Impaired Face Feature-to-Location Statistical Learning and Single-Feature Discrimination in Developmental Prosopagnosia
Abstract
:1. Introduction
1.1. Specialised Face Processing and the Current Study
1.2. Face Perception Impairments in Prosopagnosia
1.3. Individual Differences, Mild Prosopagnosia, and Hypotheses
2. Materials and Methods
2.1. Participants
2.2. Diagnostic Criteria
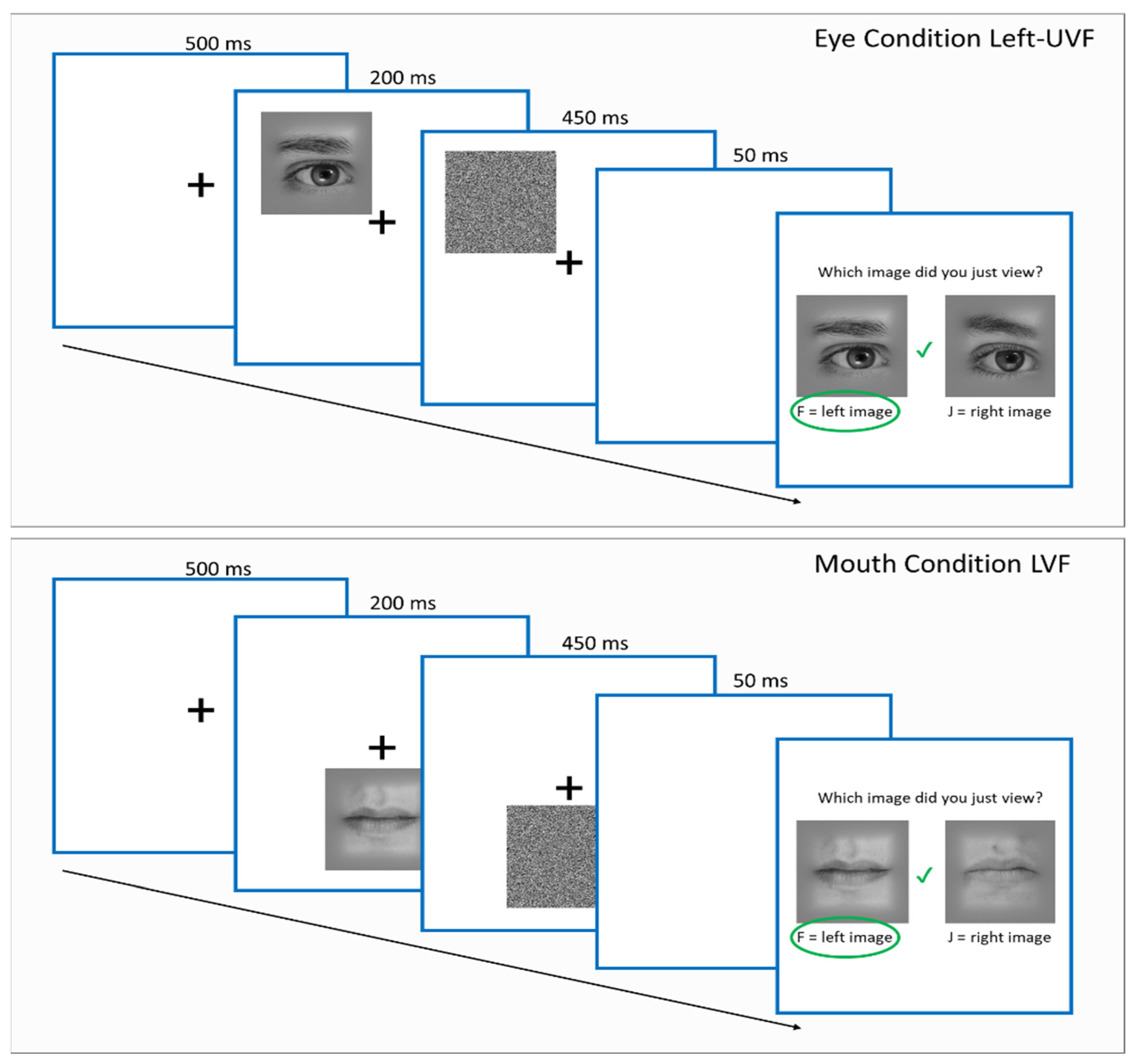
2.3. Procedure
2.4. Stimuli
3. Results
3.1. Screening Test Results
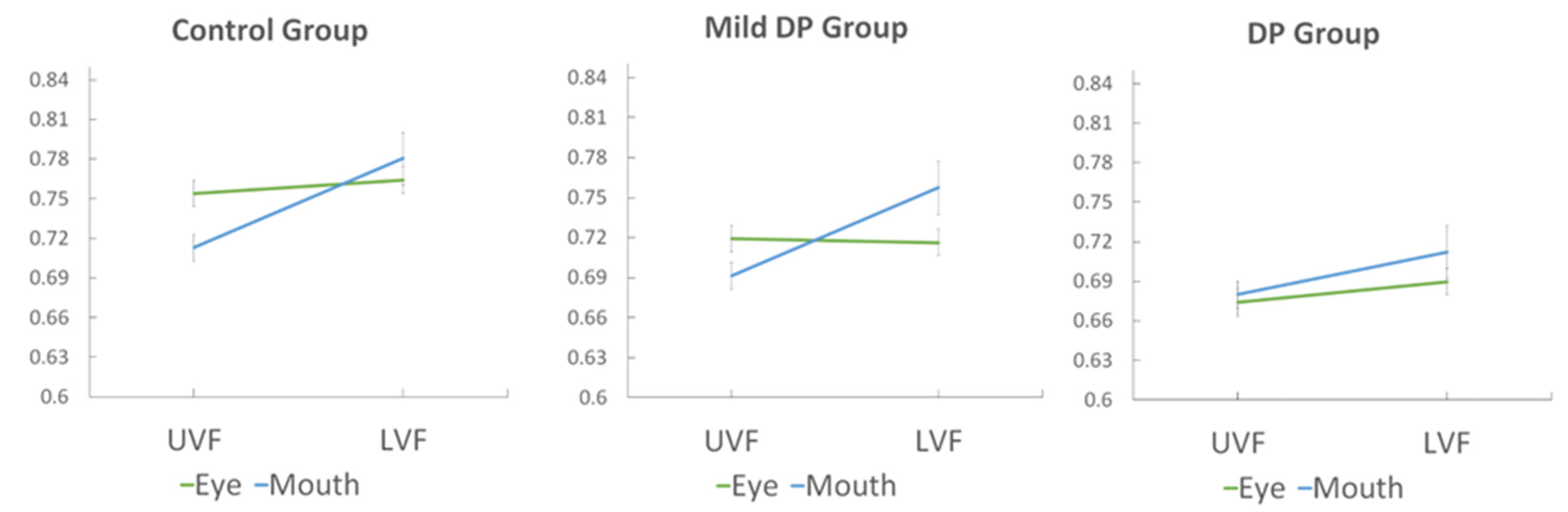
3.2. Face Feature Discrimination Accuracy
3.3. Response Times
3.4. Effects of Age in Control Participants
3.5. Controlling for Age in Group Comparisons
3.6. Partial Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Towler, J.; Eimer, M. Electrophysiological studies of face processing in developmental prosopagnosia: Neuropsychological and neurodevelopmental perspectives. Cogn. Neuropsychol. 2012, 29, 503–529. [Google Scholar] [CrossRef]
- Susilo, T.; Duchaine, B. Advances in developmental prosopagnosia research. Curr. Opin. Neurobiol. 2013, 23, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Towler, J.; Fisher, K.; Eimer, M. The cognitive and neural basis of developmental prosopagnosia. Q. J. Exp. Psychol. 2017, 70, 316–344. [Google Scholar] [CrossRef]
- Geskin, J.; Behrmann, M. Congenital prosopagnosia without object agnosia? A literature review. Cogn. Neuropsychol. 2017, 35, 4–54. [Google Scholar] [CrossRef]
- De Haas, B.; Schwarzkopf, D.S.; Alvarez, I.; Lawson, R.P.; Henriksson, L.; Kriegeskorte, N.; Rees, G. Perception and processing of faces in the human brain is tuned to typical feature locations. J. Neurosci. 2016, 36, 9289–9302. [Google Scholar] [CrossRef] [PubMed]
- Young, A.W.; Hellawell, D.; Hay, D.C. Configurational information in face perception. Perception 1987, 16, 747–759. [Google Scholar] [CrossRef]
- Tanaka, J.W.; Farah, M.J. Parts and wholes in face recognition. Q. J. Exp. Psychol. Sect. A 1993, 46, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Rossion, B. The composite face illusion: A whole window into our understanding of holistic face perception. Vis. Cogn. 2013, 21, 139–253. [Google Scholar] [CrossRef]
- Richler, J.J.; Gauthier, I. A meta-analysis and review of holistic face processing. Psychol. Bull. 2014, 140, 1281–1302. [Google Scholar] [CrossRef]
- Tanaka, J.W.; Simonyi, D. The “parts and wholes” of face recognition: A review of the literature. Q. J. Exp. Psychol. 2016, 69, 1876–1889. [Google Scholar] [CrossRef]
- Rezlescu, C.; Susilo, T.; Wilmer, J.B.; Caramazza, A. The inversion, part-whole, and composite effects reflect distinct perceptual mechanisms with varied relationships to face recognition. J. Exp. Psychol. Hum. Percept. Perform. 2017, 43, 1961–1973. [Google Scholar] [CrossRef] [PubMed]
- Boutet, I.; Nelson, E.A.; Watier, N.; Cousineau, D.; Béland, S.; Collin, C.A. Different measures of holistic face processing tap into distinct but partially overlapping mechanisms. Atten. Percept. Psychophys. 2021, 83, 2905–2923. [Google Scholar] [CrossRef]
- Peterson, M.F.; Eckstein, M.P. Looking just below the eyes is optimal across face recognition tasks. Proc. Natl. Acad. Sci. USA 2012, 109, E3314–E3323. [Google Scholar] [CrossRef]
- Peterson, M.F.; Lin, J.; Zaun, I.; Kanwisher, N. Individual differences in face-looking behavior generalize from the lab to the world. J. Vis. 2016, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Duchaine, B.; Yovel, G.; Nakayama, K. No global processing deficit in the Navon task in 14 developmental prosopagnosics. Soc. Cogn. Affect. Neurosci. 2007, 2, 104–113. [Google Scholar] [CrossRef]
- Towler, J.; Eimer, M. Electrophysiological evidence for parts and wholes in visual face memory. Cortex 2016, 83, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.K. Looking at upside-down faces. J. Exp. Psychol. 1969, 81, 141–145. [Google Scholar] [CrossRef]
- DeGutis, J.; Cohan, S.; Mercado, R.J.; Wilmer, J.; Nakayama, K. Holistic processing of the mouth but not the eyes in developmental prosopagnosia. Cogn. Neuropsychol. 2012, 29, 419–446. [Google Scholar] [CrossRef]
- Avidan, G.; Tanzer, M.; Behrmann, M. Impaired holistic processing in congenital prosopagnosia. Neuropsychologia 2011, 49, 2541–2552. [Google Scholar] [CrossRef]
- Davies-Thompson, J.; Morgan, D.; Davis, J.P.; Towler, J.R. Face Feature Change Detection Ability in Developmental Prosopagnosia and Super-Recognisers. Brain Sci. 2024, 14, 561. [Google Scholar] [CrossRef]
- Bate, S.; Bennetts, R.J.; Gregory, N.; Tree, J.J.; Murray, E.; Adams, A.; Bobak, A.K.; Penton, T.; Yang, T.; Banissy, M.J. Objective patterns of face recognition deficits in 165 adults with self-reported developmental prosopagnosia. Brain Sci. 2019, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.J.; Gaunt, E.; Kidane, B.; Hunter, L.; Pulford, J. A new approach to diagnosing and researching developmental prosopagnosia: Excluded cases are impaired too. Behav. Res. Methods 2022, 55, 4291–4314. [Google Scholar] [CrossRef] [PubMed]
- Duchaine, B.; Nakayama, K. The Cambridge Face Memory Test: Results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia 2005, 44, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Gaule, A.; Sowden, S.; Bird, G.; Cook, R. The 20-item prosopagnosia index (PI20): A self-report instrument for identifying developmental prosopagnosia. R. Soc. Open Sci. 2015, 2, 140343. [Google Scholar] [CrossRef] [PubMed]
- Anwyl-Irvine, A.L.; Massonnié, J.; Flitton, A.; Kirkham, N.; Evershed, J.K. Gorilla in our midst: An online behavioral experiment builder. Behav. Res. Methods 2020, 52, 388–407. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, L.; Mur, M.; Kriegeskorte, N. FaciotopyA face-feature map with face-like topology in the human occipital face area. Cortex 2015, 72, 156–167. [Google Scholar] [CrossRef]
- Fry, R.; Tanaka, J.; Cohan, S.; Wilmer, J.B.; Germine, L.; DeGutis, J. Effects of age on face perception: Reduced eye region discrimination ability but intact holistic processing. PsyArXiv 2023, 38, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Bobak, A.K.; Parris, B.A.; Gregory, N.J.; Bennetts, R.J.; Bate, S. Eye-Movement Strategies in Developmental Prosopagnosia and Super Face Recognition. Q. J. Exp. Psychol. 2017, 70, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Ramon, M.; Busigny, T.; Rossion, B. Impaired holistic processing of unfamiliar individual faces in acquired prosopagnosia. Neuropsychologia 2010, 48, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Schyns, P.G.; Bonnar, L.; Gosselin, F. Show Me the Features! Understanding Recognition From the Use of Visual Information. Psychol. Sci. 2002, 13, 402–409. [Google Scholar] [CrossRef]
- Slessor, G.; Riby, D.M.; Finnerty, A.N. Age-related differences in processing face configuration: The importance of the eye region. J. Gerontol. Ser. B 2012, 68, 228–231. [Google Scholar] [CrossRef] [PubMed]
| CFPT | CFMT | FFT | PI-20 | |
|---|---|---|---|---|
| DP | −0.96 | −2.78 | −4.63 | 3.84 |
| Mild DP | −0.31 | −1.44 | −3.75 | 3.71 |
| p-values | 0.00003 | 0.00000 | 0.00045 | 0.09362 |
| Eye Upper | Eye Lower | Mouth Upper | Mouth Lower | |
|---|---|---|---|---|
| Younger Controls | 78.85 | 78.55 | 71.92 | 77.01 |
| Older Controls | 71.84 | 74.25 | 70.66 | 79.12 |
| p-values | 0.002 | 0.044 | 0.592 | 0.395 |
| CFPT | CFMT | FFT | PI20 | |
|---|---|---|---|---|
| Eye Upper | 0.26 | 0.36 | 0.31 | −0.28 |
| Eye Lower | 0.17 | 0.42 | 0.33 | −0.29 |
| Mouth Upper | 0.06 | 0.20 | 0.11 | −0.12 |
| Mouth Lower | 0.21 | 0.36 | 0.28 | −0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Towler, J.R.; Morgan, D.; Davies-Thompson, J. Impaired Face Feature-to-Location Statistical Learning and Single-Feature Discrimination in Developmental Prosopagnosia. Brain Sci. 2024, 14, 815. https://doi.org/10.3390/brainsci14080815
Towler JR, Morgan D, Davies-Thompson J. Impaired Face Feature-to-Location Statistical Learning and Single-Feature Discrimination in Developmental Prosopagnosia. Brain Sciences. 2024; 14(8):815. https://doi.org/10.3390/brainsci14080815
Chicago/Turabian StyleTowler, John R., Daniel Morgan, and Jodie Davies-Thompson. 2024. "Impaired Face Feature-to-Location Statistical Learning and Single-Feature Discrimination in Developmental Prosopagnosia" Brain Sciences 14, no. 8: 815. https://doi.org/10.3390/brainsci14080815






