Functional Network Connectivity for Components of Depression-Related Psychological Fragility
Abstract
:1. Introduction
1.1. Resilience and Fragility
1.2. Neural Networks and Functional Connectivity
2. Materials and Methods
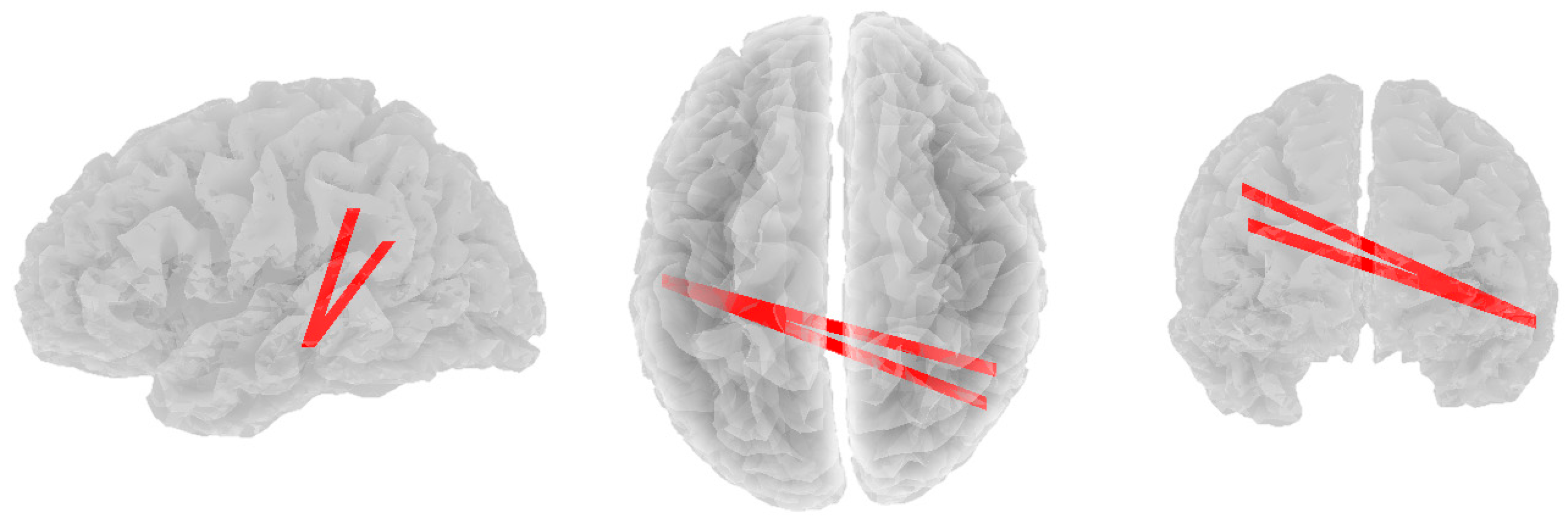
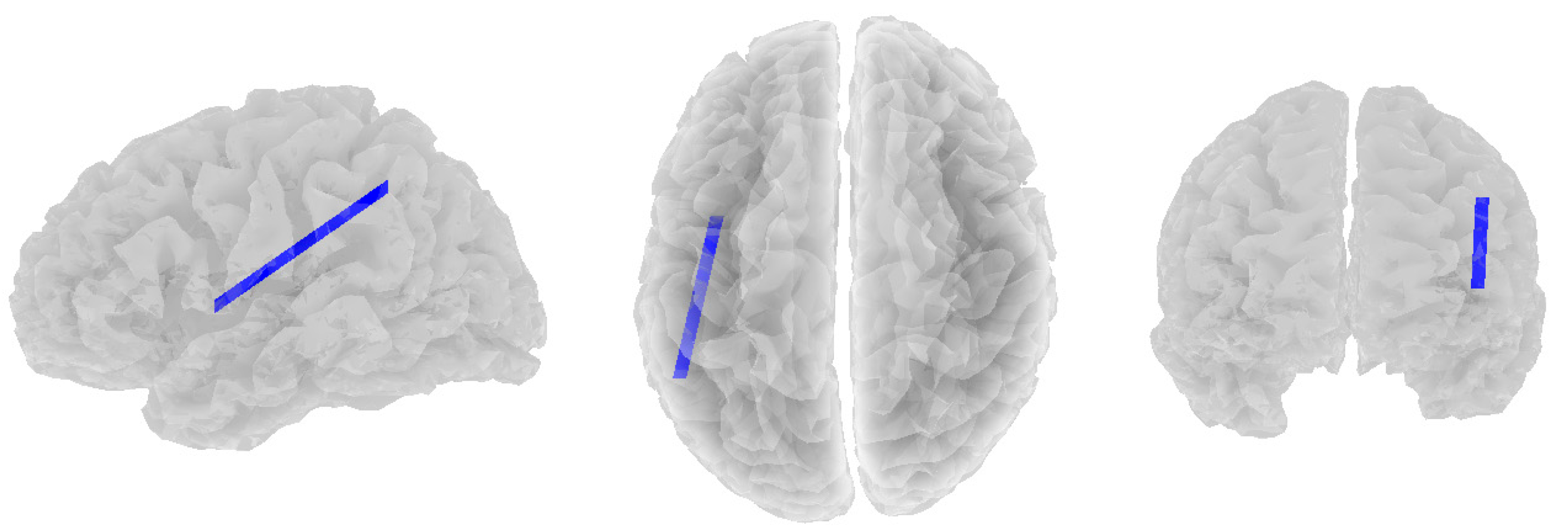
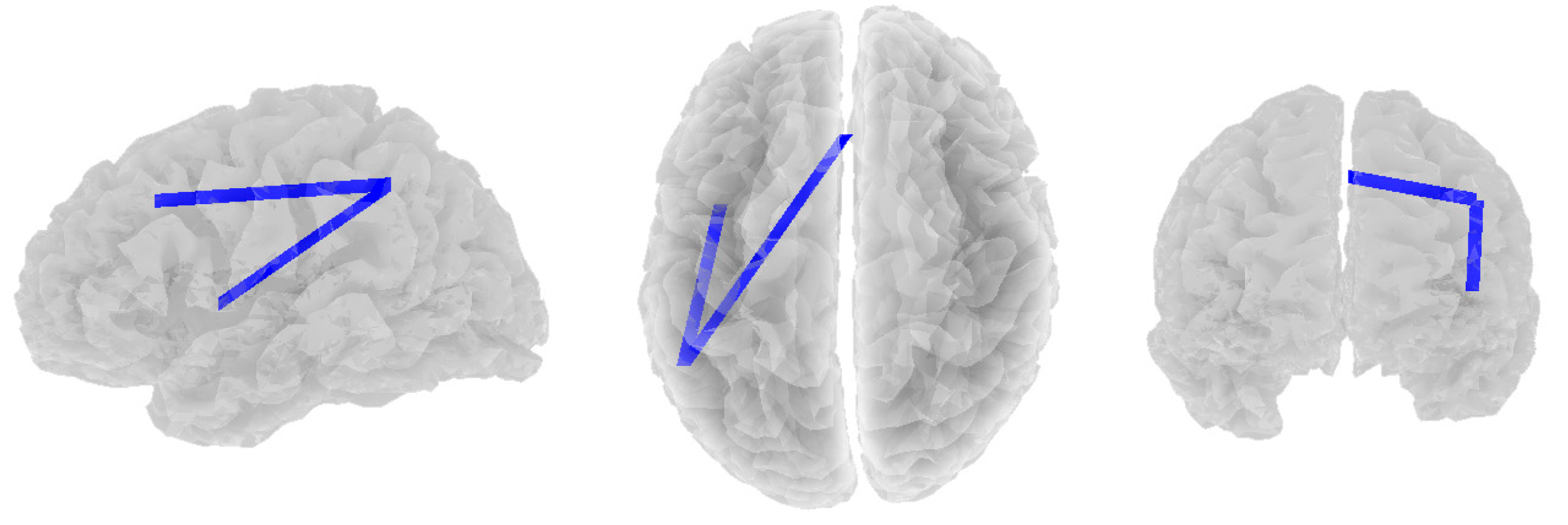
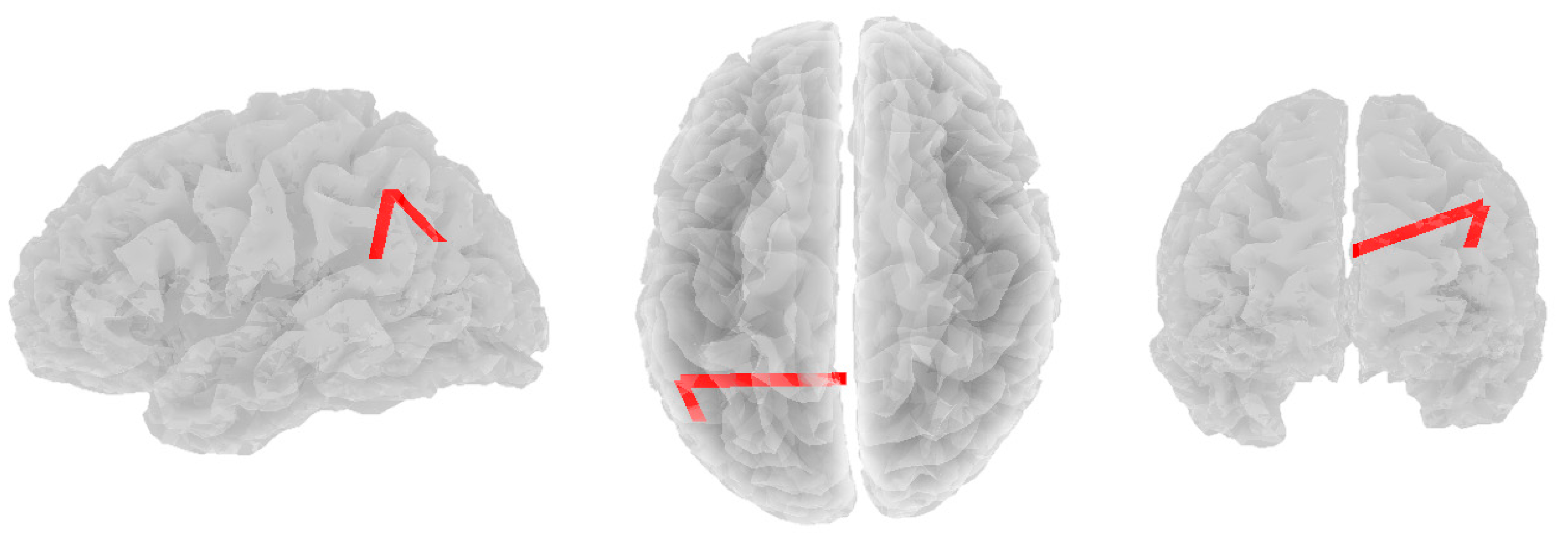
3. Results
3.1. Data
3.2. Alpha Band
3.3. Beta Band
3.4. Theta Band
4. Discussion
4.1. Theta
4.2. Alpha
4.3. Beta
4.4. Depression-Based Differences
4.5. The Depressed Group
4.6. The Non-Depressed Group
4.7. Limitations
4.8. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostergaard, S.; Jensen, S.; Bech, P. The heterogeneity of the depressive syndrome: When numbers get serious. Acta Psychiatr. Scand. 2011, 124, 495–496. [Google Scholar] [CrossRef]
- APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Text Revision; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Baumeister, H.; Parker, G. Meta-review of depressive subtyping models. J. Affect. Disord. 2012, 139, 126–140. [Google Scholar]
- Ventriglio, A.; Bhugra, D.; Sampogna, G.; Luciano, M.; De Berardis, D.; Sani, G.; Fiorillo, A. From dysthymia to treatment-resistant depression: Evolution of a psychopathological construct. Int. Rev. Psychiatry 2020, 32, 471–476. [Google Scholar] [CrossRef]
- Parker, G. Beyond major depression. Psychol. Med. 2005, 35, 467–474. [Google Scholar] [CrossRef]
- Sharpley, C.F.; Bitsika, V. Differences in neurobiological pathways of four “clinical content” subtypes of depression. Behav. Brain Res. 2013, 256, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.; van Loo, H.; Wardenaar, K.; Bossarte, R.; Brenner, L.; Ebert, D.; de Jonge, P.; Nierenberg, A.; Rosellini, A.; Sampson, N.; et al. Using patient self-reports to study heterogeneity of treatment effects in major depressive disorder. Epidemiol. Psychiatr. Sci. 2017, 26, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Yang, X.; Yang, H.; Zhou, P.; Ma, H.; Teng, C.; Chen, H.; Ou, H.; Li, J.; Mathews, C.A. Psychological resilience as a protective factor for depression and anxiety among the public during the outbreak of COVID-19. Front. Psychol. 2021, 11, 618509. [Google Scholar] [CrossRef]
- Fletcher, D.; Sarkar, M. Psychological Resilience. Eur. Psychol. 2013, 18, 12–23. [Google Scholar] [CrossRef]
- Favale, D.; Nacci, M.; Bellomo, A.; Torales, J.; Castaldelli-Maia, J.M.; Ventriglio, A. Hope and resilience among patients affected by unipolar and bipolar depression. Int. J. Soc. Psychiatry 2023, 69, 967–975. [Google Scholar] [CrossRef]
- Lau, W.K. The role of resilience in depression and anxiety symptoms: A three-wave cross-lagged study. Stress Health 2022, 38, 804–812. [Google Scholar] [CrossRef]
- Leys, C.; Kotsou, I.; Shankland, R.; Firmin, M.; Péneau, S.; Fossion, P. Resilience predicts lower anxiety and depression and greater recovery after a vicarious trauma. Int. J. Environ. Res. Public Health 2021, 18, 12608. [Google Scholar] [CrossRef]
- Fischer, A.S.; Hagan, K.E.; Gotlib, I.H. Functional neuroimaging biomarkers of resilience in major depressive disorder. Curr. Opin. Psychiatry 2021, 34, 22–28. [Google Scholar] [CrossRef]
- Cooper, J.A.; Nuutinen, M.R.; Lawlor, V.M.; DeVries, B.A.; Barrick, E.M.; Hossein, S.; Leonard, C.V.; Hahn, E.C.; Teer, A.P.; Shields, G.S.; et al. Reduced adaptation of glutamatergic stress response is associated with pessimistic expectations in depression. Nat. Commun. 2021, 12, 3166. [Google Scholar] [CrossRef]
- Connor, K.; Davidson, J. Development of a new resilience scale: The Connor-Davidson Resilience Scale (CD-RISC). Depress. Anxiety 2003, 18, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Sharpley, C.; Bitsika, V.; Christie, D. ‘Steeling’ Effects in the Association between Psychological Resilience and Cancer Treatment in Prostate Cancer patients. Psycho-Oncology 2021, 30, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Fang, H.; Liu, Y.; Zheng, L.; Zhu, X.; Duan, H.; Wu, J. Neurocognitive correlates of psychological resilience: Event-related potential studies. J. Affect. Disord. 2022, 312, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Hasan, R.A.; Ali SS, A.; Tang, T.B.; Yusoff, M.S.B. Finding the EEG Footprint of Stress Resilience. In Proceedings of the International Conference on Artificial Intelligence for Smart Community: AISC 2020, Universiti Teknologi Petronas, Seri Iskandar, Malaysia, 17–18 December 2020; Springer Nature: Singapore, 2022. [Google Scholar]
- Paban, V.; Modolo, J.; Mheich, A.; Hassan, M. Psychological resilience correlates with EEG source-space brain network flexibility. Netw. Neurosci. 2019, 3, 539–550. [Google Scholar] [CrossRef]
- Greicius, M.D.; Flores, B.H.; Menon, V.; Glover, G.H.; Solvason, H.B.; Kenna, H.; Reiss, A.L.; Schatzberg, A.F. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry 2007, 62, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Northoff, G.; Wiebking, C.; Feinberg, T.; Panksepp, J. The “resting-state hypothesis” of major depressive disorder—A translational subcortical–cortical framework for a system disorder. Neurosci. Biobehav. Rev. 2011, 35, 1929–1945. [Google Scholar] [CrossRef]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef]
- Fingelkurts, A.A.; Fingelkurts, A.A. Three-dimensional components of selfhood in treatment-naive patients with major depressive disorder: A resting-state qEEG imaging study. Neuropsychologia 2017, 99, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Knyazev, G.G.; Savostyanov, A.N.; Bocharov, A.V.; Brak, I.V.; Osipov, E.A.; Filimonova, E.A.; Saprigyn, A.E.; Aftanas, L.I. Task-positive and task-negative networks in major depressive disorder: A combined fMRI and EEG study. J. Affect. Disord. 2018, 235, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.M.; Yahya, N.; Kamel, N.; Faye, I. Automated diagnosis of major depressive disorder using brain effective connectivity and 3D convolutional neural network. IEEE Access 2021, 9, 8835–8846. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Supekar, K.S.; Ryali, S.; Menon, V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J. Neurosci. 2011, 31, 18578–18589. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.P.; Furman, D.J.; Chang, C.; Thomason, M.E.; Dennis, E.; Gotlib, I.H. Default-mode and task-positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biol. Psychiatry 2011, 70, 327–333. [Google Scholar] [CrossRef]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Lehmann, D.; Koukkou, M.; Kochi, K.; Anderer, P.; Saletu, B.; Tanaka, H.; Hirata, K.; John, E.R.; Prichep, L.; et al. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 3768–3784. [Google Scholar] [CrossRef]
- Miljevic, A.; Bailey, N.W.; Murphy, O.W.; Perera, M.P.N.; Fitzgerald, P.B. Alterations in EEG functional connectivity in individuals with depression: A systematic review. J. Affect. Disord. 2023, 328, 287–302. [Google Scholar] [CrossRef]
- Olbrich, S.; Tränkner, A.; Chittka, T.; Hegerl, U.; Schönknecht, P. Functional connectivity in major depression: Increased phase synchronization between frontal cortical EEG-source estimates. Psychiatry Res. Neuroimaging 2014, 222, 91–99. [Google Scholar] [CrossRef]
- Whitton, A.E.; Deccy, S.; Ironside, M.L.; Kumar, P.; Beltzer, M.; Pizzagalli, D.A. Electroencephalography source functional connectivity reveals abnormal high-frequency communication among large-scale functional networks in depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 50–58. [Google Scholar] [CrossRef]
- Zung, W. A self-rating depression scale. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zung, W. From art to science: The diagnosis and treatment of depression. Arch. Gen. Psychiatry 1973, 29, 328–337. [Google Scholar] [CrossRef] [PubMed]
- DeJonge, J.; Baneke, J. The Zung Self-rating Depression Scale: A replication study on reliability, validity and prediction. Psychol. Rep. 1989, 64, 833–834. [Google Scholar] [CrossRef]
- Gabrys, J.; Peters, K. Reliability, discriminant and predictive validity of the Zung Self-Rating Depression Scale. Psychol. Rep. 1985, 57, 1091–1096. [Google Scholar] [CrossRef]
- Schaefer, A.; Brown, J.; Watson, C.; Plenel, D.; DeMotts, J.; Howard, M.; Petrik, N.; Ballweg, B. Comparison of the validities of the Beck, Zung and MMPI depression scales. J. Consult. Clin. Psychol. 1985, 53, 415–418. [Google Scholar] [CrossRef]
- Marco-Pallarés, J.; Grau, C.; Ruffini, G. Combined ICA-LORETA analysis of mismatch negativity. Neuroimage 2005, 25, 471–477. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Esslen, M.; Kochi, K.; Lehmann, D. Functional imaging with low resolution brain electromagnetic tomography (LORETA): Review, new comparisons, and new validation. Jpn. J. Clin. Neurophysiol. 2002, 30, 81–94. [Google Scholar]
- Mazziotta, J.; Toga, A.; Evans, A.; Fox, P.; Lancaster, J.; Zilles, K.; Woods, R.; Paus, T.; Simpson, G.; Pike, B.; et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2001, 356, 1293–1322. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E. The restless brain. Brain Connect. 2011, 1, 3–12. [Google Scholar] [CrossRef]
- Sharpley, C.; Evans, I.; Bitsika, V.; Arnold, W.; Jesulola, E.; Agnew, L. Frontal Alpha Asymmetry Confirms the Heterogeneity of Psychological Resilience. Brain Sci. 2023, 13, 1354. [Google Scholar] [CrossRef]
- Iseger, T.A.; Korgaonkar, M.S.; Kenemans, J.L.; Grieve, S.M.; Baeken, C.; Fitzgerald, P.B.; Arns, M. EEG connectivity between the subgenual anterior cingulate and prefrontal cortices in response to antidepressant medication. Eur. Neuropsychopharmacol. 2017, 27, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Han, D.H.; Hong, J.S.; Min, K.J.; Lee, Y.S.; Hahm, B.J.; Kim, S.M. Features of resting-state electroencephalogram theta coherence in somatic symptom disorder compared with major depressive disorder: A pilot study. Psychosom. Med. 2017, 79, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Dell’Acqua, C.; Ghiasi, S.; Benvenuti, S.M.; Greco, A.; Gentili, C.; Valenza, G. Increased functional connectivity within alpha and theta frequency bands in dysphoria: A resting-state EEG study. J. Affect. Disord. 2021, 281, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Orgo, L.; Bachmann, M.; Kalev, K.; Hinrikus, H.; Järvelaid, M. Brain functional connectivity in depression: Gender differences in EEG. In Proceedings of the 2016 IEEE EMBS Conference on Biomedical Engineering and Sciences (IECBES), Kuala Lumpur, Malaysia, 4–8 December 2016; pp. 270–273. [Google Scholar]
- Klimesch, W. Memory processes, brain oscillations and EEG synchronization. Int. J. Psychophysiol. 1996, 24, 61–100. [Google Scholar] [CrossRef]
- Pfurtscheller, G. Induced oscillations in the alpha band: Functional meaning. Epilepsia 2003, 44, 2–8. [Google Scholar] [CrossRef]
- Thut, G.; Miniussi, C.; Gross, J. The functional importance of rhythmic activity in the brain. Curr. Biol. 2012, 22, R658–R663. [Google Scholar] [CrossRef]
- Bonnefond, M.; Kastner, S.; Jensen, O. Communication between brain areas based on nested oscillations. eneuro 2017, 4. [Google Scholar] [CrossRef]
- Hasanzadeh, F.; Mohebbi, M.; Rostami, R. Graph theory analysis of directed functional brain networks in major depressive disorder based on EEG signal. J. Neural Eng. 2020, 17, 026010. [Google Scholar] [CrossRef]
- Knott, V.; Mahoney, C.; Kennedy, S.; Evans, K. EEG power, frequency, asymmetry and coherence in male depression. Psychiatry Res. Neuroimaging 2001, 106, 123–140. [Google Scholar] [CrossRef]
- McVoy, M.; Aebi, M.E.; Loparo, K.; Lytle, S.; Morris, A.; Woods, N.; Deyling, E.; Tatsuoka, C.; Kaffashi, F.; Lhatoo, S.; et al. Resting-state quantitative electroencephalography demonstrates differential connectivity in adolescents with major depressive disorder. J. Child Adolesc. Psychopharmacol. 2019, 29, 370–377. [Google Scholar] [CrossRef]
- Park, C.A.; Kwon, R.J.; Kim, S.; Jang, H.R.; Chae, J.H.; Kim, T.; Jeong, J. Decreased phase synchronization of the EEG in patients with major depressive disorder. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering 2006, Seoul, Republic of Korea, 27 August–1 September 2006; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1095–1098. [Google Scholar]
- Damborská, A.; Honzírková, E.; Barteček, R.; Hořínková, J.; Fedorová, S.; Ondruš, Š.; Michel, C.M.; Rubega, M. Altered directed functional connectivity of the right amygdala in depression: High-density EEG study. Sci. Rep. 2020, 10, 4398. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.W.; Frey, L.C.; Hopp, J. The normal EEG. In Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants; American Epilepsy Society: Chicago, IL, USA, 2016. [Google Scholar]
- Gu, X.; Hof, P.R.; Friston, K.J.; Fan, J. Anterior insular cortex and emotional awareness. J. Comp. Neurol. 2013, 521, 3371–3388. [Google Scholar] [CrossRef] [PubMed]
- Pavuluri, M.; May, A. I feel, therefore, I am: The insula and its role in human emotion, cognition and the sensory-motor system. Aims Neurosci. 2015, 2. [Google Scholar] [CrossRef]
- Scalabrini, A.; Vai, B.; Poletti, S.; Damiani, S.; Mucci, C.; Colombo, C.; Zanardi, R.; Benedetti, F.; Northoff, G. All roads lead to the default-mode network—Global source of DMN abnormalities in major depressive disorder. Neuropsychopharmacology 2020, 45, 2058–2069. [Google Scholar] [CrossRef]
- Engel, A.K.; Fries, P. Beta-band oscillations—Signalling the status quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef]
- Neuner, I.; Arrubla, J.; Werner, C.J.; Hitz, K.; Boers, F.; Kawohl, W.; Shah, N.J. The default mode network and EEG regional spectral power: A simultaneous fMRI-EEG study. PLoS ONE 2014, 9, e88214. [Google Scholar] [CrossRef]
- Dunlop, B.W.; Cha, J.; Choi, K.S.; Rajendra, J.K.; Nemeroff, C.B.; Craighead, W.E.; Mayberg, H.S. Shared and unique changes in brain connectivity among depressed patients after remission with pharmacotherapy versus psychotherapy. Am. J. Psychiatry 2023, 180, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Fonzo, G.A.; Goodkind, M.S.; Oathes, D.J.; Zaiko, Y.V.; Harvey, M.; Peng, K.K.; Weisse, M.E.; Thompson, A.L.; Zack, S.E.; Lindley, S.E.; et al. Amygdala and insula connectivity changes following psychotherapy for post-traumatic stress disorder: A randomized clinical trial. Biol. Psychiatry 2021, 89, 857–867. [Google Scholar] [CrossRef]
- Linden, D.E. How psychotherapy changes the brain–the contribution of functional neuroimaging. Mol. Psychiatry 2006, 11, 528–538. [Google Scholar] [CrossRef]
- Barsaglini, A.; Sartori, G.; Benetti, S.; Pettersson-Yeo, W.; Mechelli, A. The effects of psychotherapy on brain function: A systematic and critical review. Prog. Neurobiol. 2014, 114, 1–14. [Google Scholar] [CrossRef]
- Yuan, H.; Young, K.D.; Phillips, R.; Zotev, V.; Misaki, M.; Bodurka, J. Resting-state functional connectivity modulation and sustained changes after real-time functional magnetic resonance imaging neurofeedback training in depression. Brain Connect. 2014, 4, 690–701. [Google Scholar] [CrossRef]
- Taylor, J.E.; Yamada, T.; Kawashima, T.; Kobayashi, Y.; Yoshihara, Y.; Miyata, J.; Kawato, M.; Motegi, T. Depressive symptoms reduce when dorsolateral prefrontal cortex-precuneus connectivity normalizes after functional connectivity neurofeedback. Sci. Rep. 2022, 12, 2581. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.; Shand, F.; Tighe, J.; Laurent, S.; Bryant, S. Road to resilience: A systematic review and meta-analysis of resilience training programmes and interventions. BMJ Open 2018, 8, e017858. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.; Fikretoglu, D. Building resilience: The conceptual basis and research evidence for resilience training programs. Rev. Gen. Psychol. 2018, 22, 452–468. [Google Scholar] [CrossRef]
- Keynan, J.N.; Cohen, A.; Jackont, G.; Green, N.; Goldway, N.; Davidov, A.; Meir-Hasson, Y.; Raz, G.; Intrator, N.; Fruchter, E.; et al. Electrical fingerprint of the amygdala guides neurofeedback training for stress resilience. Nat. Hum. Behav. 2019, 3, 63–73. [Google Scholar] [CrossRef]
- Kallen, V.; van Wouwe, N.; Delahaij, R.; Boeschoten, M.; Vermetten, E. Using Neurological Feedback to Enhance Resilience and Recuperation. In Proceedings of the NATO Human Factors and Medicine Panel (HFM) Symposium, Bergen, Norway, 11–13 April 2011. [Google Scholar]

| CDRISC Factors | CDRISC Items |
|---|---|
| 1: Personal competence, high standards, and tenacity | 10. I give my best effort no matter what |
| 11. I can achieve my goals | |
| 12. When things look hopeless, I don’t give up | |
| 16. I am not easily discouraged by failure | |
| 17. I think of myself as a strong person | |
| 23. I like challenges | |
| 24. I work to attain my goals | |
| 25. I have pride in my achievements | |
| 2: Trust in one’s instincts, tolerance of negative affect, and strengthening effects of stress | 6. I can see the humorous side of things |
| 7. I believe that coping with stress strengthens me | |
| 14. When I’m under pressure, I can focus and think clearly | |
| 15. I prefer to take the lead in problem solving | |
| 18. I make unpopular or difficult decisions | |
| 19. I can handle unpleasant feelings | |
| 20. I have to act on a hunch | |
| 3: Positive acceptance of change and secure relationships | 1. I am able to adapt to change |
| 2. I have close and secure relationships | |
| 4. I can deal with whatever comes | |
| 5. Past success gives me confidence for new challenges | |
| 8. I tend to bounce back after illness or hardship | |
| 4: Control | 13. I know where to turn for help |
| 21. I have a strong sense of purpose | |
| 22. I am in control of my life | |
| 5: Spiritual Influences | 3. Sometimes fate or God can help me |
| 9. Things happen for a reason |
| Network | Location | MNI (X, Y, Z) |
|---|---|---|
| Default Mode Network | posterior cingulate | 0, −52, 27 |
| medial prefrontal cortex | −1, 54, 27 | |
| left lateral parietal lobule | −46, −66, 30 | |
| right lateral parietal lobule | 49, −63, 33 | |
| left inferior temporal lobule | −61, −24, −9 | |
| right inferior temporal lobule | 58, −24, −9 | |
| Central Executive Network | dorsal medial prefrontal cortex | 0, 24, 46 |
| left anterior prefrontal cortex | −44, 45, 0 | |
| right anterior prefrontal cortex | 44, 45, 0 | |
| left superior parietal lobule | −50, −51, 45 | |
| right superior parietal lobule | 50, −51, 45 | |
| Salience Network | dorsal anterior cingulate | 0, −21, 36 |
| left anterior prefrontal cortex | −35, 45, 30 | |
| right anterior prefrontal cortex | 32, 45, 30 | |
| left insula | −41, 3, 6 | |
| right insula | 41, 3, 6 | |
| left lateral parietal lobule | −62, −45, 30 | |
| right lateral parietal lobule | 62, −45, 30 |
| Resilience Factor | Group | Mean | SD | SEM | 5% Trimmed Mean |
|---|---|---|---|---|---|
| 1 | ND | 4.18 | 0.51 | 0.06 | 4.20 |
| D | 3.28 | 0.66 | 0.12 | 3.27 | |
| 2 | ND | 3.63 | 0.39 | 0.05 | 3.64 |
| D | 2.95 | 0.55 | 0.10 | 2.95 | |
| 3 | ND | 4.36 | 0.49 | 0.06 | 4.38 |
| D | 3.25 | 0.75 | 0.13 | 3.25 | |
| 4 | ND | 4.09 | 0.67 | 0.08 | 4.13 |
| D | 3.10 | 0.88 | 0.15 | 3.09 | |
| 5 | ND | 2.13 | 0.80 | 0.10 | 2.15 |
| D | 2.05 | 0.84 | 0.15 | 2.06 |
| Resilience Factor | Group Difference | 95% CI | t | p | Cohen’s d |
|---|---|---|---|---|---|
| 1 | 0.899 | 0.66–1.14 | 7.4520 | <0.001 | 1.59 |
| 2 | 0.681 | 0.49–0.87 | 7.1460 | <0.001 | 1.52 |
| 3 | 1.11 | 0.86–1.36 | 8.8370 | <0.001 | 1.88 |
| 4 | 0.988 | 0.67–1.30 | 6.2350 | <0.001 | 1.33 |
| 5 | 0.084 | −0.26–0.43 | 0.4830 | 0.63 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, I.D.; Sharpley, C.F.; Bitsika, V.; Vessey, K.A.; Jesulola, E.; Agnew, L.L. Functional Network Connectivity for Components of Depression-Related Psychological Fragility. Brain Sci. 2024, 14, 845. https://doi.org/10.3390/brainsci14080845
Evans ID, Sharpley CF, Bitsika V, Vessey KA, Jesulola E, Agnew LL. Functional Network Connectivity for Components of Depression-Related Psychological Fragility. Brain Sciences. 2024; 14(8):845. https://doi.org/10.3390/brainsci14080845
Chicago/Turabian StyleEvans, Ian D., Christopher F. Sharpley, Vicki Bitsika, Kirstan A. Vessey, Emmanuel Jesulola, and Linda L. Agnew. 2024. "Functional Network Connectivity for Components of Depression-Related Psychological Fragility" Brain Sciences 14, no. 8: 845. https://doi.org/10.3390/brainsci14080845
APA StyleEvans, I. D., Sharpley, C. F., Bitsika, V., Vessey, K. A., Jesulola, E., & Agnew, L. L. (2024). Functional Network Connectivity for Components of Depression-Related Psychological Fragility. Brain Sciences, 14(8), 845. https://doi.org/10.3390/brainsci14080845





