The Association between Long Non-Coding RNAs and Alzheimer’s Disease
Abstract
1. Introduction
1.1. Significance of lncRNAs
1.2. Biological Functions of LncRNAs in Health and Disease
1.3. LncRNAs Dysregulation in Alzheimer’s Disease
1.4. LncRNAs as Potential Therapeutic Targets in Alzheimer’s Disease
1.5. Gender Specific lncRNAs in AD
1.6. LncRNA Therapy
1.7. Diagnostic Potential of LncRNAs in AD
2. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Idda, M.L.; Munk, R.; Abdelmohsen, K.; Gorospe, M. Noncoding RNAs in Alzheimer’s disease. Wiley Interdiscip. Rev. RNA 2018, 9, e1463. [Google Scholar] [CrossRef] [PubMed]
- Doxtater, K.; Tripathi, M.K.; Khan, M.M. Recent advances on the role of long non-coding RNAs in Alzheimer’s disease. Neural Regen. Res. 2020, 15, 2253–2254. [Google Scholar] [PubMed]
- Riva, P.; Ratti, A.; Venturin, M. The Long Non-Coding RNAs in Neurodegenerative Diseases: Novel Mechanisms of Pathogenesis. Curr. Alzheimer Res. 2016, 13, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Beam, C.R.; Kaneshiro, C.; Jang, J.Y.; Reynolds, C.A.; Pedersen, N.L.; Gatz, M. Differences Between Women and Men in Incidence Rates of Dementia and Alzheimer’s Disease. J. Alzheimers Dis. 2018, 64, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xia, P.; Yang, L.; Lu, C.; Lu, Z. The roles of long non-coding RNAs in Alzheimer’s disease diagnosis, treatment, and their involvement in Alzheimer’s disease immune responses. Noncoding RNA Res. 2024, 9, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Canoy, R.J.; Sy, J.C.; Deguit, C.D.; Castro, C.B.; Dimaapi, L.J.; Panlaqui, B.G.; Perian, W.; Yu, J.; Velasco, J.M.; Sevilleja, J.E.; et al. Non-coding RNAs involved in the molecular pathology of Alzheimer’s disease: A systematic review. Front. Neurosci. 2024, 18, 1421675. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, M.S. Non-Coding RNAs in Neurological and Neuropsychiatric Disorders: Unraveling the Hidden Players in Disease Pathogenesis. Cells 2024, 13, 1063. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Lu, L.; Li, J. Long non-coding RNAs with essential roles in neurodegenerative disorders. Neural Regen. Res. 2024, 19, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Munro, T.; Mattick, J.S. The potential of long noncoding RNA therapies. Trends Pharmacol. Sci. 2022, 43, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Marchese, F.P.; Raimondi, I.; Huarte, M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017, 18, 206. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Lauretti, E.; Dabrowski, K.; Pratico, D. The neurobiology of non-coding RNAs and Alzheimer’s disease pathogenesis: Pathways, mechanisms and translational opportunities. Ageing Res. Rev. 2021, 71, 101425. [Google Scholar] [CrossRef]
- Srinivas, T.; Mathias, C.; Oliveira-Mateos, C.; Guil, S. Roles of lncRNAs in brain development and pathogenesis: Emerging therapeutic opportunities. Mol. Ther. 2023, 31, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, H.; Niu, M.; Wang, Y.; Xu, R.; Guo, Y.; Zhang, C. Roles of long noncoding RNAs in human inflammatory diseases. Cell Death Discov. 2024, 10, 235. [Google Scholar] [CrossRef]
- Faghihi, M.A.; Modarresi, F.; Khalil, A.M.; Wood, D.E.; Sahagan, B.G.; Morgan, T.E.; Finch, C.E.; St Laurent, G., 3rd; Kenny, P.J.; Wahlestedt, C. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat. Med. 2008, 14, 723–730. [Google Scholar] [CrossRef]
- Ma, P.; Li, Y.; Zhang, W.; Fang, F.; Sun, J.; Liu, M.; Li, K.; Dong, L. Long Non-coding RNA MALAT1 Inhibits Neuron Apoptosis and Neuroinflammation While Stimulates Neurite Outgrowth and Its Correlation With MiR-125b Mediates PTGS2, CDK5 and FOXQ1 in Alzheimer’s Disease. Curr. Alzheimer Res. 2019, 16, 596–612. [Google Scholar] [CrossRef]
- Cao, M.; Li, H.; Zhao, J.; Cui, J.; Hu, G. Identification of age- and gender-associated long noncoding RNAs in the human brain with Alzheimer’s disease. Neurobiol. Aging 2019, 81, 116–126. [Google Scholar] [CrossRef]
- Zhang, Z. Long non-coding RNAs in Alzheimer’s disease. Curr. Top. Med. Chem. 2016, 16, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Liao, Y.T.; He, J.C.; Xie, C.L.; Chen, S.Y.; Fan, H.H.; Su, Z.P.; Wang, Z. Plasma long non-coding RNA BACE1 as a novel biomarker for diagnosis of Alzheimer disease. BMC Neurol. 2018, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Khodayi, M.; Khalaj-Kondori, M.; Hoseinpour Feizi, M.A.; Jabarpour Bonyadi, M.; Talebi, M. Plasma lncRNA profiling identified BC200 and NEAT1 lncRNAs as potential blood-based biomarkers for late-onset Alzheimer’s disease. EXCLI J. 2022, 21, 772–785. [Google Scholar] [PubMed]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Pereira Fernandes, D.; Bitar, M.; Jacobs, F.M.J.; Barry, G. Long Non-Coding RNAs in Neuronal Aging. Noncoding RNA 2018, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.W.; Luo, T.; Zou, S.S.; Wu, A.S. The Role of Long Noncoding RNAs in Central Nervous System and Neurodegenerative Diseases. Front. Behav. Neurosci. 2018, 12, 175. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.; Zheng, D.; Qing, H. Regulatory Roles of Long Non-Coding RNAs in the Central Nervous System and Associated Neurodegenerative Diseases. Front. Cell Neurosci. 2017, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Begliarzade, S.; Beilerli, A.; Sufianov, A.; Tamrazov, R.; Kudriashov, V.; Ilyasova, T.; Liang, Y.; Beylerli, O. Long non-coding RNAs as promising biomarkers and therapeutic targets in cervical cancer. Noncoding RNA Res. 2023, 8, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, T.; Doss, C.G. Non-coding RNAs in human health and disease: Potential function as biomarkers and therapeutic targets. Funct. Integr. Genom. 2023, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F. Non-Coding RNA-Targeted Therapy: A State-of-the-Art Review. Int. J. Mol. Sci. 2024, 25, 3630. [Google Scholar] [CrossRef] [PubMed]
- Fardi, F.; Khasraghi, L.B.; Shahbakhti, N.; Salami Naseriyan, A.; Najafi, S.; Sanaaee, S.; Alipourfard, I.; Zamany, M.; Karamipour, S.; Jahani, M.; et al. An interplay between non-coding RNAs and gut microbiota in human health. Diabetes Res. Clin. Pract. 2023, 201, 110739. [Google Scholar] [CrossRef]
- Agliano, F.; Rathinam, V.A.; Medvedev, A.E.; Vanaja, S.K.; Vella, A.T. Long Noncoding RNAs in Host-Pathogen Interactions. Trends Immunol. 2019, 40, 492–510. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Li, Q.; Zhao, J.; Yi, D.; Ding, J.; Zhao, F.; Hu, S.; Zhou, J.; Deng, T.; et al. Influenza Virus Exploits an Interferon-Independent lncRNA to Preserve Viral RNA Synthesis through Stabilizing Viral RNA Polymerase PB1. Cell Rep. 2019, 27, 3295–3304.e4. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Chen, J.; Yin, X.; Zhou, J. Current understanding on long non-coding RNAs in immune response to COVID-19. Virus Res. 2023, 323, 198956. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, H.; Jafari, H.; Moravveji, S.S.; Abbasi Faranghizadeh, F.; Talebi, M.; Ghanavi, J.; Esfandi, F.; Najafi, S.; Nasiri Moghadam, M.; Farnia, P.; et al. Non-coding RNA in SARS-CoV-2: Progress toward therapeutic significance. Int. J. Biol. Macromol. 2022, 222, 1538–1550. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xia, Z.A.; Zhong, B.; Xiong, X.; Sheng, C.; Wang, Y.; Gong, W.; Cao, Y.; Wang, Z.; Peng, W. Distinct Hippocampal Expression Profiles of Long Non-coding RNAs in an Alzheimer’s Disease Model. Mol. Neurobiol. 2017, 54, 4833–4846. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, X.; Li, Z.; He, Y. Relationship between long non-coding RNAs and Alzheimer’s disease: A systematic review. Pathol. Res. Pract. 2019, 215, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Dinger, M.E.; Mariani, J.; Kosik, K.S.; Mehler, M.F.; Mattick, J.S. Noncoding RNAs in Long-Term Memory Formation. Neuroscientist 2008, 14, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Su, W.; Zhuo, Y. The Role of Long Noncoding RNAs in Neurodegenerative Diseases. Mol. Neurobiol. 2017, 54, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Harrow, J.; Frankish, A.; Gonzalez, J.M.; Tapanari, E.; Diekhans, M.; Kokocinski, F.; Aken, B.L.; Barrell, D.; Zadissa, A.; Searle, S.; et al. GENCODE: The reference human genome annotation for The ENCODE Project. Genome Res. 2012, 22, 1760–1774. [Google Scholar] [CrossRef] [PubMed]
- Carlevaro-Fita, J.; Johnson, R. Global Positioning System: Understanding Long Noncoding RNAs through Subcellular Localization. Mol. Cell 2019, 73, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.A.; Ahmad, S.M.; Mumtaz, P.T.; Malik, A.A.; Dar, M.A.; Urwat, U.; Shah, R.A.; Ganai, N.A. Long non-coding RNAs: Mechanism of action and functional utility. Noncoding RNA Res. 2016, 1, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Oo, J.A.; Brandes, R.P.; Leisegang, M.S. Long non-coding RNAs: Novel regulators of cellular physiology and function. Pflug. Arch. 2022, 474, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Chang, H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. Linking deregulation of non-coding RNA to the core pathophysiology of Alzheimer’s disease: An integrative review. Prog. Neurobiol. 2017, 156, 1–68. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhuang, Y.; Zhao, X.; Li, X. Long Non-coding RNA in Neuronal Development and Neurological Disorders. Front. Genet. 2018, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Diaz Duran, R.; Wei, H.; Kim, D.H.; Wu, J.Q. Invited Review: Long non-coding RNAs: Important regulators in the development, function and disorders of the central nervous system. Neuropathol. Appl. Neurobiol. 2019, 45, 538–556. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Chen, Y.; Jin, J.; Xu, Y.; Zhu, X. Long Non-coding RNA: Insight Into Mechanisms of Alzheimer’s Disease. Front. Mol. Neurosci. 2021, 14, 821002. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, A.K.; Vij, P.; Lopez, S.; Leslie, S.M.; Doxtater, K.; Khan, M.M.; Yallapu, M.M.; Chauhan, S.C.; Maestre, G.E.; Tripathi, M.K. Long Non-Coding RNAs: New Insights in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 2268. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Q.; Fu, P.; Yao, C.; Zhu, L.S.; Hou, T.Y.; Chen, J.G.; Lu, Y.; Liu, D.; Zhu, L.Q. Long Non-coding RNAs, Novel Culprits, or Bodyguards in Neurodegenerative Diseases. Mol. Ther. Nucleic Acids 2018, 10, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Bernard, D.; Prasanth, K.V.; Tripathi, V.; Colasse, S.; Nakamura, T.; Xuan, Z.; Zhang, M.Q.; Sedel, F.; Jourdren, L.; Coulpier, F.; et al. A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J. 2010, 29, 3082–3093. [Google Scholar] [CrossRef] [PubMed]
- Chanda, K.; Jana, N.R.; Mukhopadhyay, D. Long non-coding RNA MALAT1 protects against Abeta(1-42) induced toxicity by regulating the expression of receptor tyrosine kinase EPHA2 via quenching miR-200a/26a/26b in Alzheimer’s disease. Life Sci. 2022, 302, 120652. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Feng, P.; Zhu, X.; He, S.; Duan, J.; Zhou, D. Long non-coding RNA Malat1 promotes neurite outgrowth through activation of ERK/MAPK signalling pathway in N2a cells. J. Cell Mol. Med. 2016, 20, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, S.; Li, B.; Zhang, Y. MALAT1 inhibits proliferation and promotes apoptosis of SH-SY5Y cells induced by Abeta25-35 via blocking PI3K/mTOR/GSK3beta pathway. Xi Bao Yu Fen. Zi Mian Yi Xue Za Zhi 2018, 34, 434–441. [Google Scholar] [PubMed]
- Yao, J.; Wang, X.Q.; Li, Y.J.; Shan, K.; Yang, H.; Wang, Y.N.; Yao, M.D.; Liu, C.; Li, X.M.; Shen, Y.; et al. Long non-coding RNA MALAT1 regulates retinal neurodegeneration through CREB signaling. EMBO Mol. Med. 2016, 8, 1113. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, Z. lncRNA NEAT1: Key player in neurodegenerative diseases. Ageing Res. Rev. 2023, 86, 101878. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Yang, Z.; Yang, F.; Wang, X.; Tan, J.; Liao, B. Long Noncoding RNA NEAT1 Aggravates Abeta-Induced Neuronal Damage by Targeting miR-107 in Alzheimer’s Disease. Yonsei Med. J. 2019, 60, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhao, J.; Wang, W.; Zhou, J.; Zhang, J. Depletion of LncRNA NEAT1 Rescues Mitochondrial Dysfunction Through NEDD4L-Dependent PINK1 Degradation in Animal Models of Alzheimer’s Disease. Front. Cell Neurosci. 2020, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.B.; Anderson, A.G.; Lauzon, S.N.; Davis, M.N.; Hauser, R.M.; Roberts, S.C.; Rodriguez-Nunez, I.; Trausch-Lowther, K.; Barinaga, E.A.; Hall, P.I.; et al. Neuronal MAPT expression is mediated by long-range interactions with cis-regulatory elements. Am. J. Hum. Genet. 2024, 111, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Simone, R.; Javad, F.; Emmett, W.; Wilkins, O.G.; Almeida, F.L.; Barahona-Torres, N.; Zareba-Paslawska, J.; Ehteramyan, M.; Zuccotti, P.; Modelska, A.; et al. MIR-NATs repress MAPT translation and aid proteostasis in neurodegeneration. Nature 2021, 594, 117–123. [Google Scholar] [CrossRef]
- Bhagat, R.; Minaya, M.A.; Renganathan, A.; Mehra, M.; Marsh, J.; Martinez, R.; Eteleeb, A.M.; Nana, A.L.; Spina, S.; Seeley, W.W.; et al. Long non-coding RNA SNHG8 drives stress granule formation in tauopathies. Mol. Psychiatry 2023, 28, 4889–4901. [Google Scholar] [CrossRef]
- Fotuhi, S.N.; Khalaj-Kondori, M.; Hoseinpour Feizi, M.A.; Talebi, M. Long Non-coding RNA BACE1-AS May Serve as an Alzheimer’s Disease Blood-Based Biomarker. J. Mol. Neurosci. 2019, 69, 351–359. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, H.; Wu, Q.; Xu, W.; Xia, M. Knockdown of BACE1-AS by siRNA improves memory and learning behaviors in Alzheimer’s disease animal model. Exp. Ther. Med. 2018, 16, 2080–2086. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Faghihi, M.A.; Magistri, M.; Alvarez-Garcia, O.; Lotz, M.; Wahlestedt, C. Antisense RNA controls LRP1 Sense transcript expression through interaction with a chromatin-associated protein, HMGB2. Cell Rep. 2015, 11, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Ciarlo, E.; Massone, S.; Penna, I.; Nizzari, M.; Gigoni, A.; Dieci, G.; Russo, C.; Florio, T.; Cancedda, R.; Pagano, A. An intronic ncRNA-dependent regulation of SORL1 expression affecting Abeta formation is upregulated in post-mortem Alzheimer’s disease brain samples. Dis. Model. Mech. 2013, 6, 424–433. [Google Scholar] [PubMed]
- Asadi, M.R.; Hassani, M.; Kiani, S.; Sabaie, H.; Moslehian, M.S.; Kazemi, M.; Ghafouri-Fard, S.; Taheri, M.; Rezazadeh, M. The Perspective of Dysregulated LncRNAs in Alzheimer’s Disease: A Systematic Scoping Review. Front. Aging Neurosci. 2021, 13, 709568. [Google Scholar] [CrossRef]
- Li, H.; Zheng, L.; Jiang, A.; Mo, Y.; Gong, Q. Identification of the biological affection of long noncoding RNA BC200 in Alzheimer’s disease. Neuroreport 2018, 29, 1061–1067. [Google Scholar] [CrossRef]
- Liu, N.X.; Li, Q.H. LncRNA BC200 regulates neuron apoptosis and neuroinflammation via PI3K/AKT pathway in Alzheimer’s disease. J. Biol. Regul. Homeost. Agents 2020, 34, 2255–2261. [Google Scholar]
- Mus, E.; Hof, P.R.; Tiedge, H. Dendritic BC200 RNA in aging and in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2007, 104, 10679–10684. [Google Scholar] [CrossRef]
- Parenti, R.; Paratore, S.; Torrisi, A.; Cavallaro, S. A natural antisense transcript against Rad18, specifically expressed in neurons and upregulated during beta-amyloid-induced apoptosis. Eur. J. Neurosci. 2007, 26, 2444–2457. [Google Scholar] [CrossRef] [PubMed]
- Massone, S.; Ciarlo, E.; Vella, S.; Nizzari, M.; Florio, T.; Russo, C.; Cancedda, R.; Pagano, A. NDM29, a RNA polymerase III-dependent non coding RNA, promotes amyloidogenic processing of APP and amyloid beta secretion. Biochim. Biophys. Acta 2012, 1823, 1170–1177. [Google Scholar] [CrossRef]
- Knauss, J.L.; Miao, N.; Kim, S.N.; Nie, Y.; Shi, Y.; Wu, T.; Pinto, H.B.; Donohoe, M.E.; Sun, T. Long noncoding RNA Sox2ot and transcription factor YY1 co-regulate the differentiation of cortical neural progenitors by repressing Sox2. Cell Death Dis. 2018, 9, 799. [Google Scholar] [CrossRef] [PubMed]
- Arisi, I.; D’Onofrio, M.; Brandi, R.; Felsani, A.; Capsoni, S.; Drovandi, G.; Felici, G.; Weitschek, E.; Bertolazzi, P.; Cattaneo, A. Gene expression biomarkers in the brain of a mouse model for Alzheimer’s disease: Mining of microarray data by logic classification and feature selection. J. Alzheimers Dis. 2011, 24, 721–738. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, C.J.; Zhao, Z.Y.; Gao, Y.Y.; Zhao, J.T.; Li, X.X.; Zhang, M.; Wang, H. Mechanisms underlying LncRNA SNHG1 regulation of Alzheimer’s disease involve DNA methylation. J. Toxicol. Environ. Health Part A 2024, 87, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, T.; Qu, Q.; Kang, T.; Yang, Q. Long Noncoding RNA SNHG1 Promotes Neuroinflammation in Parkinson’s Disease via Regulating miR-7/NLRP3 Pathway. Neuroscience 2018, 388, 118–127. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.Q.; Wei, X.Z.; Lee, C.; Huo, D.S.; Wang, H.; Zhao, Z.Y. Differentially expressed long-chain noncoding RNAs in human neuroblastoma cell line (SH-SY5Y): Alzheimer’s disease cell model. J. Toxicol. Environ. Health Part A 2019, 82, 1052–1060. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, N.; Lv, C.; Li, N.; Li, X.; Li, W. lncRNA SNHG1 Knockdown Alleviates Amyloid-beta-Induced Neuronal Injury by Regulating ZNF217 via Sponging miR-361-3p in Alzheimer’s Disease. J. Alzheimers Dis. 2020, 77, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Luan, W.; Shen, X.; Wang, Z.; Cao, Y. LncRNA BDNF-AS as ceRNA regulates the miR-9-5p/BACE1 pathway affecting neurotoxicity in Alzheimer’s disease. Arch. Gerontol. Geriatr. 2022, 99, 104614. [Google Scholar] [CrossRef] [PubMed]
- Massone, S.; Vassallo, I.; Fiorino, G.; Castelnuovo, M.; Barbieri, F.; Borghi, R.; Tabaton, M.; Robello, M.; Gatta, E.; Russo, C.; et al. 17A, a novel non-coding RNA, regulates GABA B alternative splicing and signaling in response to inflammatory stimuli and in Alzheimer disease. Neurobiol. Dis. 2011, 41, 308–317. [Google Scholar] [CrossRef]
- Yue, D.; Guanqun, G.; Jingxin, L.; Sen, S.; Shuang, L.; Yan, S.; Minxue, Z.; Ping, Y.; Chong, L.; Zhuobo, Z.; et al. Silencing of long noncoding RNA XIST attenuated Alzheimer’s disease-related BACE1 alteration through miR-124. Cell Biol. Int. 2020, 44, 630–636. [Google Scholar] [CrossRef]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics—Challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, I.A.; Mehler, M.F. Long non-coding RNAs: Novel targets for nervous system disease diagnosis and therapy. Neurotherapeutics 2013, 10, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, M.; Liu, H. LncRNA17A regulates autophagy and apoptosis of SH-SY5Y cell line as an in vitro model for Alzheimer’s disease. Biosci. Biotechnol. Biochem. 2019, 83, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Wang, J.; Li, Y.; Li, G.; Wang, Y. LINC00472 Regulates Ferroptosis of Neurons in Alzheimer’s Disease via FOXO1. Dement. Geriatr. Cogn. Disord. 2024, 53, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Balusu, S.; Horre, K.; Thrupp, N.; Craessaerts, K.; Snellinx, A.; Serneels, L.; T’Syen, D.; Chrysidou, I.; Arranz, A.M.; Sierksma, A.; et al. MEG3 activates necroptosis in human neuron xenografts modeling Alzheimer’s disease. Science 2023, 381, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Shou, F.; Li, G.; Morshedi, M. Long Non-coding RNA ANRIL and Its Role in the Development of Age-Related Diseases. Mol. Neurobiol. 2024, 24, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Safari, M.; Taheri, M.; Samadian, M. Expression of Linear and Circular lncRNAs in Alzheimer’s Disease. J. Mol. Neurosci. 2022, 72, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Li, L.; Qiu, X.; Wu, J.; Xu, L.; Shao, W. Long non-coding RNA ANRIL knockdown suppresses apoptosis and pro-inflammatory cytokines while enhancing neurite outgrowth via binding microRNA-125a in a cellular model of Alzheimer’s disease. Mol. Med. Rep. 2020, 22, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhong, M.B.; Zhang, L.; Zhang, B.; Cai, D. Sex Differences in Alzheimer’s Disease: Insights From the Multiomics Landscape. Biol. Psychiatry 2022, 91, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, L.; Qin, C. Long non-coding RNAs in brain development, synaptic biology, and Alzheimer’s disease. Brain Res. Bull. 2017, 132, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.H.; Androsavich, J.R.; So, N.; Jenkins, M.P.; MacCormack, D.; Prigodich, A.; Welch, V.; True, J.M.; Dolsten, M. Breaking the mold with RNA-a “RNAissance” of life science. npj Genom. Med. 2024, 9, 2. [Google Scholar] [CrossRef]
- Dibaj, M.; Haghi, M.; Safaralizadeh, R.; Saberi, A. The role of EZH2 and its regulatory lncRNAs as a serum-based biomarker in Alzheimer’s disease. Mol. Biol. Rep. 2024, 51, 866. [Google Scholar] [CrossRef]
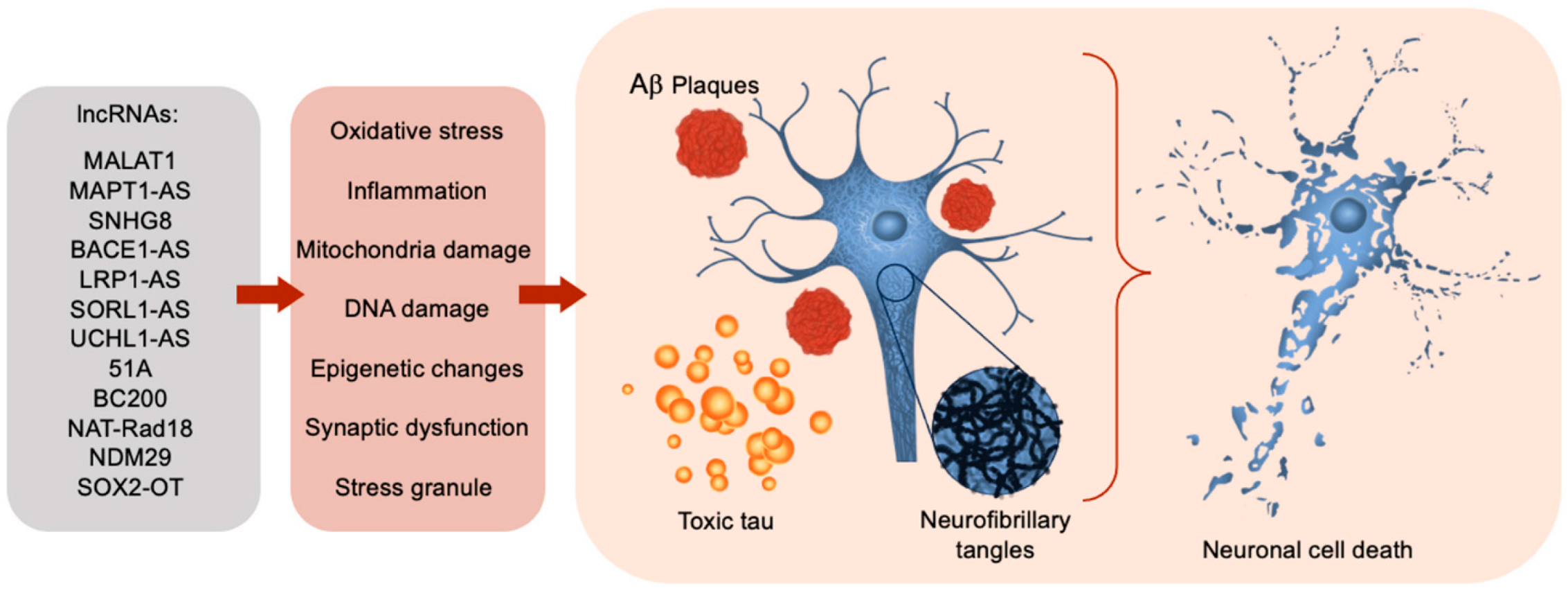
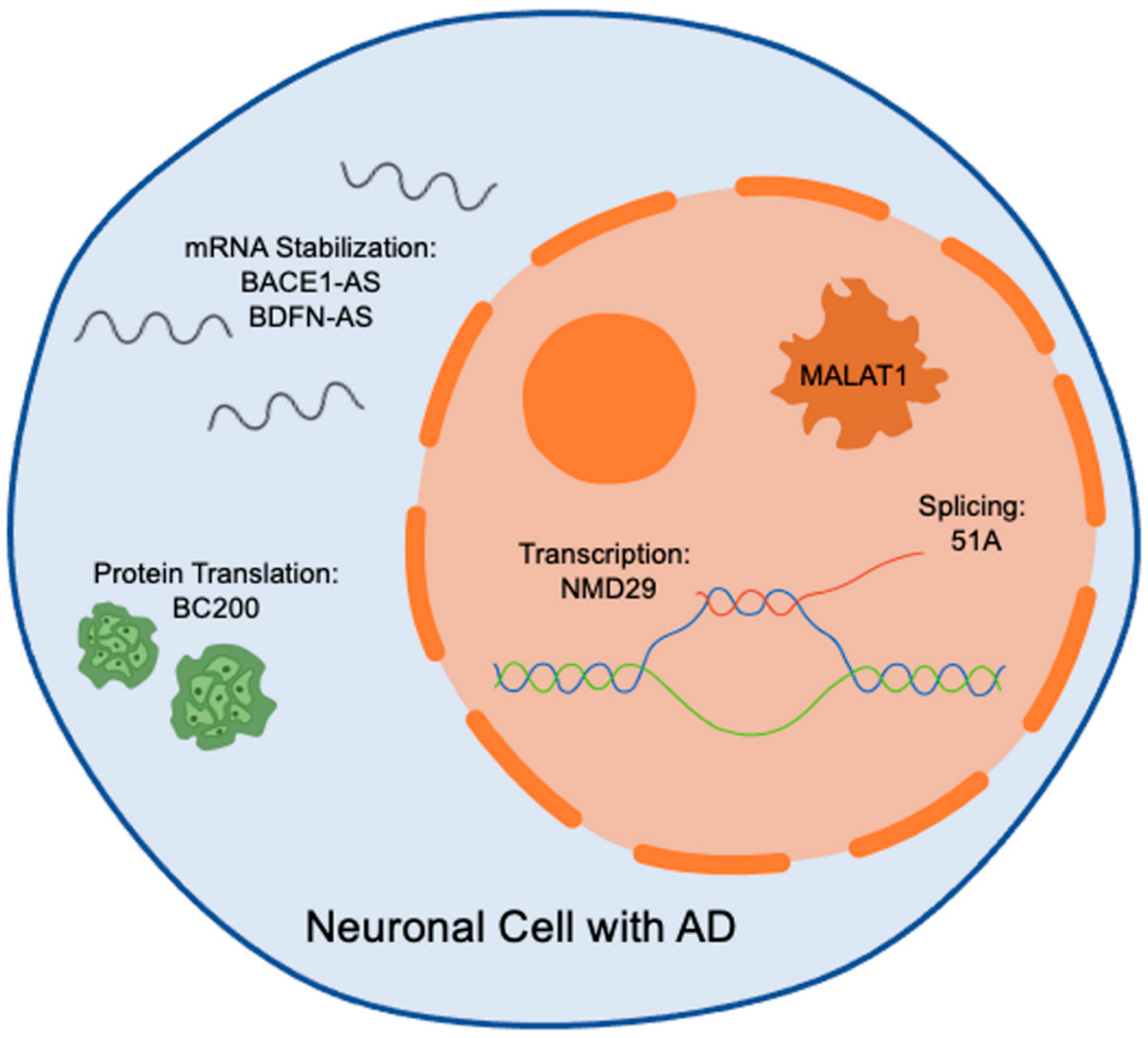
| LncRNA ID | Trend | Proposed Function | Reference(s) |
|---|---|---|---|
| MALAT1 | ↓ in AD | Inhibit neuronal apoptosis & neuroinflammation, neurite outgrowth stimulation | [17,51,52,53,54,55] |
| NEAT1 | ↑ in AD | Limits Aβ production and ameliorates cognitive deficits | [21,56,57,58] |
| MAPT1-AS | ↓ in AD | Inhibit tau translation by rRNA pairing | [59,60] |
| SNHG8 | ↓ in AD | Interacts with TIA1, an RNA binding protein associated with tau and stress granules | [61] |
| BACE1-AS | ↑ in AD | Amyloid precursor protein cleaving β-secretase → increased Aβ peptides | [18,20,21,62,63] |
| LRP1-AS | ↑ in AD | Lowers LRP1’s expression, influencing Aβ agglomeration | [64] |
| SORL1-AS | ↑ in AD | Creation of deleterious splicing isoforms of SORL1 → increase Aβ levels | [23] |
| UCHL1-AS | ↓ in AD | Ubiquitinates Aβ protein → plaque deconstruction | [24] |
| 51A | ↑ in AD | Downregulates SORL1, Impaired processing of APP, increased Aβ formation | [5,35,65,66] |
| BC200 | ↑ in AD | Regulating neuronal protein translation → amyloid plaque formation and AD pathogenesis | [21,67,68,69] |
| NAT-Rad18 | ↑ in AD | Increases neuronal apoptosis by lowering neuronal DNA damage compensatory abilities | [25,70] |
| NDM29 | ↑ in AD | Increased Aβ peptides | [71] |
| SOX2-OT | ↓ in AD | Promotes neurogenesis and neuronal differentiation | [66,72,73] |
| SNHG1 | ↑ in AD | Regulate neuroinflammation, DNA methylation and apoptosis | [74,75,76,77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Black, C.M.; Braden, A.A.; Nasim, S.; Tripathi, M.; Xiao, J.; Khan, M.M. The Association between Long Non-Coding RNAs and Alzheimer’s Disease. Brain Sci. 2024, 14, 818. https://doi.org/10.3390/brainsci14080818
Black CM, Braden AA, Nasim S, Tripathi M, Xiao J, Khan MM. The Association between Long Non-Coding RNAs and Alzheimer’s Disease. Brain Sciences. 2024; 14(8):818. https://doi.org/10.3390/brainsci14080818
Chicago/Turabian StyleBlack, Carson M., Anneliesse A. Braden, Samia Nasim, Manish Tripathi, Jianfeng Xiao, and Mohammad Moshahid Khan. 2024. "The Association between Long Non-Coding RNAs and Alzheimer’s Disease" Brain Sciences 14, no. 8: 818. https://doi.org/10.3390/brainsci14080818
APA StyleBlack, C. M., Braden, A. A., Nasim, S., Tripathi, M., Xiao, J., & Khan, M. M. (2024). The Association between Long Non-Coding RNAs and Alzheimer’s Disease. Brain Sciences, 14(8), 818. https://doi.org/10.3390/brainsci14080818






