kovic, M.; Mussa-Ivaldi, F.A. Evaluation of robotic training forces that either enhance or reduce error in chronic hemiparetic stroke survivors. Exp. Brain Res. 2006, 168, 368–383. [Google Scholar] [CrossRef]
- O’brien, K.; Crowell, C.R.; Schmiedeler, J. Error augmentation feedback for lateral weight shifting. Gait Posture 2017, 54, 178–182. [Google Scholar] [CrossRef]
- Qiao, M.; Richards, J.T.; Franz, J.R. Visuomotor error augmentation affects mediolateral head and trunk stabilization during walking. Hum. Mov. Sci. 2019, 68, 102525. [Google Scholar] [CrossRef]
- Liu, L.Y.; Li, Y.; Lamontagne, A. The effects of error-augmentation versus error-reduction paradigms in robotic therapy to enhance upper extremity performance and recovery post-stroke: A systematic review. J. Neuroeng. Rehabil. 2018, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.-C.; Srivastava, S.; Agrawal, S.K.; Scholz, J.P. Effect of robotic performance-based error-augmentation versus error-reduction training on the gait of healthy individuals. Gait Posture 2013, 37, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.C.; Srivastava, S.; Higginson, J.S.; Agrawal, S.K.; Scholz, J.P. Short-term Performance-based Error-augmentation versus Error-reduction Robotic Gait Training for Individuals with Chronic Stroke: A Pilot Study. Phys. Med. Rehabil. Int. 2015, 2, 1066. [Google Scholar] [PubMed]
- DePaul, V.; Chow, F.; Thomas, K.; Chan, P.; Azuma, L.; Poon, V.; Mansfield, A.; Patterson, K. Self-assessment of gait symmetry in individuals with unilateral stroke: A pilot study. In Proceedings of the International Society for Posture and Gait Research World Congress, Seville, Spain, 28 June–2 July 2015. [Google Scholar]
- Dettmann, M.; Linder, M.; Sepic, S. Relationships among walking performance, postural stability, and functional assessments of the hemiplegic patient. Am. J. Phys. Med. 1987, 66, 77–90. [Google Scholar] [PubMed]
- Kim, C.; Eng, J.J. Symmetry in vertical ground reaction force is accompanied by symmetry in temporal but not distance variables of gait in persons with stroke. Gait Posture 2003, 18, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Petry, N.M. A Comparison of Young, Middle-Aged, and Older Adult Treatment-Seeking Pathological Gamblers. Gerontologist 2002, 42, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Fung, J.; Richards, C.L.; Malouin, F.; McFadyen, B.J.; Lamontagne, A. A treadmill and motion coupled virtual reality system for gait training post-stroke. CyberPsychology Behav. 2006, 9, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Fesliansstudios. Available online: https://www.fesliyanstudios.com/ (accessed on 4 August 2024).
- Patterson, K.K.; Gage, W.H.; Brooks, D.; Black, S.E.; McIlroy, W.E. Evaluation of gait symmetry after stroke: A comparison of current methods and recommendations for standardization. Gait Posture 2010, 31, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Mazumdar, M.; Memtsoudis, S.G. Beyond repeated measures ANOVA: Advanced statistical methods for the analysis of longitudinal data in anesthesia research. Reg. Anesth. Pain Med. 2012, 37, 99–105. [Google Scholar] [CrossRef]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]
- Chiaramonte, R.; D’amico, S.; Caramma, S.; Grasso, G.; Pirrone, S.; Ronsisvalle, M.G.; Bonfiglio, M. The Effectiveness of Goal-Oriented Dual Task Proprioceptive Training in Subacute Stroke: A Retrospective Observational Study. Ann. Rehabil. Med. 2024, 48, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, R.; Rauter, G.; Riener, R.; Wolf, P. Augmented visual, auditory, haptic, and multimodal feedback in motor learning: A review. Psychon. Bull. Rev. 2013, 20, 21–53. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.B.; Warren, D.H. Immediate perceptual response to intersensory discrepancy. Psychol. Bull. 1980, 88, 638–667. [Google Scholar] [CrossRef] [PubMed]
- Freides, D. Human information processing and sensory modality: Cross-modal functions, information complexity, memory, and deficit. Psychol. Bull. 1974, 81, 284–310. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A. Kinetics: Forces and Moments of Force. In Biomechanics and Motor Control of Human Movement, 3rd ed.; Winter, D.A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 107–138. [Google Scholar]
- Crosby, L.D.; Chen, J.L.; Grahn, J.A.; Patterson, K.K. Perceptions of an over-ground induced temporal gait asymmetry by healthy young adults. Hum. Mov. Sci. 2021, 78, 102806. [Google Scholar] [CrossRef]
- Brandstater, M.; Debruin, H.; Gowland, C.; Clark, B. Hemiplegic gait: Analysis of temporal variables. Arch. Phys. Med. Rehabil. 1983, 64, 583–587. [Google Scholar]
- Wang, Y.; Mukaino, M.; Ohtsuka, K.; Otaka, Y.; Tanikawa, H.; Matsuda, F.; Tsuchiyama, K.; Yamada, J.; Saitoh, E. Gait characteristics of post-stroke hemiparetic patients with different walking speeds. Int. J. Rehabil. Res. 2020, 43, 69–75. [Google Scholar] [CrossRef]



| Healthy Young Adult (n = 12) | Mean ± 1 SD |
|---|---|
| Age (years) | 26.4 ± 4.68 |
| Sex (male/female) | 5/7 * |
| Dominant side ** (right/left) | 11/1 * |
| Height (cm) | 171.1 ± 10.329 |
| Weight (kg) | 66.8 ± 18.44 |
| Comfortable walking speed (m/s) | 1.52 ± 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.Y.; Sangani, S.; Patterson, K.K.; Fung, J.; Lamontagne, A. Application of an Auditory-Based Feedback Distortion to Modify Gait Symmetry in Healthy Individuals. Brain Sci. 2024, 14, 798. https://doi.org/10.3390/brainsci14080798
Liu LY, Sangani S, Patterson KK, Fung J, Lamontagne A. Application of an Auditory-Based Feedback Distortion to Modify Gait Symmetry in Healthy Individuals. Brain Sciences. 2024; 14(8):798. https://doi.org/10.3390/brainsci14080798
Chicago/Turabian StyleLiu, Le Yu, Samir Sangani, Kara K. Patterson, Joyce Fung, and Anouk Lamontagne. 2024. "Application of an Auditory-Based Feedback Distortion to Modify Gait Symmetry in Healthy Individuals" Brain Sciences 14, no. 8: 798. https://doi.org/10.3390/brainsci14080798






