The Relationship between Clinical and Psychophysical Assessments of Visual Perceptual Disturbances in Individuals at Clinical High Risk for Psychosis: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
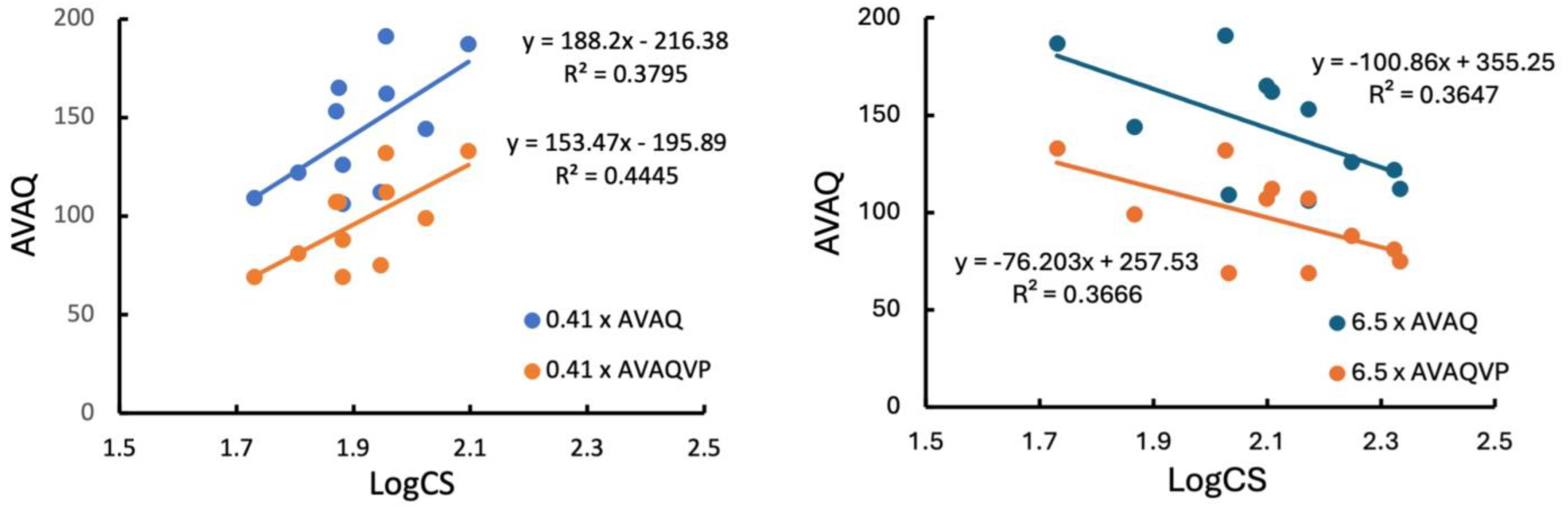
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yung, A.R.; McGorry, P.D.; McFarlane, C.A.; Jackson, H.J.; Patton, G.C.; Rakkar, A. Monitoring and care of young people at incipient risk of psychosis. Schizophr. Bull. 1996, 22, 283–303. [Google Scholar] [CrossRef] [PubMed]
- Schultze-Lutter, F.; Michel, C.; Schmidt, S.J.; Schimmelmann, B.G.; Maric, N.P.; Salokangas, R.K.; Riecher-Rossler, A.; van der Gaag, M.; Nordentoft, M.; Raballo, A.; et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur. Psychiatry 2015, 30, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Salazar de Pablo, G.; Woods, S.W.; Drymonitou, G.; de Diego, H.; Fusar-Poli, P. Prevalence of Individuals at Clinical High-Risk of Psychosis in the General Population and Clinical Samples: Systematic Review and Meta-Analysis. Brain Sci. 2021, 11, 1544. [Google Scholar] [CrossRef] [PubMed]
- McGhie, A.; Chapman, J. Disorders of attention and perception in early schizophrenia. Br. J. Med. Psychol. 1961, 34, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Klosterkotter, J.; Hellmich, M.; Steinmeyer, E.M.; Schultze-Lutter, F. Diagnosing schizophrenia in the initial prodromal phase. Arch. Gen. Psychiatry 2001, 58, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Uhlhaas, P.J.; Mishara, A.L. Perceptual anomalies in schizophrenia: Integrating phenomenology and cognitive neuroscience. Schizophr. Bull. 2007, 33, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, S.M.; Demmin, D.; Skodlar, B. Space and Objects: On the Phenomenology and Cognitive Neuroscience of Anomalous Perception in Schizophrenia (Ancillary Article to EAWE Domain 1). Psychopathology 2017, 50, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Addington, J.; Stowkowy, J.; Weiser, M. Screening tools for clinical high risk for psychosis. Early Interv. Psychiatry 2015, 9, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, J.M.; Meyhoefer, I.; Antonucci, L.A.; Kambeitz-Ilankovic, L.; Surmann, M.; Bienek, O.; Romer, G.; Dannlowski, U.; Hahn, T.; Korda, A.; et al. The impact of visual dysfunctions in recent-onset psychosis and clinical high-risk state for psychosis. Neuropsychopharmacology 2022, 47, 2051–2060. [Google Scholar] [CrossRef]
- Perez, V.B.; Shafer, K.M.; Cadenhead, K.S. Visual information processing dysfunction across the developmental course of early psychosis. Psychol. Med. 2012, 42, 2167–2179. [Google Scholar] [CrossRef]
- Phillipson, O.T.; Harris, J.P. Perceptual changes in schizophrenia: A questionnaire survey. Psychol. Med. 1985, 15, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, S.M. Visual Perception Disturbances in Schizophrenia: A Unified Model. Neuropsychopathology Schizophr. Mol. Brain Syst. Motiv. Cogn. 2016, 63, 77–132. [Google Scholar] [CrossRef]
- Zemon, V.; Herrera, S.; Gordon, J.; Revheim, N.; Silipo, G.; Butler, P.D. Contrast sensitivity deficits in schizophrenia: A psychophysical investigation. Eur. J. Neurosci. 2021, 53, 1155–1170. [Google Scholar] [CrossRef] [PubMed]
- Butler, P.D.; Javitt, D.C. Early-stage visual processing deficits in schizophrenia. Curr. Opin. Psychiatry 2005, 18, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Keri, S.; Antal, A.; Szekeres, G.; Benedek, G.; Janka, Z. Spatiotemporal visual processing in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 2002, 14, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.P.; Shaqiri, A.; Brand, A.; Nogueira, R.L.; Herzog, M.H.; Roinishvili, M.; Santos, N.A.; Chkonia, E. Schizophrenia patients using atypical medication perform better in visual tasks than patients using typical medication. Psychiatry Res. 2019, 275, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Herrera, S.N.; Zemon, V.; Revheim, N.; Silipo, G.; Gordon, J.; Butler, P.D. Cognitive function mediates the relationship between visual contrast sensitivity and functional outcome in schizophrenia. J. Psychiatr. Res. 2021, 144, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Butler, P.D.; Zemon, V.; Schechter, I.; Saperstein, A.M.; Hoptman, M.J.; Lim, K.O.; Revheim, N.; Silipo, G.; Javitt, D.C. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch. Gen. Psychiatry 2005, 62, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Kiss, I.; Fabian, A.; Benedek, G.; Keri, S. When doors of perception open: Visual contrast sensitivity in never-medicated, first-episode schizophrenia. J. Abnorm. Psychol. 2010, 119, 586–593. [Google Scholar] [CrossRef]
- Kelemen, O.; Kiss, I.; Benedek, G.; Keri, S. Perceptual and cognitive effects of antipsychotics in first-episode schizophrenia: The potential impact of GABA concentration in the visual cortex. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 47, 13–19. [Google Scholar] [CrossRef]
- Kadivar, A.; Ilapakurti, M.; Dobkins, K.; Cadenhead, K.S. Visual contrast sensitivity in clinical high risk and first episode psychosis. Schizophr. Res. 2024, 271, 186–193. [Google Scholar] [CrossRef]
- Keri, S.; Benedek, G. Visual contrast sensitivity alterations in inferred magnocellular pathways and anomalous perceptual experiences in people at high-risk for psychosis. Vis. Neurosci. 2007, 24, 183–189. [Google Scholar] [CrossRef]
- Pokorny, J.; Smith, V.C. Psychophysical signatures associated with magnocellular and parvocellular pathway contrast gain. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 1997, 14, 2477–2486. [Google Scholar] [CrossRef]
- Kaplan, E. The M, P, and K pathways of the primate visual system. Vis. Neurosci. 2004, 1, 481–493. [Google Scholar]
- Legge, G.E. Sustained and transient mechanisms in human vision: Temporal and spatial properties. Vis. Res. 1978, 18, 69–81. [Google Scholar] [CrossRef] [PubMed]
- McGlashan, T.; Miller, T.; Woods, S.; Rosen, J.; Hoffman, R.; Davidson, L. Structured Interview for Prodromal Syndromes; PRIME Research Clinic: New Haven, CT, USA, 2001. [Google Scholar]
- Miller, T.J.; McGlashan, T.H.; Rosen, J.L.; Cadenhead, K.; Cannon, T.; Ventura, J.; McFarlane, W.; Perkins, D.O.; Pearlson, G.D.; Woods, S.W. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: Predictive validity, interrater reliability, and training to reliability. Schizophr. Bull. 2003, 29, 703–715. [Google Scholar] [CrossRef]
- Zemon, V.; Butler, P.D.; Legatt, M.E.; Gordon, J. The spatial contrast sensitivity function and its neurophysiological bases. Vis. Res. 2023, 210, 108266. [Google Scholar] [CrossRef] [PubMed]
- Nikitova, N.; Keane, B.P.; Demmin, D.; Silverstein, S.M.; Uhlhaas, P.J. The Audio-Visual Abnormalities Questionnaire (AVAQ): Development and validation of a new instrument for assessing anomalies in sensory perception in schizophrenia spectrum disorders. Schizophr. Res. 2019, 209, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Abrham, Y.; Butler, P.D.; Hu, B.; Keane, B.P. When do contrast sensitivity deficits (or enhancements) depend on spatial frequency? Two ways to avoid spurious interactions. Eur. J. Neurosci. 2023, 57, 351–359. [Google Scholar] [CrossRef]
- Merigan, W.H.; Maunsell, J.H. How parallel are the primate visual pathways? Annu. Rev. Neurosci. 1993, 16, 369–402. [Google Scholar] [CrossRef]
- Burbeck, C.A.; Kelly, D.H. Spatiotemporal characteristics of visual mechanisms: Excitatory-inhibitory model. J. Opt. Soc. Am. 1980, 70, 1121–1126. [Google Scholar] [CrossRef]
- Silverstein, S.M.; Demmin, D.L.; Bednar, J.A. Computational Modeling of Contrast Sensitivity and Orientation Tuning in First-Episode and Chronic Schizophrenia. Comput. Psychiatry 2017, 1, 102–131. [Google Scholar] [CrossRef] [PubMed]
- Owsley, C.; Sloane, M.E. Contrast sensitivity, acuity, and the perception of ‘real-world’ targets. Br. J. Ophthalmol. 1987, 71, 791–796. [Google Scholar] [CrossRef]
- Costen, N.P.; Parker, D.M.; Craw, I. Spatial content and spatial quantisation effects in face recognition. Perception 1994, 23, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Maurer, D. A comparison of spatial frequency tuning for the recognition of facial identity and facial expressions in adults and children. Vis. Res. 2011, 51, 508–519. [Google Scholar] [CrossRef]
- Fiorentini, A.; Maffei, L.; Sandini, G. The role of high spatial frequencies in face perception. Perception 1983, 12, 195–201. [Google Scholar] [CrossRef]
- Collin, C.A.; Liu, C.H.; Troje, N.F.; McMullen, P.A.; Chaudhuri, A. Face recognition is affected by similarity in spatial frequency range to a greater degree than within-category object recognition. J. Exp. Psychol. Hum. Percept. Perform. 2004, 30, 975–987. [Google Scholar] [CrossRef]
- Addington, J.; Penn, D.; Woods, S.W.; Addington, D.; Perkins, D.O. Facial affect recognition in individuals at clinical high risk for psychosis. Br. J. Psychiatry 2008, 192, 67–68. [Google Scholar] [CrossRef]
- Amminger, G.P.; Schafer, M.R.; Papageorgiou, K.; Klier, C.M.; Schlogelhofer, M.; Mossaheb, N.; Werneck-Rohrer, S.; Nelson, B.; McGorry, P.D. Emotion recognition in individuals at clinical high-risk for schizophrenia. Schizophr. Bull. 2012, 38, 1030–1039. [Google Scholar] [CrossRef]
- Osborne, K.J.; Kraus, B.; Curran, T.; Earls, H.; Mittal, V.A. An Event-Related Potential Investigation of Early Visual Processing Deficits during Face Perception in Youth at Clinical High Risk for Psychosis. Schizophr. Bull. 2022, 48, 90–99. [Google Scholar] [CrossRef]
- Wolwer, W.; Brinkmeyer, J.; Stroth, S.; Streit, M.; Bechdolf, A.; Ruhrmann, S.; Wagner, M.; Gaebel, W. Neurophysiological correlates of impaired facial affect recognition in individuals at risk for schizophrenia. Schizophr. Bull. 2012, 38, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, C.M.; Keilp, J.G.; Kayser, J.; Klim, C.; Butler, P.D.; Bruder, G.E.; Gur, R.C.; Javitt, D.C. Emotion recognition deficits as predictors of transition in individuals at clinical high risk for schizophrenia: A neurodevelopmental perspective. Psychol. Med. 2015, 45, 2959–2973. [Google Scholar] [CrossRef] [PubMed]
- Frenette, B.; Mergler, D.; Bowler, R. Contrast-sensitivity loss in a group of former microelectronics workers with normal visual acuity. Optom. Vis. Sci. 1991, 68, 556–560. [Google Scholar] [CrossRef]
- Mergler, D.; Huel, G.; Bowler, R.; Benoit, F.; Cone, J. Visual dysfunction among former microelectronics assembly workers. Arch. Environ. Health 1991, 46, 326–334. [Google Scholar] [CrossRef]
- Bowler, R.M.; Mergler, D.; Huel, G.; Harrison, R.; Cone, J. Neuropsychological impairment among former microelectronics workers. Neurotoxicology 1991, 12, 87–103. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ifrah, C.; Herrera, S.N.; Silverstein, S.M.; Corcoran, C.M.; Gordon, J.; Butler, P.D.; Zemon, V. The Relationship between Clinical and Psychophysical Assessments of Visual Perceptual Disturbances in Individuals at Clinical High Risk for Psychosis: A Preliminary Study. Brain Sci. 2024, 14, 819. https://doi.org/10.3390/brainsci14080819
Ifrah C, Herrera SN, Silverstein SM, Corcoran CM, Gordon J, Butler PD, Zemon V. The Relationship between Clinical and Psychophysical Assessments of Visual Perceptual Disturbances in Individuals at Clinical High Risk for Psychosis: A Preliminary Study. Brain Sciences. 2024; 14(8):819. https://doi.org/10.3390/brainsci14080819
Chicago/Turabian StyleIfrah, Chloe, Shaynna N. Herrera, Steven M. Silverstein, Cheryl M. Corcoran, James Gordon, Pamela D. Butler, and Vance Zemon. 2024. "The Relationship between Clinical and Psychophysical Assessments of Visual Perceptual Disturbances in Individuals at Clinical High Risk for Psychosis: A Preliminary Study" Brain Sciences 14, no. 8: 819. https://doi.org/10.3390/brainsci14080819





