Cognitive Stimulation with Music in Older Adults with Cognitive Impairment: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Are there any differences in outcomes in participants with mild onset of cognitive decline (MCI) compared to participants with an already existing dementia diagnosis? What are the outcome differences between mild and moderate stages of dementia?
- (2)
- Which types of music-based interventions are the most effective for cognitive stimulation, and how well defined are they in research articles?
- (3)
- Do specific music-based protocols designed for the training of certain cognitive functions depending on the specific cognitive deficits of patients exist?
- (4)
- Are there any assessment tools used in the majority of studies, and how far do they go in assessing the effects of an intervention on a specific cognitive function?
2.1. Search Strategy and Identification of Relevant Studies
2.2. Inclusion and Exclusion Criteria
- -
- Population: people with cognitive impairment related to aging, MCI, or dementia. Participants could be experiencing a range of disease stages, from MCI to moderate stages of dementia (the sample could include patients with Alzheimer’s disease-type dementia or any other defined type), but the severity should be clearly defined.
- -
- Intervention: Music-based interventions (i.e., music therapy or other music-based activities): the idea was to select only interventions based on the use of music, characterized by group or individual sessions conducted by a professional figure according to a process idea, not single exposures to sound–musical stimuli.
- -
- Comparison: the selection of interventions without the presence of a control group but with relevant results could be considered, keeping in mind, however, the greater reliability of studies comparing music-based treatments with a control group. In this regard, control groups treated with both standard care and other interventions based on other music-based or other non-pharmacological interventions were accepted in the selection process.
- -
- Outcomes: test scores evaluating the global cognitive situation or performance tasks linked to specific cognitive areas or neuroimaging studies showing the effects of the intervention on a cortical activation level.
- -
- Study design: given the nature of the review, no restrictions on study designs to be taken into consideration were placed. RCTs and CCTs were favored, but crossover, observational, cohort, and case studies could also be included in the review. Literature reviews and meta-analyses were additionally used as resources for selecting further relevant studies.
2.3. Study Selection
2.4. Data Extraction
3. Results
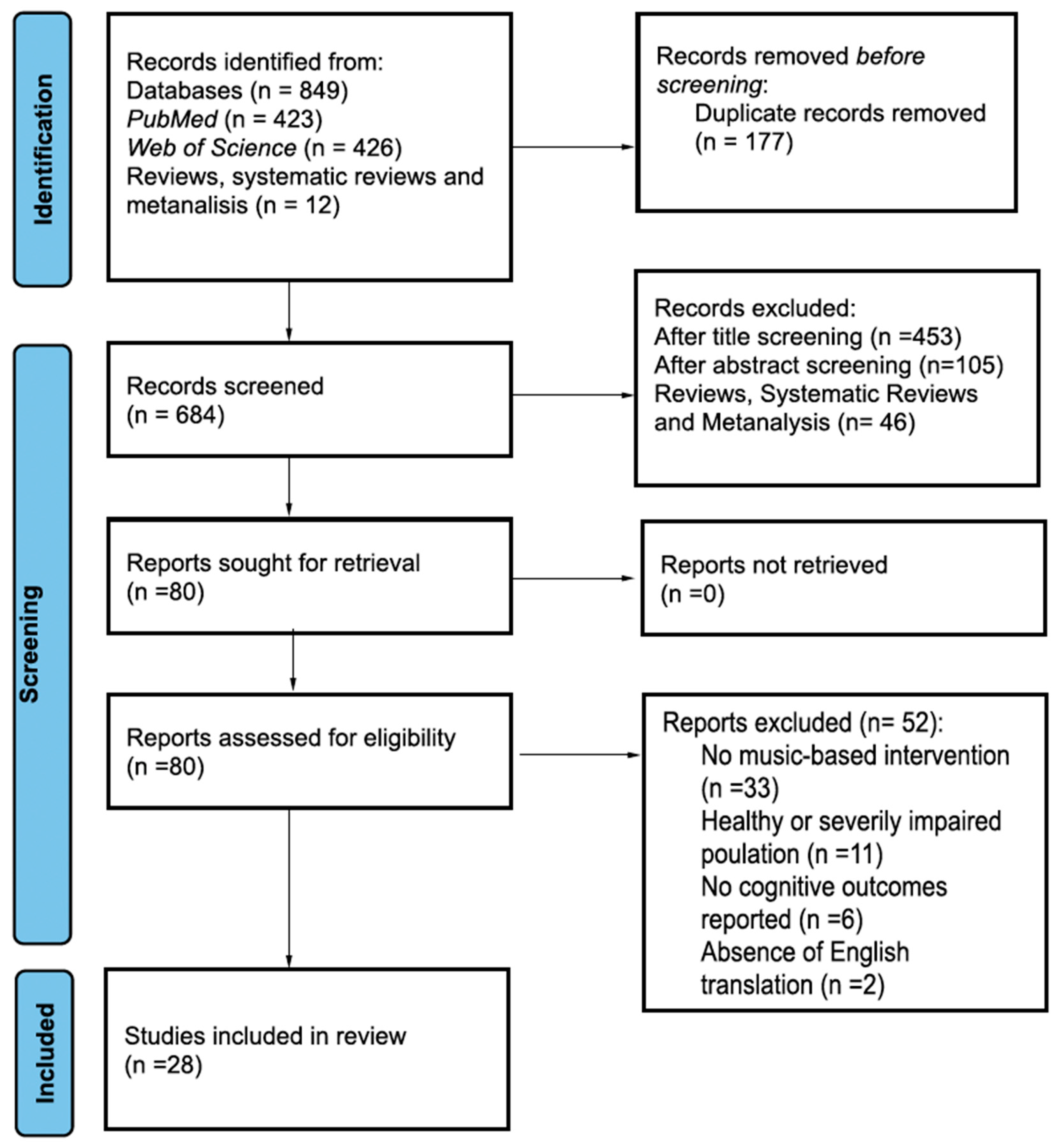
3.1. Search Results
3.2. Studies Characteristics and Outcomes
4. Discussion
4.1. Implications for Practice
4.2. Limits and Future Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wurm, R.; Stögmann, E. Epidemiology of dementia—The epidemic we saw coming. Wien Med. Wochenschr. 2021, 171, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Cahill, S. WHO’s global action plan on the public health response to dementia: Some challenges and opportunities. Aging Ment. Health 2020, 24, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K.; Touchon, J. Mild cognitive impairment: Conceptual basis and current nosological status. Lancet 2000, 355, 225–228. [Google Scholar] [CrossRef]
- Anand, S.; Schoo, C. Mild Cognitive Impairment; StatPearls: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK599514/ (accessed on 15 August 2024).
- Bruscoli, M.; Lovestone, S. Is MCI really just early dementia? A systematic review of conversion studies. Int. Psychogeriatr. 2004, 16, 129–140. [Google Scholar] [CrossRef]
- Mango, D.; Saidi, A.; Cisale, G.Y.; Feligioni, M.; Corbo, M.; Nisticò, R. Targeting Synaptic Plasticity in Experimental Models of Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Belleville, S.; Bherer, L. Biomarkers of cognitive training effects in aging. Curr. Trans. Geriatr. Exp. Gerontol. Rep. 2012, 1, 104–110. [Google Scholar] [CrossRef]
- Bahar-Fuchs, A.; Clare, L.; Woods, B. Cognitive training and cognitive rehabilitation for persons with mild to moderate dementia of the Alzheimer’s or vascular type: A review. Alzheimers Res. Ther. 2013, 5, 35. [Google Scholar] [CrossRef]
- Spector, A.; Orrell, M.; Woods, B. Cognitive Stimulation Therapy (CST): Effects on different areas of cognitive function for people with dementia. Int. J. Geriatr. Psychiatry 2010, 25, 1253–1258. [Google Scholar] [CrossRef]
- Saragih, I.D.; Tonapa, S.I.; Saragih, I.S.; Lee, B.O. Effects of cognitive stimulation therapy for people with dementia: A systematic review and meta-analysis of randomized controlled studies. Int. J. Nurs. Stud. 2022, 1, 104181. [Google Scholar] [CrossRef]
- Cafferata, R.M.; Hicks, B.; von Bastian, C.C. Effectiveness of cognitive stimulation for dementia: A systematic review and meta-analysis. Psychol. Bull. 2021, 147, 455. [Google Scholar] [CrossRef]
- Sun, Y.; Ji, M.; Leng, M.; Li, X.; Zhang, X.; Wang, Z. Comparative efficacy of 11 non-pharmacological interventions on depression, anxiety, quality of life, and caregiver burden for informal caregivers of people with dementia: A systematic review and network meta-analysis. Int. J. Nurs. Stud. 2022, 129, 104204. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E.; Gardini, S.; Pastore, M.; Piras, F.; Vincenzi, M.; Borella, E. Cognitive Stimulation Therapy for Older Adults with Mild-to-Moderate Dementia in Italy: Effects on Cognitive Functioning, and on Emotional and Neuropsychiatric Symptoms. J. Gerontol. B 2021, 76, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Capotosto, E.; Belacchi, C.; Gardini, S.; Faggian, S.; Piras, F.; Mantoan, V.; Borella, E. Cognitive stimulation therapy in the Italian context: Its efficacy in cognitive and non-cognitive measures in older adults with dementia. Int. J. Geriatr. Psychiatry 2017, 32, 331–340. [Google Scholar] [PubMed]
- Nordahl, C.W.; Ranganath, C.; Yonelinas, A.P.; DeCarli, C.; Reed, B.R.; Jagust, W.J. Different mechanisms of episodic memory failure in mild cognitive impairment. Neuropsychologia 2005, 43, 1688–1697. [Google Scholar] [CrossRef]
- Belleville, S.; Gilbert, B.; Fontaine, F.; Gagnon, L.; Menard, E.; Gauthier, S. Improvement of episodic memory in persons with mild cognitive impairment and healthy older adults: Evidence from a cognitive intervention program. Dement. Geriatr. Cogn. Disord. 2006, 22, 486–499. [Google Scholar] [CrossRef]
- Olchik, M.R.; Farina, J.; Steibel, N.; Teixeira, A.R.; Yassuda, M.S. Memory training (MT) in mild cognitive impairment (MCI) generates change in cognitive performance. Arch. Gerontol. Geriatr. 2013, 56, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; Chambon, C.; Michel, B.F.; Paban, V.; Alescio-Lautier, B. Positive effects of computer-based cognitive training in adults with mild cognitive impairment. Neuropsychologia 2012, 50, 1871–1881. [Google Scholar] [CrossRef]
- Tappen, R.M.; Hain, D. The effect of in-home cognitive training on functional performance of individuals with mild cognitive impairment and early-stage Alzheimer’s disease. Res. Gerontol. Nurs. 2014, 7, 14–24. [Google Scholar] [CrossRef]
- Strobach, T.; Frensch, P.; Müller, H.; Schubert, T. Evidence for the acquisition of dual-task coordination skills in older adults. Acta Psychol. 2015, 160, 104–116. [Google Scholar] [CrossRef]
- Vidovich, M.R.; Lautenschlager, N.T.; Flicker, L.; Clare, L.; McCaul, K.; Almeida, O.P. The PACE study: A randomized clinical trial of cognitive activity strategy training for older people with mild cognitive impairment. Am. J. Geriatr. Psychiatry 2015, 23, 360–372. [Google Scholar] [CrossRef]
- Ceccato, E.; Vigato, G.; Bonetto, C.; Bevilacqua, A.; Pizziolo, P.; Crociani, S.; Zanfretta, E.; Pollini, L.; Caneva, P.A.; Baldin, L.; et al. STAM protocol in dementia: A multicenter, single-blind, randomized, and controlled trial. Am. J. Alzheimers Dis. Other Demen. 2012, 27, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Irish, M.; Cunningham, C.J.; Walsh, J.B.; Coakley, D.; Lawlor, B.A.; Robertson, I.H.; Coen, R.F. Investigating the enhancing effect of music on autobiographical memory in mild Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2006, 22, 108–120. [Google Scholar] [CrossRef]
- Chanda, M.L.; Levitin, D.J. The neurochemistry of music. Trends Cogn. Sci. 2013, 17, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Levitin, D.J.; Tirovolas, A.K. Current advances in the cognitive neuroscience of music. Ann. NY Acad. Sci. 2009, 1156, 211–231. [Google Scholar] [CrossRef] [PubMed]
- Koelsch, S. Brain correlates of music-evoked emotions. Nat. Rev. Neurosci. 2014, 15, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Altenmüller, E.; Furuya, S. Brain plasticity and the concept of metaplasticity in skilled musicians. Adv. Exp. Med. Biol. 2016, 957, 197–208. [Google Scholar]
- Herholz, S.C.; Zatorre, R.J. Musical training as a framework for brain plasticity: Behavior, function, and structure. Neuron 2012, 76, 486–502. [Google Scholar] [CrossRef]
- Bugos, J.A.; Perlstein, W.M.; McCrae, C.S.; Brophy, T.S.; Bedenbaugh, P.H. Individualized piano instruction enhances executive functioning and working memory in older adults. Aging Ment. Health 2007, 11, 464–471. [Google Scholar] [CrossRef]
- Seinfeld, S.; Figueroa, H.; Ortiz-Gil, J.; Sanchez-Vives, M.V. Effects of music learning and piano practice on cognitive function, mood and quality of life in older adults. Front. Psychol. 2013, 4, 61506. [Google Scholar] [CrossRef]
- Ferreri, L.; Moussard, A.; Bigand, E.; Tillmann, B. Music and the aging brain. In The Oxford Handbook of Music and the Brain; Thaut, M.H., Hodges, D.A., Eds.; Oxford University Press: Oxford, UK, 2019; pp. 623–644. [Google Scholar]
- Bian, X.; Wang, Y.; Zhao, X.; Zhang, Z.; Ding, C. Does music therapy affect the global cognitive function of patients with dementia? A meta-analysis. NeuroRehabilitation 2022, 48, 553–562. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, J.Y.; Kim, B. Person-centered care in persons living with dementia: A systematic review and meta-analysis. The Gerontologist 2022, 62, e253–e264. [Google Scholar] [CrossRef]
- Bleibel, M.; El Cheikh, A.; Sadier, N.S.; Abou-Abbas, L. The effect of music therapy on cognitive functions in patients with Alzheimer’s disease: A systematic review of randomized controlled trials. Alzheimers Res. Ther. 2023, 15, 65. [Google Scholar] [CrossRef]
- Ito, E.; Nouchi, R.; Dinet, J.; Cheng, C.H.; Husebø, B.S. The Effect of Music-Based Intervention on General Cognitive and Executive Functions, and Episodic Memory in People with Mild Cognitive Impairment and Dementia: A Systematic Review and Meta-Analysis of Recent Randomized Controlled Trials. Healthcare 2022, 10, 1462. [Google Scholar] [CrossRef] [PubMed]
- Foster, N.A.; Valentine, E.R. The effect of auditory stimulation on autobiographical recall in dementia. Exp. Aging Res. 2001, 27, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.G.; Moulin, C.J.; Hayre, S.; Jones, R.W. Music enhances category fluency in healthy older adults and Alzheimer’s disease patients. Exp. Aging Res. 2005, 31, 91–99. [Google Scholar] [CrossRef]
- Prickett, C.; Moore, R. The use of music to aid memory of Alzheimer’s patients. J. Music Ther. 1991, 28, 101–110. [Google Scholar] [CrossRef]
- Brotons, M.; Marti, P. Music therapy with Alzheimer’s patients and their family caregivers: A pilot project. J. Music Ther. 2003, 40, 138–150. [Google Scholar] [CrossRef]
- Fusar-Poli, L.; Bieleninik, Ł.; Brondino, N.; Chen, X.J.; Gold, C. The effect of music therapy on cognitive functions in patients with dementia: A systematic review and meta-analysis. Aging Ment. Health 2018, 22, 1103–1112. [Google Scholar] [CrossRef]
- Särkämö, T. Cognitive, emotional, and neural benefits of musical leisure activities in aging and neurological rehabilitation: A critical review. Ann. Phys. Rehabil. Med. 2018, 61, 414–418. [Google Scholar] [CrossRef]
- Raglio, A. Music therapy interventions in Parkinson’s disease: The state of-the-art. Front. Neurol. 2015, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Laffont, I.; Dalla Bella, S. Music, rhythm, rehabilitation and the brain: From pleasure to synchronization of biological rhythms. Ann. Phys. Rehabil. Med. 2018, 61, 363–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Wen, X.N.; Chen, Y.; Xu, N.; Zhang, J.H.; Hou, X.; Liu, J.P.; Li, P.; Chen, J.Y.; Wang, J.H.; et al. Effects of movement training based on rhythmic auditory stimulation in cognitive impairment: A meta-analysis of randomized controlled clinical trial. Front. Neurosci. 2024, 18, 1360935. [Google Scholar] [CrossRef] [PubMed]
- Thaut, M.H. Rhythm, Music and the Brain: Scientific Foundations and Clinical Applications; Taylor & Francis Group: New York, NY, USA, 2005; pp. 1–247. [Google Scholar]
- Galińska, E. Music therapy in neurological rehabilitation. Psychiatr. Pol. 2015, 49, 835–846. [Google Scholar] [CrossRef]
- Schlaug, G.; Altenmüller, E.; Thaut, M. Music listening and music making in the treatment of neurological disorders and impairments [editorial]. Music Percept. 2010, 27, 249–250. [Google Scholar] [CrossRef]
- Park, J.K.; Kim, S.J. Dual-task-based drum playing with rhythmic cueing on motor and attention control in patients with Parkinson’s disease: A preliminary randomized study. Int. J. Environ. Res. Public Health 2021, 18, 10095. [Google Scholar] [CrossRef]
- Hars, M.; Herrmann, F.R.; Gold, G.; Rizzoli, R.; Trombetti, A. Effect of music-based multitask training on cognition and mood in older adults. Age Ageing 2014, 43196, 200. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Mak, S.; Thomas, A. Steps for Conducting a Scoping Review. J. Grad. Med. Educ. 2022, 14, 565–567. [Google Scholar] [CrossRef]
- Biasutti, M.; Mangiacotti, A. Music Training Improves Depressed Mood Symptoms in Elderly People: A Randomized Controlled Trial. Int. J. Aging. Hum. Dev. 2021, 92, 115–133. [Google Scholar] [CrossRef]
- Biasutti, M.; Mangiacotti, A. Assessing a cognitive music training for older participants: A randomised controlled trial. Int. J. Geriatr. Psychiatry 2018, 33, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Pei, Y.C. Musical dual-task training in patients with mild-to-moderate dementia: A randomized controlled trial. Neuropsychiatr. Dis. Treat. 2018, 14, 1381–1393. [Google Scholar] [CrossRef]
- Chéour, S.; Chéour, C.; Gendreau, T.; Bouazizi, M.; Singh, K.P.; Saeidi, A.; Tao, D.; Supriya, R.; Bragazzi, N.L.; Baker, J.S.; et al. Remediation of cognitive and motor functions in Tunisian elderly patients with mild Alzheimer’s disease: Implications of music therapy and/or physical rehabilitation. Front. Aging Neurosci. 2023, 15, 1216052. [Google Scholar] [CrossRef]
- Cheung, D.S.K.; Lai, C.K.Y.; Wong, F.K.Y.; Leung, M.C.P. The effects of the music-with-movement intervention on the cognitive functions of people with moderate dementia: A randomized controlled trial. Aging Ment. Health 2018, 22, 306–315. [Google Scholar] [CrossRef]
- Doi, T.; Verghese, J.; Makizako, H.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Suzuki, T.; Shimada, H. Effects of Cognitive Leisure Activity on Cognition in Mild Cognitive Impairment: Results of a Randomized Controlled Trial. J. Am. Med. Dir. Ass. 2017, 18, 686–691. [Google Scholar] [CrossRef]
- Feng, L.; Romero-Garcia, R.; Suckling, J.; Tan, J.; Larbi, A.; Cheah, I.; Wong, G.; Tsakok, M.; Lanskey, B.; Lim, D.; et al. Effects of choral singing versus health education on cognitive decline and aging: A randomized controlled trial Presbyterian Community Services. Aging 2020, 12, 24798–24816. [Google Scholar] [CrossRef]
- Fraile, E.; Bernon, D.; Rouch, I.; Pongan, E.; Tillmann, B.; Lévêque, Y. The effect of learning an individualized song on autobiographical memory recall in individuals with Alzheimer’s disease: A pilot study. J. Clin. Exp. Neuropsychol. 2019, 41, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Giovagnoli, A.R.; Manfredi, V.; Parente, A.; Schifano, L.; Oliveri, S.; Avanzini, G. Cognitive training in Alzheimer’s disease: A controlled randomized study. Neurol. Sci. 2017, 38, 1485–1493. [Google Scholar] [CrossRef]
- Gómez-Gallego, M.; Cándido Gómez-Gallego, J.; Gallego-Mellado, M.; García-García, J.; Vasefi, M.; Tchounwou, P.B. Comparative Efficacy of Active Group Music Intervention versus Group Music Listening in Alzheimer’s Disease. Int. J. Environ. Res. Public Health 2021, 18, 8067. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Park, J.; Kim, H.; Jo, G.; Do, H.K.; Lee, B.I. Cognitive intervention with musical stimuli using digital devices on mild cognitive impairment: A pilot study. Healthcare 2020, 8, 45. [Google Scholar] [CrossRef]
- Kim, H.S.; Kang, J.S. Effect of a group music intervention on cognitive function and mental health outcomes among nursing home residents: A randomized controlled pilot study. Geriatr. Nurs. 2021, 42, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Liu, C.K.; Yang, Y.H.; Chou, M.C.; Chen, C.H.; Lai, C.L. Adjunct effect of music therapy on cognition in alzheimer’s disease in Taiwan: A pilot study. Neuropsychiatr. Dis. Treat. 2015, 11, 291–296. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, C.; Ying, F. Effect of Music Therapy on the Rehabilitation of Elderly People with Dementia. Front. Artif. Intell. Appl. 2024, 383, 459–466. [Google Scholar]
- Lyu, J.; Zhang, J.; Mu, H.; Li, W.; Champ, M.; Xiong, Q.; Gao, T.; Xie, L.; Jin, W.; Yang, W.; et al. The Effects of Music Therapy on Cognition, Psychiatric Symptoms, and Activities of Daily Living in Patients with Alzheimer’s Disease. J. Alzheimers. Dis. 2018, 64, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Maguire, L.E.; Wanschura, P.B.; Battaglia, M.M.; Howell, S.N.; Flinn, J.M. Participation in active singing leads to cognitive improvements in individuals with dementia. J. Am. Geriatr. Soc. 2015, 63, 815. [Google Scholar] [CrossRef]
- Mahendran, R.; Gandhi, M.; Moorakonda, R.B.; Wong, J.; Kanchi, M.M.; Fam, J.; Rawtaer, I.; Kumar, A.P.; Feng, L.; Kua, E.H. Art therapy is associated with sustained improvement in cognitive function in the elderly with mild neurocognitive disorder: Findings from a pilot randomized controlled trial for art therapy and music reminiscence activity versus usual care. Trials 2018, 19, 615. [Google Scholar] [CrossRef]
- Moreira, S.V.; Justi, F.R.D.R.; Gomes, C.F.A.; Moreira, M. Music Therapy Enhances Episodic Memory in Alzheimer’s and Mixed Dementia: A Double-Blind Randomized Controlled Trial. Healthcare 2023, 11, 2912. [Google Scholar] [CrossRef]
- Murabayashi, N.; Akahoshi, T.; Ishimine, R.; Saji, N.; Takeda, C.; Nakayama, H.; Noro, M.; Fujimoto, H.; Misaki, M.; Miyamoto, K.; et al. Effects of Music Therapy in Frail Elderlies: Controlled Crossover Study. Demen. Geriatr. Cogn. Dis. Extra 2019, 9, 87–99. [Google Scholar] [CrossRef]
- Pérez-Ros, P.; Cubero-Plazas, L.; Mejías-Serrano, T.; Cunha, C.; Martínez-Arnau, F.M. Preferred Music Listening Intervention in Nursing Home Residents with Cognitive Impairment: A Randomized Intervention Study. J. Alzheimers Dis. 2019, 70, 431–440. [Google Scholar] [CrossRef]
- Pongan, E.; Tillmann, B.; Leveque, Y.; Trombert, B.; Getenet, J.C.; Auguste, N.; Dauphinot, V.; el Haouari, H.; Navez, M.; Dorey, J.M.; et al. Can Musical or Painting Interventions Improve Chronic Pain, Mood, Quality of Life, and Cognition in Patients with Mild Alzheimer’s Disease? Evidence from a Randomized Controlled Trial. J. Alzheimers Dis. 2017, 60, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Särkämö, T.; Laitinen, S.; Numminen, A.; Kurki, M.; Johnson, J.K.; Rantanen, P. Clinical and demographic factors associated with the cognitive and emotional efficacy of regular musical activities in dementia. J. Alzheimers Dis. 2016, 49, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Särkämö, T.; Tervaniemi, M.; Laitinen, S.; Numminen, A.; Kurki, M.; Johnson, J.K.; Rantanen, P. Cognitive, emotional, and social benefits of regular musical activities in early dementia: Randomized controlled study. The Gerontologist 2014, 54, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Yuba, T.; Tabei, K.I.; Okubo, Y.; Kida, H.; Sakuma, H.; Tomimoto, H. Music Therapy Using Singing Training Improves Psychomotor Speed in Patients with Alzheimer’s Disease: A Neuropsychological and fMRI Study. Dement. Geriatr. Cogn. Dis. Extra 2015, 5, 296–308. [Google Scholar] [CrossRef]
- Shimizu, N.; Umemura, T.; Matsunaga, M.; Hirai, T. Effects of movement music therapy with a percussion instrument on physical and frontal lobe function in older adults with mild cognitive impairment: A randomized controlled trial. Aging Ment. Health 2018, 22, 1614–1626. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Y.; Yang, S.; Thomas, W.K.S.; Smith, G.D.; Yang, Z.; Yuan, L.; Chung, J.W. Effect of music intervention on apathy in nursing home residents with dementia. Geriatr. Nurs. 2018, 39, 471–476. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Xie, J.; Wang, T.; Yu, C.; An, N. Music therapy improves cognitive function and behavior in patients with moderate Alzheimer’s disease. Int. J. Clin. Exp. Med. 2018, 11, 4808–4814. [Google Scholar]
- Xue, B.; Meng, X.; Liu, Q.; Luo, X. The effect of receptive music therapy on older adults with mild cognitive impairment and depression: A randomized controlled trial. Sci. Rep. 2023, 13, 22159. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatric Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Reitan, R.M. Manual for Administration of Neuropsychological Test Batteries for Adults and Children; Neuropsychology Laboratory: Tucson, AZ, USA, 1979. [Google Scholar]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B. The FAB: A Frontal Assessment Battery at bedside. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef]
- Mondini, S.; Mapelli, D.; Vestri, A.; Arcara, G.; Bisiacchi, P.S. Esame Neuropsicologico Breve 2 (ENB-2); Raffaello Cortina Editore: Milan, Italy, 2011. [Google Scholar]
- Gómez-Soria, I.; Iguacel, I.; Aguilar-Latorre, A.; Peralta-Marrupe, P.; Latorre, E.; Zaldívar, J.N.C.; Calatayud, E. Cognitive stimulation and cognitive results in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2023, 104, 104807. [Google Scholar] [CrossRef]
- Liang, Y.; Qu, L.B.; Liu, H. Non-linear associations between sleep duration and the risks of mild cognitive impairment/dementia and cognitive decline: A dose–response meta-analysis of observational studies. Aging Clin. Exp. Res. 2019, 31, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Orfanos, S.; Gibbor, L.; Carr, C.; Spector, A. Group-based cognitive stimulation therapy for dementia: A qualitative study on experiences of group interactions. Aging Ment. Health 2020, 25, 991–998. [Google Scholar] [CrossRef]
- Rodakowski, J.; Saghafi, E.; Butters, M.A.; Skidmore, E. RNon-pharmacological interventions for adults with mild cognitive impairment and early stage dementia: An updated scoping review. Mol. Aspects Med. 2015, 43, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Clement, F.; Belleville, S. Compensation and disease severity on the memory-related activations in mild cognitive impairment. Biol. Psychiatry 2010, 68, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Belleville, S.; Fouquet, C.; Duchesne, S.; Collins, D.L.; Hudon, C. Detecting early preclinical Alzheimer’s disease via cognition, neuropsychiatry, and neuroimaging: Qualitative review and recommendations for testing. J. Alzheimers Dis. 2014, 42, S375–S382. [Google Scholar] [CrossRef] [PubMed]
- van der Steen, J.T.; Smaling, H.J.; van der Wouden, J.C.; Bruinsma, M.S.; Scholten, R.J.; Vink, A.C. Music-based therapeutic interventions for people with dementia. Cochrane Database Syst. Rev. 2018, 7, CD003477. [Google Scholar] [CrossRef]
| Study and Year [Ref.] | Study Design | Country | No. of Participants | Mean Age | Female | Severity of Cognitive Impairment | Type of Music-Based Intervention | Control Type | Intervention Period | Intervention Frequency | Intervention Duration | Cognitive Outcomes | Cognitive Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biasutti and Mangiacotti 2021 [52] | 1 RCT | Italy | 45 | 84.5 | 29/64.4% | 2 MCI and mild dementia | Music training (improvisation exercises) | Gymnastic activity | 6 weeks | 2 per week | 70 min | 3 MMSE | Improvement |
| Biasutti and Mangiacotti 2018 [53] | RCT | Italy | 35 | 83.57 | 23/65.7% | MCI and mild dementia | Music training (improvisation exercises) | Gymnastic activity (45 min) | 6 weeks | 2 per week | 70 min | MMSE, 4 VFT, 5 TMT-A, attentional matrices, 6 CDT | Improvement in MMSE, VFT, CDT |
| Chen 2018 [54] | RCT | Taiwan | 28 | 77.3 | 14/50% | Mild–moderate dementia | Musical dual-task training (7 NMT) | Dual-task training | 8 weeks | 1 per week | 60 min | TMT-A | Improvement |
| Chéour 2023 [55] | RCT | Tunisia | 28 | 72.8 | 12/42.85% | Mild 8 AD | Music listening | Physical rehabilitation PR + ML, control | 4 months | 3 times per week | 60 min | MMSE, 9 ADAS- Cog | Improvement (in ML, PR, and PR + ML) |
| Cheung 2018 [56] | RCT | China | 165 | 85.3 | 125/75.75% | Moderate dementia | Movement music Therapy (MM) | Music listening (ML), Social activity | 6 weeks | 2 per week | 40 min | MMSE; 10 FOME; Mod-VFT; Digit Span | Improvement (MM, ML) in MMSE; memory storage and recall; VFT improvement in MM group |
| Doi 2017 [57] | RCT | Japan | 201 | 76 | 104/51.7% | MCI | Playing instrument (percussions) | Dance, control | 10 months | 1 per week | 60 min | MMSE, TMT-A, TMT-B, story, and word memory | MMSE improvement (music group); improvement memory recall (dance group only) |
| Feng 2020 [58] | RCT | Malaysia | 93 | 70 | 73/78.5% | At risk of dementia (early cognitive impairment in 5/10 cognitive tests) | Choral singing | Health education | 2 years | 1 per week | 60 min | 11 CCTS, 12 SM-MMSE 13 MRI, oxidative damage/ immunosenescence | Improvement without intergroup difference; no difference in bio- markers |
| Fraile 2019 [59] | 14 CCT | France | 12 | 83.83 | 7/60% | Mild–moderate AD | Learning an individualized song (lyrics writing) | Control | 5 weeks + 5 weeks (Waitlist) | 2 per week | 20 min | Cued autobiographical recall, phonological, and semantic fluency; 15 EFCL (verbal, memory, executive process) | Improvement in autobio- graphical memory retrieval and general cognitive abilities |
| Giovagnoli 2017 [60] | RCT | Italy | 39 | 73.6 | 24/61.5% | Mild–moderate AD | Active music therapy | Active control (cognitive training CT, neuro- education NE) | 12 weeks | 2 per week | 45 min | TMT, 16 DSST | No significant changes; clinically significant improvement rates = CT: (62%) AMT: (8%) NE: (none) |
| Gómez- Gallego 2021 [61] | Quasi- experi- mental design (Randomization of nursing homes) | Spain | 90 | 80.9 | 55/61.1% | Mild–moderate dementia (probable AD) | Active music intervention (AMI); receptive music Intervention (RMI) | Control pharmacological therapy, cognitive stimulation | 12 weeks | 2 per week | 45 min | MMSE | Improve- ment in AMI (higher than in RMI) |
| Han 2020 [62] | RCT, pilot | Korea | 24 | 73.12 | 11/45.8% | MCI | Song-based cognitive stimulation protocol | Control | 10 weeks | 2 per week | 60 min | MMSE-DS 17 MoCA-K | Improvement |
| Kim and Kang 2021 [63] | RCT, pilot | South Korea | 49/40 | 81.6 | 31/77.5% | Mild–moderate dementia | Active music intervention (rhythmic exercises) | Control (usual care) | 12 weeks | 2 per week | 50 min | MMSE- Korean version | Improvement |
| Li 2015 [64] | Quasi- experi- mental trial design | Taiwan | 41 | 78.75 | 28/68.29% | Mild AD | Music listening (2 different pieces) | Control | 6 months | 2 daily | 30 min | 18 CASI, CASI- estimated MMSE | No improvement (less decreased score than controls) |
| Liu 2024 [65] | RCT | China | 24 | 69.45 | 12/50% | Mild–moderate Dementia | Group music therapy (activities change across sessions) | Control (usual care) | 5 months | 1 per week | 40 min | MMSE | Improved scores (without statistics) |
| Lyu 2018 [66] | RCT | China | 298 | 69.7 | 173/58.1% | 10 AD (mild, moderate, severe) | Singing (S) | Reading (R), control | 12 weeks | 2 per week (2 per day) | 30–40 min | VFT, 19 AVLT, MMSE | Improvement in VFT (S and R groups) and in immediate recall (singing) |
| Maguire 2015 [67] | No rando- mization (voluntary partici- pation) | USA | 45 | 70–99 | 38/85% | MCI and mild–moderate dementia | Singing (vocal training) | Music listening | 16 weeks | 3 times per week | 50 min | MMSE, CDT | Improvement in MMSE (both groups, larger effect in singing group) |
| Mahendran 2018 [68] | RCT, pilot | Malaysia | 68 | 71.1 | 38/55.9% | MCI | Music listening ML (reminiscence) | Art therapy, control | 12 weeks | 1 per week | 65 min | AVLT | Improvement in ML group |
| Moreira 2023 [69] | RCT | Brazil | 43 | 76.49 | 39/91% | MCI and mild–moderate dementia | Neurologic music therapy | Control | 6 weeks | 2 per week | 30–40 min | 20 WAIS-III Digit subtest; Corsi block-tapping test; 21 FMT; 22 SASMET; CDT; MMSE | Improvement in episodic memory tests only |
| Murabayashi 2019 [70] | CCT | Japan | 115 | 81.3 | 109/93.6% | Frail elderlies (with dementia or other care needs) | Music listening and singing | Control | 12 weeks +12 weeks (Waitlist) | 1 per week | 45–50 min | VFT: 23 YKSST | No significant difference observed either in the period- effect or treatment for cognition |
| Perez-Ros 2019 [71] | RCT | Spain | 119 | 80.52 | 61/51.26% | Mild–moderate dementia | Preferred music listening + occupational therapy | Occupatio- nal therapy | 8 weeks | 5 days per week | 60 min | MMSE | Mainte- nance (worse- ning in controls) |
| Pongan 2017 [72] | RCT | France | 59 | 79.5 | 39/66.1% | Mild AD | Singing | Paint | 12 weeks | 1 per week | 120 min | TMT-A, 24 FAB | Improvement (digit, inhibitory processes) in both groups; singing group: verbal memory stable |
| Särkämö 2016 [73] | RCT | Finland | 89 | 78.3 | 55/51.4% | Mild–moderate dementia | Singing groups, music listening groups (ML) | Standard Care | 10 weeks | 1 per week | 90 min | MMSE, 25 WMS-III FAB, WAIS-III, TMT-A, 26 BNT; 27 WAB | Singing: improvement in working memory; maintenance: executive function, orientation (mild dementia); ML-supported general cognition, working memory (moderate dementia) |
| Särkämö 2014 [74] | RCT | Finland | 89 | 78.8 | 60/67.4% | Mild–moderate dementia | Singing groups, music listening groups | Standard Care | 10 weeks | 1 per week | 90 min | MMSE, WMS-III, FAB, WAIS-III, TMT-A, BNT, WAB | Experimental groups: improvement orientation, remote episodic memory; attention, executive function, general cognition slightly improved; effect of singing also in short-term and working memory |
| Satoh 2015 [75] | CT | Japan | 20 | 77.55 | 14/70% | Mild–moderate AD | Singing training | Control | 6 months | 1 per week | 60 min | MMSE, 28 RCPM, 29 RBMT, FAB, fMRI assessment | Time for RCPM completion significant reduction; increased activity in the right angular gyrus and the left lingual gyrus (fMRI) |
| Shimizu 2018 [76] | RCT | Japan | 45 | 74.64 | 38/84.4% | MCI | Movement music therapy (MMT) | Gymnastic activity | 12 weeks | 1 per week | 65 min | FAB, 30 CBF with 31 NIRS | Improvement (FAB) in MMT; significant increase in CBF |
| Tang 2018 [77] | RCT | China | 77 | 75.88 | 38/49.4% | Mild–moderate AD | Listening, singing, and playing instruments | Control | 12 weeks | 3 times per week | 50 min | MMSE | Mainte- nance (decrease in controls) |
| Wang 2018 [78] | RCT | China | 60 | 69.75 | 38/63.3% | Mild AD | Listening and singing familiar songs (+pharmacolo- gical therapy) | Pharmacological therapy only | 12 weeks | 3 times per day | 30–50 min | MMSE, MoCA | Improvement (MMSE); listening group. MoCA significant increase (both groups) |
| Xue 2023 [79] | RCT | China | 80 | 74.93 | 62/77.5% | MCI | Receptive music therapy (RMT) | Standard Care | 8 weeks | 4 times per week | 20 min (+time for the last two session phases) | MoCA | Improvement in RMT (especially memory, attention, abstraction) |
| Study and Year [Ref.] | Music Intervention Provider | Group or Individual Intervention |
|---|---|---|
| Biasutti and Mangiacotti 2021 [52] | Music psychologist | Group |
| Biasutti and Mangiacotti 2018 [53] | Music psychologist | Group |
| Chen 2018 [54] | Music therapist | Individual |
| Chéour 2023 [55] | Music therapist | Group (with caregivers) |
| Cheung 2018 [56] | Health care professional | Group (4–6 people) |
| Doi 2017 [57] | Music teacher | Group |
| Feng 2020 [58] | Professional musicians | Group |
| Fraile 2019 [59] | Language and speech therapist trainees | Individual |
| Giovagnoli 2017 [60] | Music therapist | Individual |
| Gómez-Gallego 2021 [61] | Art therapists (specialized in music therapy) | Group (6–9 people) |
| Han 2020 [62] | Music therapist (and neurologist) | Group |
| Kim and Kang 2021 [63] | Music therapist | Group |
| Li 2015 [64] | Self-administration (with the help of caregivers) | Individual |
| Liu 2024 [65] | Music therapist | Group |
| Lyu 2018 [66] | Music therapist | Group (6 people) |
| Maguire 2015 [67] | Vocal teacher (+DVD instructor) | Group |
| Mahendran 2018 [68] | Art therapist (specialized in music) | Group |
| Moreira 2023 [69] | Music therapist (and psychologist) | Individual (with caregiver presence) |
| Murabayashi 2019 [70] | Music therapist | Group |
| Perez-Ros 2019 [71] | Nurses | Group |
| Pongan 2017 [72] | Choir conductor (with a psychologist) | Group |
| Särkämö 2016 [73] | Music therapist and Vocal teacher | Group (10 people = 5 caregiver-patient dyads) |
| Särkämö 2014 [74] | Music therapist and Vocal teacher | Group (10 people = 5 caregiver-patient dyads) |
| Satoh 2015 [75] | Vocal teacher (+a pianist) | Group |
| Shimizu 2018 [76] | Instructor (trained in protocol administration) | Group |
| Tang 2018 [77] | Music therapist | Group (9 people) |
| Wang 2018 [78] | Music therapist | Group |
| Xue 2023 [79] | Music therapist | Group |
| Cognitive Area | Cognitive Measures | No. of Studies |
|---|---|---|
| Global Cognition (or composite score cognitive test) | MMSE | 20 |
| MoCA | 3 | |
| AVLT | 2 | |
| CASI | 2 | |
| ADAS-Cog | 1 | |
| CCTS | 1 | |
| Memory | AVLT | 2 |
| Digit Span | 2 | |
| WMS | 2 | |
| Corsi block-tapping test | 1 | |
| Cued autobiographical recall | 1 | |
| FMT | 1 | |
| FOME | 1 | |
| RBMT | 1 | |
| SASMET | 1 | |
| Story and word memory | 1 | |
| Attention (Speed processing) | TMT-A | 7 |
| Attentional matrices | 1 | |
| DSST | 1 | |
| YKSST | 1 | |
| Executive Functions | FAB | 5 |
| CDT | 3 | |
| Digit Span Backward | 2 | |
| TMT-B | 2 | |
| Reasoning | WAIS | 3 |
| RCPM | 1 | |
| Language | VFT | 4 |
| BNT | 2 | |
| EFCL | 1 | |
| Phonological and semantic fluency | 1 | |
| WAB | 1 | |
| Neuroimaging techniques | fMRI (cerebral activity, immunosenescence) | 2 |
| CBF with NIRS | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raglio, A.; Figini, C.; Bencivenni, A.; Grossi, F.; Boschetti, F.; Manera, M.R. Cognitive Stimulation with Music in Older Adults with Cognitive Impairment: A Scoping Review. Brain Sci. 2024, 14, 842. https://doi.org/10.3390/brainsci14080842
Raglio A, Figini C, Bencivenni A, Grossi F, Boschetti F, Manera MR. Cognitive Stimulation with Music in Older Adults with Cognitive Impairment: A Scoping Review. Brain Sciences. 2024; 14(8):842. https://doi.org/10.3390/brainsci14080842
Chicago/Turabian StyleRaglio, Alfredo, Camilla Figini, Alice Bencivenni, Federica Grossi, Federica Boschetti, and Marina Rita Manera. 2024. "Cognitive Stimulation with Music in Older Adults with Cognitive Impairment: A Scoping Review" Brain Sciences 14, no. 8: 842. https://doi.org/10.3390/brainsci14080842
APA StyleRaglio, A., Figini, C., Bencivenni, A., Grossi, F., Boschetti, F., & Manera, M. R. (2024). Cognitive Stimulation with Music in Older Adults with Cognitive Impairment: A Scoping Review. Brain Sciences, 14(8), 842. https://doi.org/10.3390/brainsci14080842






