Sensory Modality-Dependent Interplay Between Updating and Inhibition Under Increased Working Memory Load: An ERP Study
Abstract
1. Introduction
2. Method
2.1. Participants
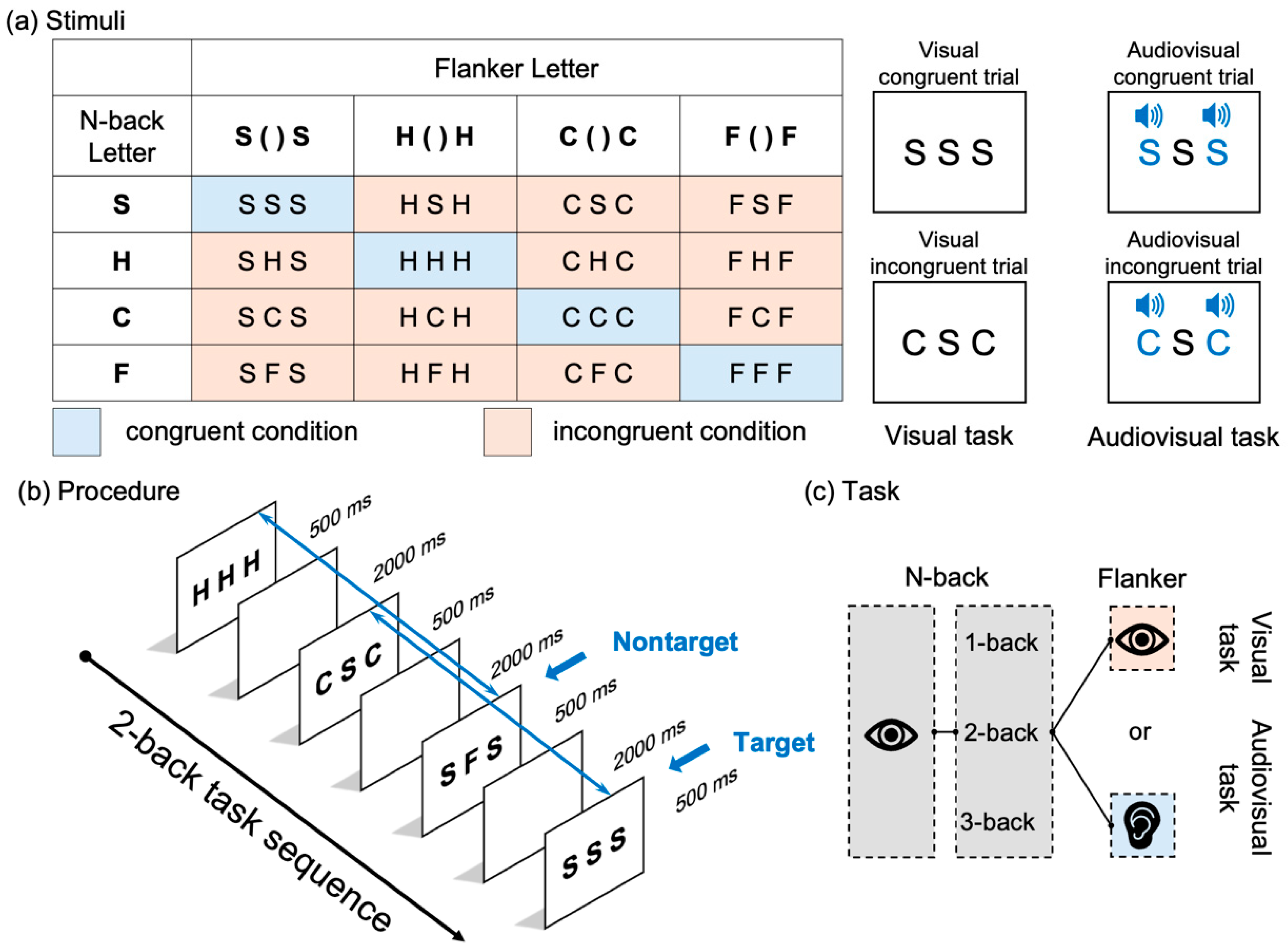
2.2. Stimuli
2.3. Design and Procedure
2.3.1. Design
2.3.2. Procedure
2.4. Data Collection and Analysis
2.4.1. Data Collection
2.4.2. Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Behavioral Results
3.1.1. Accuracy
3.1.2. Reaction Times
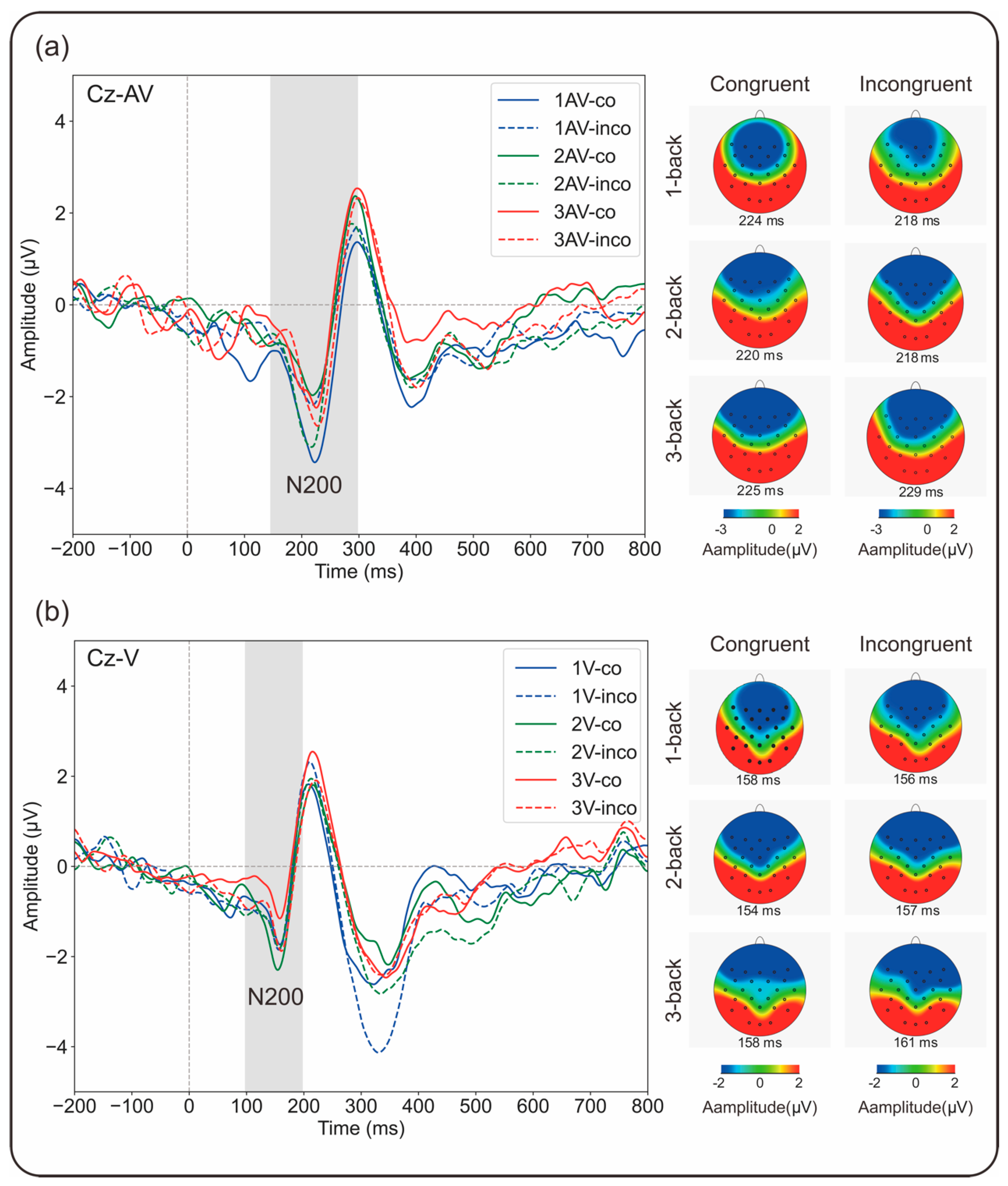
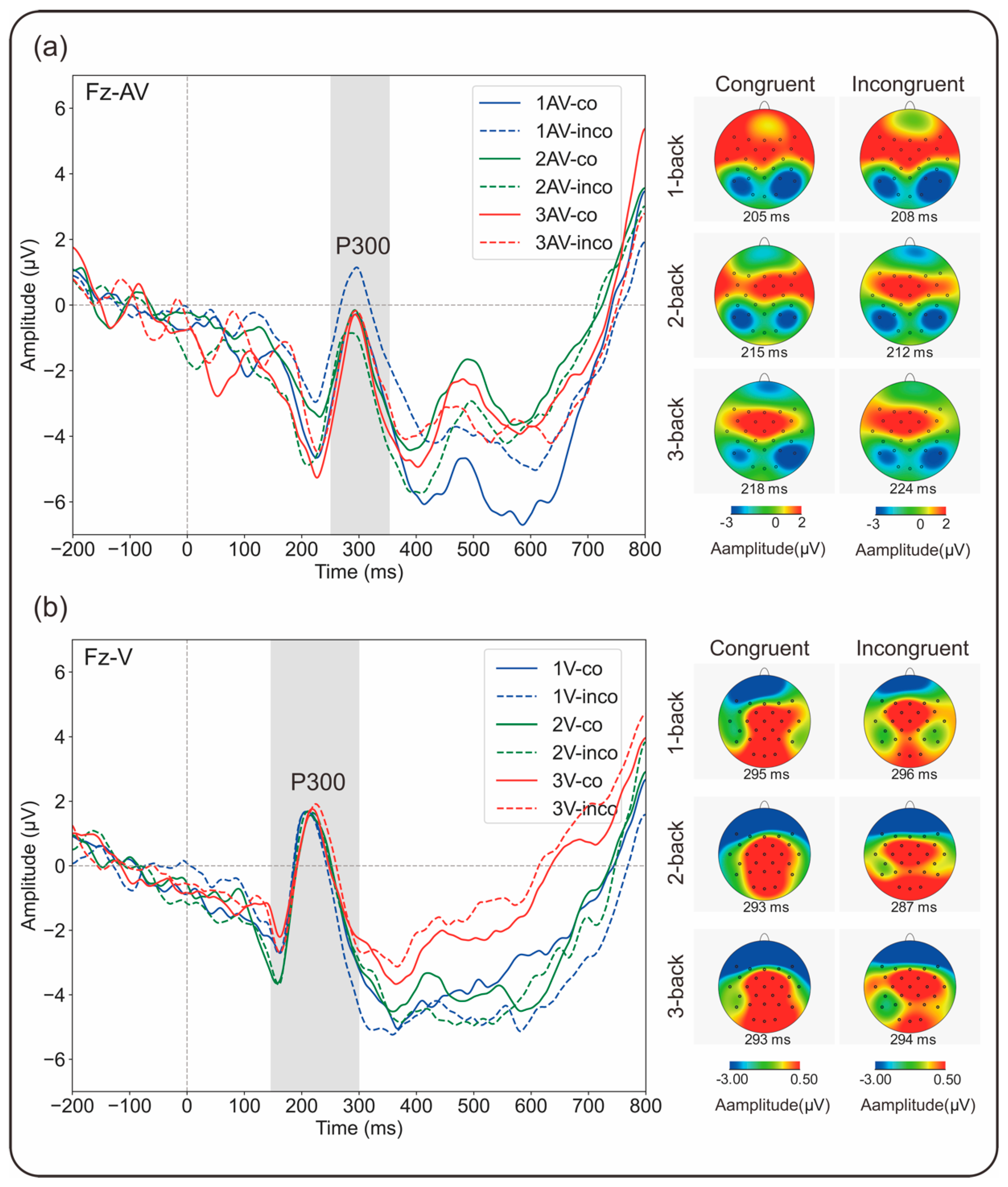
3.2. ERP Results
3.2.1. N200 Amplitude
3.2.2. P300 Amplitude
4. Discussion
5. Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, P.; Miyake, A. Models of Working Memory: An Introduction. In Models of Working Memory; Cambridge University Press: Cambridge, UK, 2012; pp. 1–27. [Google Scholar] [CrossRef]
- e Souza, R.H.C.; Naves, E.L.M. Attention Detection in Virtual Environments Using EEG Signals: A Scoping Review. Front. Physiol. 2021, 12, 727840. [Google Scholar] [CrossRef]
- Zanto, T.P.; Gazzaley, A. Selective Attention and Inhibitory Control in the Aging Brain. In Cognitive Neuroscience of Aging, 2nd ed.; Cabeza, R., Nyberg, L., Park, D.C., Eds.; Oxford University Press: New York, NY, USA, 2016; Chapter 8; pp. 207–234. [Google Scholar] [CrossRef]
- Luciana, M. Executive Function in Adolescence: A Commentary on Regulatory Control and Depression in Adolescents: Findings From Neuroimaging and Neuropsychological Research. J. Clin. Child Adolesc. Psychol. 2016, 45, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Sun, Y.; Wu, H.; Sun, H.; Zhang, D. The interaction of top–down and bottom–up attention in visual working memory. Sci. Rep. 2024, 14, 17397. [Google Scholar] [CrossRef] [PubMed]
- Rutman, A.M.; Clapp, W.C.; Chadick, J.Z.; Gazzaley, A. Early Top–Down Control of Visual Processing Predicts Working Memory Performance. J. Cogn. Neurosci. 2010, 22, 1224–1234. [Google Scholar] [CrossRef]
- Baluch, F.; Itti, L. Mechanisms of top-down attention. Trends Neurosci. 2011, 34, 210–224. [Google Scholar] [CrossRef]
- Katsuki, F.; Constantinidis, C. Bottom-Up and Top-Down Attention: Different processes and overlapping neural systems. Neuroscientist 2014, 20, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Braver, T.S.; Gray, J.R.; Burgess, G.C. Explaining the Many Varieties of Working Memory Variation: Dual Mechanisms of Cognitive Control. In Variation in Working Memory; Oxford University Press: New York, NY, USA, 2008; pp. 76–106. [Google Scholar] [CrossRef]
- Bidet-Caulet, A.; Bottemanne, L.; Fonteneau, C.; Giard, M.-H.; Bertrand, O. Brain Dynamics of Distractibility: Interaction Between Top-Down and Bottom-Up Mechanisms of Auditory Attention. Brain Topogr. 2015, 28, 423–436. [Google Scholar] [CrossRef]
- de Fockert, J.W. Beyond perceptual load and dilution: A review of the role of working memory in selective attention. Front. Psychol. 2013, 4, 287. [Google Scholar] [CrossRef]
- Ahmed, L.; de Fockert, J.W. Working memory load can both improve and impair selective attention: Evidence from the Navon paradigm. Atten. Percept. Psychophys. 2012, 74, 1397–1405. [Google Scholar] [CrossRef]
- de Fockert, J.W.; Mizon, G.A.; D’Ubaldo, M. No negative priming without cognitive control. J. Exp. Psychol. Hum. Percept. Perform. 2010, 36, 1333–1341. [Google Scholar] [CrossRef]
- De Fockert, J.W.; Bremner, A.J. Release of inattentional blindness by high working memory load: Elucidating the relationship between working memory and selective attention. Cognition 2011, 121, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, K.K.; Jha, A.P. Selective Attention Supports Working Memory Maintenance by Modulating Perceptual Processing of Distractors. J. Cogn. Neurosci. 2007, 19, 32–41. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, M.S.; Chun, M.M. Concurrent working memory load can reduce distraction. Proc. Natl. Acad. Sci. USA 2005, 102, 16524–16529. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.X.; Huang, S.; Kong, L.; Wang, S. Effects of load on the guidance of visual attention from working memory. Vis. Res. 2011, 51, 2356–2361. [Google Scholar] [CrossRef]
- Park, S.; Kim, M.-S.; Chun, M.M. Concurrent working memory load can facilitate selective attention: Evidence for specialized load. J. Exp. Psychol. Hum. Percept. Perform. 2007, 33, 1062–1075. [Google Scholar] [CrossRef]
- Navon, D.; Gopher, D. On the economy of the human-processing system. Psychol. Rev. 1979, 86, 214–255. [Google Scholar] [CrossRef]
- Schumacher, E.H.; Schwarb, H.; Lightman, E.; Hazeltine, E. Investigating the modality specificity of response selection using a temporal flanker task. Psychol. Res. 2011, 75, 499–512. [Google Scholar] [CrossRef]
- Hazeltine, E.; Bunge, S.A.; Scanlon, M.D.; Gabrieli, J.D.E. Material-dependent and material-independent selection processes in the frontal and parietal lobes: An event-related fMRI investigation of response competition. Neuropsychologia 2003, 41, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A.D.; Hitch, G. Working memory. Psychol. Learn. Motiv. Adv. Res. Theory 1974, 8, 47–89. [Google Scholar] [CrossRef]
- Baddeley, A. Working memory: Theories, models, and controversies. Annu. Rev. Psychol. 2012, 63, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Jonides, J.; Koeppe, R.A. Dissociating Verbal and Spatial Working Memory Using PET. Cereb. Cortex 1996, 6, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Collette, F.; Van der Linden, M.; Laureys, S.; Delfiore, G.; Degueldre, C.; Luxen, A.; Salmon, E. Exploring the unity and diversity of the neural substrates of executive functioning. Hum. Brain Mapp. 2005, 25, 409–423. [Google Scholar] [CrossRef]
- Nee, D.E.; Brown, J.W.; Askren, M.K.; Berman, M.G.; Demiralp, E.; Krawitz, A.; Jonides, J. A Meta-analysis of Executive Components of Working Memory. Cereb. Cortex 2013, 23, 264–282. [Google Scholar] [CrossRef]
- Scharinger, C.; Soutschek, A.; Schubert, T.; Gerjets, P. When flanker meets the n-back: What EEG and pupil dilation data reveal about the interplay between the two central-executive working memory functions inhibition and updating. Psychophysiology 2015, 52, 1293–1304. [Google Scholar] [CrossRef]
- Jaeggi, S.M.; Buschkuehl, M.; Perrig, W.J.; Meier, B. The concurrent validity of the N-back task as a working memory measure. Memory 2010, 18, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Meule, A. Reporting and Interpreting Working Memory Performance in n-back Tasks. Front. Psychol. 2017, 8, 352. [Google Scholar] [CrossRef]
- Szmalec, A.; Verbruggen, F.; Vandierendonck, A.; Kemps, E. Control of interference during working memory updating. J. Exp. Psychol. Hum. Percept. Perform. 2011, 37, 137–151. [Google Scholar] [CrossRef]
- Scharinger, C.; Soutschek, A.; Schubert, T.; Gerjets, P. Comparison of the working memory load in N-back and working memory span tasks by means of EEG frequency band power and P300 amplitude. Front. Hum. Neurosci. 2017, 11, 212526. [Google Scholar] [CrossRef]
- Nikolin, S.; Tan, Y.Y.; Schwaab, A.; Moffa, A.; Loo, C.K.; Martin, D. An investigation of working memory deficits in depression using the n-back task: A systematic review and meta-analysis. J. Affect. Disord. 2021, 284, 1–8. [Google Scholar] [CrossRef]
- Kane, M.J.; Conway, A.R.; Miura, T.K.; Colflesh, G.J. Working memory, attention control, and the N-back task: A question of construct validity. J. Exp. Psychol. Learn. Mem. Cogn. 2007, 33, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, C.W.; St James, J.D. Visual attention within and around the field of focal attention: A zoom lens model. Percept. Psychophys. 1986, 40, 225–240. [Google Scholar] [CrossRef]
- Eriksen, C.W. The Flankers Task and Response Competition: A Useful Tool for Investigating a Variety of Cognitive Problems. Vis. Cogn. 1995, 2, 101–118. [Google Scholar] [CrossRef]
- Eriksen, B.A.; Eriksen, C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974, 16, 143–149. [Google Scholar] [CrossRef]
- Kim, N.Y.; Wittenberg, E.; Nam, C.S. Behavioral and Neural Correlates of Executive Function: Interplay between Inhibition and Updating Processes. Front. Neurosci. 2017, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Pratt, N.; Willoughby, A.; Swick, D. Effects of working memory load on visual selective attention: Behavioral and electrophysiological evidence. Front. Hum. Neurosci. 2011, 5, 57. [Google Scholar] [CrossRef]
- Qi, S.; Zeng, Q.; Luo, Y.; Duan, H.; Ding, C.; Hu, W.; Li, H. Impact of Working Memory Load on Cognitive Control in Trait Anxiety: An ERP Study. PLoS ONE 2014, 9, e111791. [Google Scholar] [CrossRef]
- Pontifex, M.B.; Hillman, C.H. Neuroelectric and behavioral indices of interference control during acute cycling. Clin. Neurophysiol. 2007, 118, 570–580. [Google Scholar] [CrossRef]
- Jost, K.; Wendt, M.; Luna-Rodriguez, A.; Jacobsen, T. Electrophysiological correlates of proportion congruency manipulation in a temporal flanker task. Psychophysiology 2022, 59, e14092. [Google Scholar] [CrossRef] [PubMed]
- Vincent, V.V.; Carter, C.S. The anterior cingulate as a conflict monitor: FMRI and ERP studies. Physiol. Behav. 2002, 77, 477–482. [Google Scholar] [CrossRef]
- Watter, S.; Geffen, G.M.; Geffen, L.B. The n-back as a dual-task: P300 morphology under divided attention. Psychophysiology 2001, 38, 998–1003. [Google Scholar] [CrossRef]
- Kopp, B.; Rist, F.; Mattler, U. N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology 1996, 33, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Tillman, C.M.; Wiens, S. Behavioral and ERP indices of response conflict in Stroop and flanker tasks. Psychophysiology 2011, 48, 1405–1411. [Google Scholar] [CrossRef]
- Folstein, J.R.; Van Petten, C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology 2008, 45, 152–170. [Google Scholar] [CrossRef]
- Wei, H.; Zhou, R. High working memory load impairs selective attention: EEG signatures. Psychophysiology 2020, 57, e13643. [Google Scholar] [CrossRef]
- Larson, M.J.; Clayson, P.E.; Clawson, A. Making sense of all the conflict: A theoretical review and critique of conflict-related ERPs. Int. J. Psychophysiol. 2014, 93, 283–297. [Google Scholar] [CrossRef]
- Wei, H.; Yao, Y.; Zhou, L. Concurrent working memory task increases or decreases the flanker-related N2 amplitude. Front. Psychol. 2022, 13, 962153. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, R.; Fu, L. Working Memory Updating Function Training Influenced Brain Activity. PLoS ONE 2013, 8, e71063. [Google Scholar] [CrossRef]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Kok, A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology 2001, 38, 557–577. [Google Scholar] [CrossRef] [PubMed]
- González-Villar, A.J.; Carrillo-de-la-Peña, M.T. Brain electrical activity signatures during performance of the Multisource Interference Task. Psychophysiology 2017, 54, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Gratton, G.; Coles, M.G.H.; Donchin, E. Optimizing the Use of Information: Strategic Control of Activation of Responses. J. Exp. Psychol. Gen. 1992, 121, 480–506. [Google Scholar] [CrossRef] [PubMed]
- Botvinick, M.M.; Braver, T.S.; Barch, D.M.; Carter, C.S.; Cohen, J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001, 108, 624–652. [Google Scholar] [CrossRef] [PubMed]
- Davelaar, E.J. When the ignored gets bound: Sequential effects in the flanker task. Front. Psychol. 2013, 3, 552. [Google Scholar] [CrossRef]
- Yang, W.; Yang, X.; Guo, A.; Li, S.; Li, Z.; Lin, J.; Ren, Y.; Yang, J.; Wu, J.; Zhang, Z. Audiovisual integration of the dynamic hand-held tool at different stimulus intensities in aging. Front. Hum. Neurosci. 2022, 16, 968987. [Google Scholar] [CrossRef]
- Yang, W.; Guo, A.; Yao, H.; Yang, X.; Li, Z.; Li, S.; Chen, J.; Ren, Y.; Yang, J.; Wu, J.; et al. Effect of aging on audiovisual integration: Comparison of high- and low-intensity conditions in a speech discrimination task. Front. Aging Neurosci. 2022, 14, 1010060. [Google Scholar] [CrossRef]
- Bartholow, B.D.; Pearson, M.A.; Dickter, C.L.; Sher, K.J.; Fabiani, M.; Gratton, G. Strategic control and medial frontal negativity: Beyond errors and response conflict. Psychophysiology 2005, 42, 33–42. [Google Scholar] [CrossRef]
- Coles, M.; Smid, H.; Scheffers, M.; Otten, L. Mental chronometry and the study of human information processing. In Electrophysiology of Mind; Oxford University Press: New York, NY, USA, 1995; pp. 86–131. [Google Scholar] [CrossRef]
- Duncan, C.C.; Barry, R.J.; Connolly, J.F.; Fischer, C.; Michie, P.T.; Näätänen, R.; Polich, J.; Reinvang, I.; Van Petten, C. Event-related potentials in clinical research: Guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin. Neurophysiol. 2009, 120, 1883–1908. [Google Scholar] [CrossRef]
- Wickens, C.; Kramer, A.; Vanasse, L.; Donchin, E. Performance of Concurrent Tasks: A Psychophysiological Analysis of the Reciprocity of Information-Processing Resources. Science (1979) 1983, 221, 1080–1082. [Google Scholar] [CrossRef]
- Kramer, A.F.; Strayer, D.L. Assessing the development of automatic processing: An application of dual-task and event-related brain potential methodologies. Biol. Psychol. 1988, 26, 231–267. [Google Scholar] [CrossRef]
- Strayer, D.L.; Kramer, A.F. Attentional requirements of automatic and controlled processing. J. Exp. Psychol. Learn. Mem. Cogn. 1990, 16, 67–82. [Google Scholar] [CrossRef]
- Kramer, A.F.; Strayer, D.L.; Buckley, J. Task Versus Component Consistency in the Development of Automatic Processing: A Psychophysiological Assessment. Psychophysiology 1991, 28, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Brockhoff, L.; Vetter, L.; Bruchmann, M.; Schindler, S.; Moeck, R.; Straube, T. The effects of visual working memory load on detection and neural processing of task-unrelated auditory stimuli. Sci. Rep. 2023, 13, 4342. [Google Scholar] [CrossRef] [PubMed]
- Sörqvist, P.; Stenfelt, S.; Rönnberg, J. Working Memory Capacity and Visual–Verbal Cognitive Load Modulate Auditory–Sensory Gating in the Brainstem: Toward a Unified View of Attention. J. Cogn. Neurosci. 2012, 24, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Papadopetraki, D.; Froböse, M.I.; Westbrook, A.; Zandbelt, B.B.; Cools, R. Shielding working memory from distraction is more effortful than flexible updating. Sci. Rep. 2025, 15, 13512. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The Unity and Diversity of Executive Functions and Their Contributions to Complex ‘Frontal Lobe’ Tasks: A Latent Variable Analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef]
- Al-Ezzi, A.; Arechavala, R.J.; Butler, R.; Nolty, A.; Kang, J.J.; Shimojo, S.; Wu, D.A.; Fonteh, A.N.; Kleinman, M.T.; Kloner, R.A.; et al. Disrupted brain functional connectivity as early signature in cognitively healthy individuals with pathological CSF amyloid/tau. Commun. Biol. 2024, 7, 1037. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Guo, A.; Wu, J.; Yang, J. Sensory Modality-Dependent Interplay Between Updating and Inhibition Under Increased Working Memory Load: An ERP Study. Brain Sci. 2025, 15, 1178. https://doi.org/10.3390/brainsci15111178
Luo Y, Guo A, Wu J, Yang J. Sensory Modality-Dependent Interplay Between Updating and Inhibition Under Increased Working Memory Load: An ERP Study. Brain Sciences. 2025; 15(11):1178. https://doi.org/10.3390/brainsci15111178
Chicago/Turabian StyleLuo, Yuxi, Ao Guo, Jinglong Wu, and Jiajia Yang. 2025. "Sensory Modality-Dependent Interplay Between Updating and Inhibition Under Increased Working Memory Load: An ERP Study" Brain Sciences 15, no. 11: 1178. https://doi.org/10.3390/brainsci15111178
APA StyleLuo, Y., Guo, A., Wu, J., & Yang, J. (2025). Sensory Modality-Dependent Interplay Between Updating and Inhibition Under Increased Working Memory Load: An ERP Study. Brain Sciences, 15(11), 1178. https://doi.org/10.3390/brainsci15111178





