Glutamatergic Neurons in the Cerebellar Lateral Nucleus Contribute to Motor Deficits Induced by Chronic Sleep Disturbance
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chronic Sleep Disruption Model
2.3. EEG and EMG Electrode Implantation
2.4. Polysomnographic Recordings and Analysis
2.5. Surgeries and Viral Injections
2.6. Behavioral Assessment
2.6.1. Open-Field Test
2.6.2. Rotarod Test
2.6.3. Elevated Plus Maze Test
2.6.4. Y Maze Test
2.7. Immunofluorescence
2.8. Cell Counting
2.9. Statistical Analysis
3. Results
3.1. Chronic Sleep Disruption Alters Sleep–Wake States and Enhances Sleep Rebound
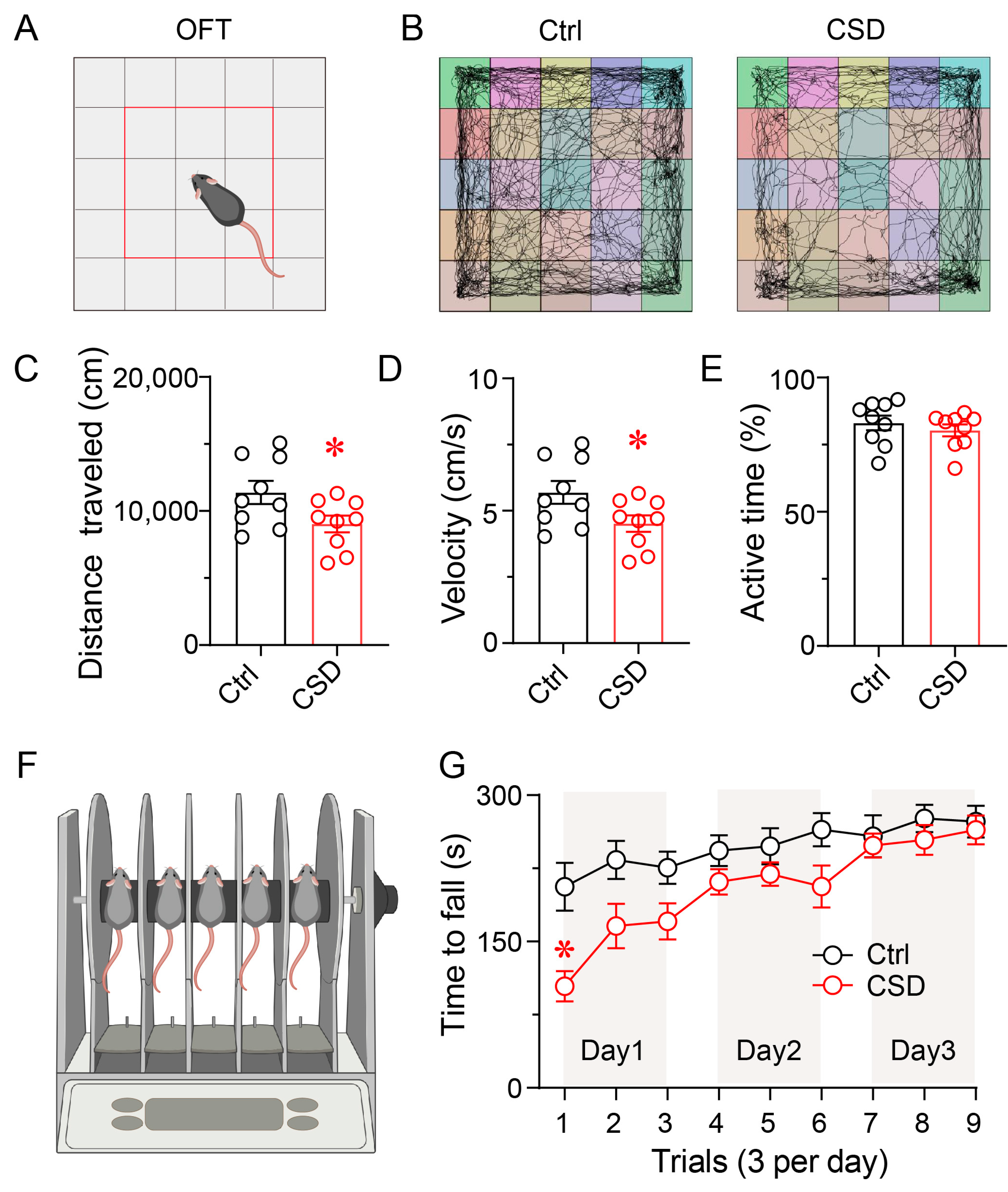
3.2. Chronic Sleep Disruption Impairs Locomotor Activity and Motor Coordination
3.3. Chronic Sleep Disruption Selectively Activates Neurons in the Lateral Nucleus
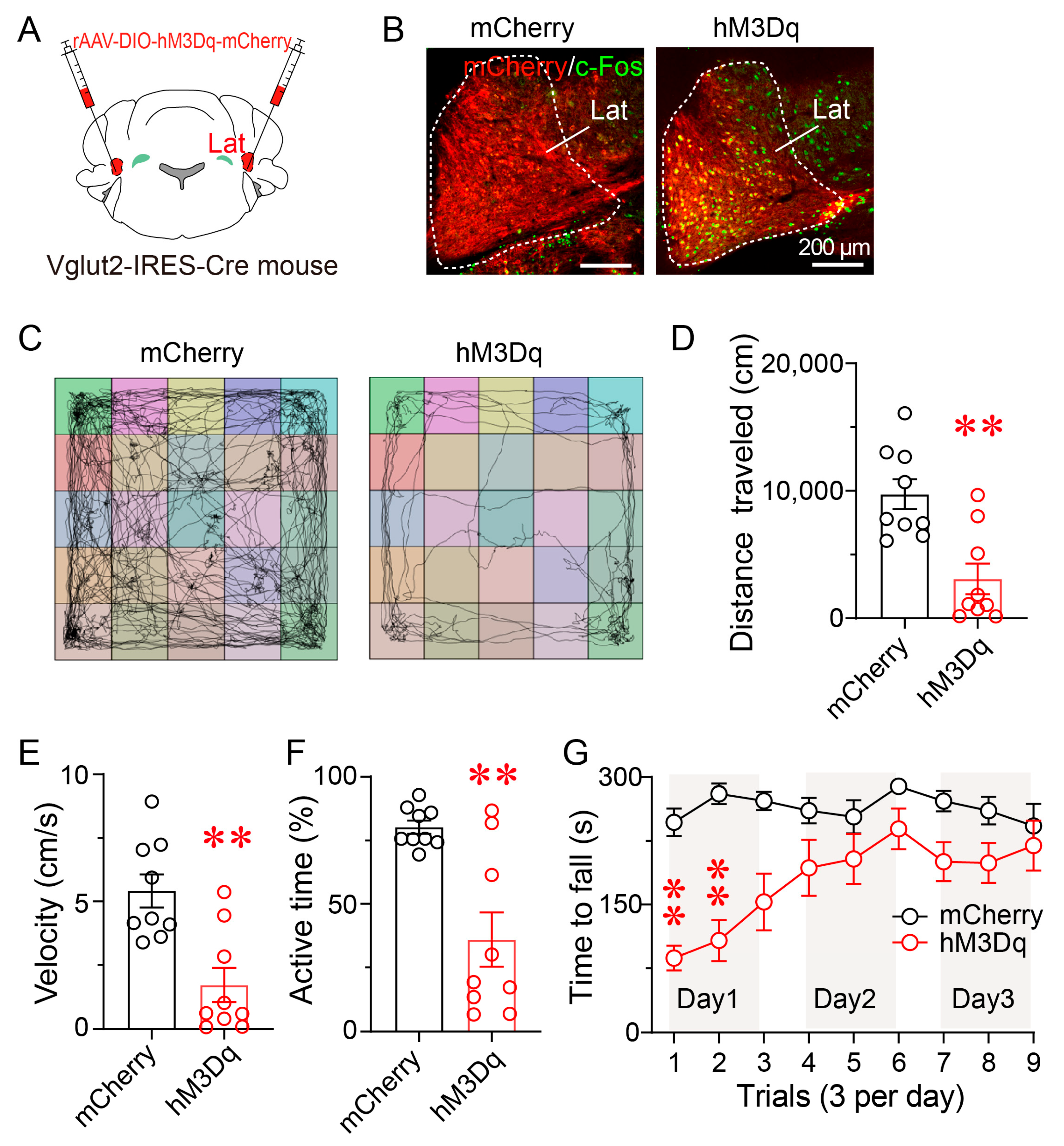
3.4. Chemogenetic Activation of LatVglut2+ Neurons Reduces Locomotor Activity and Motor Coordination
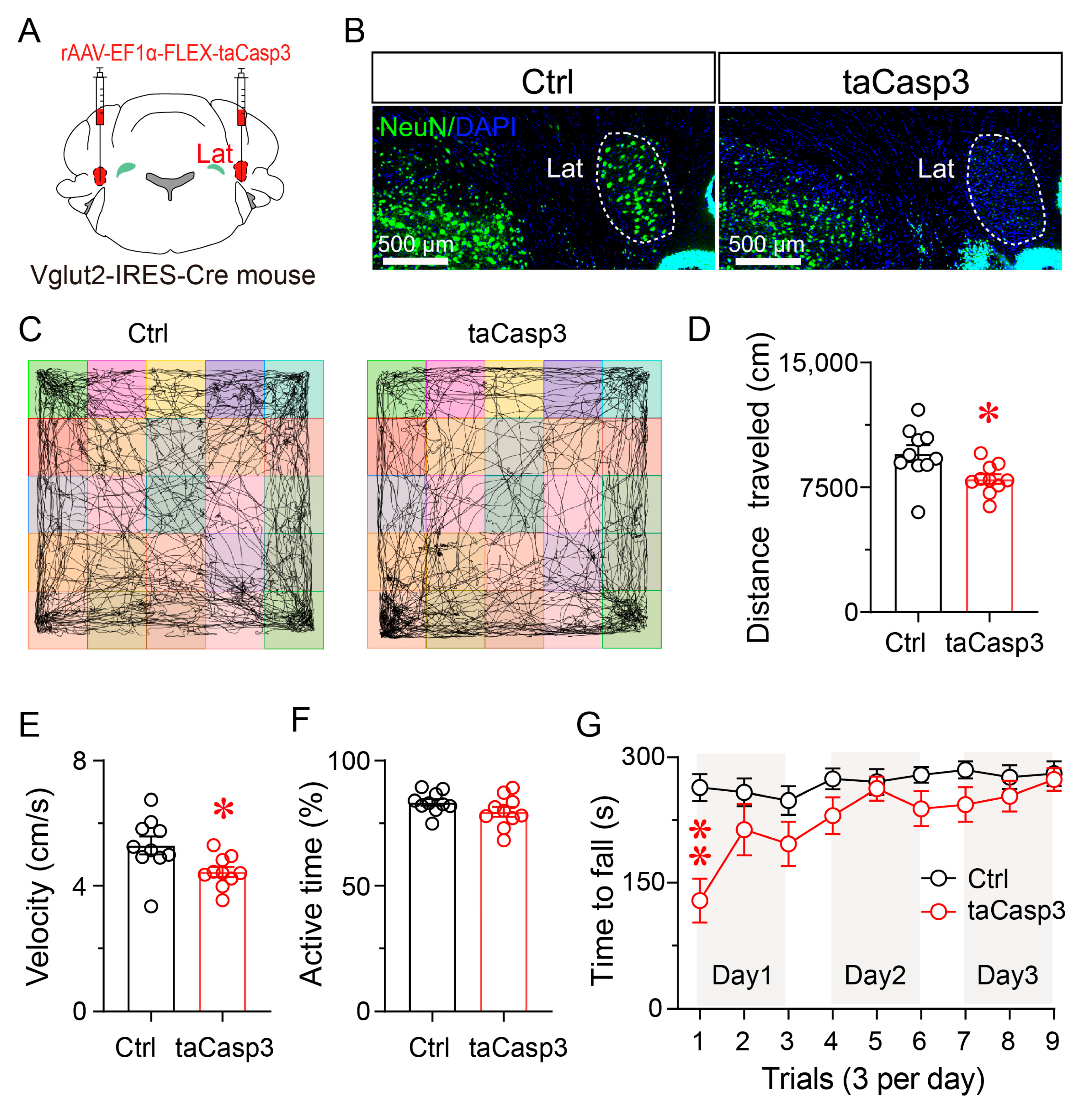
3.5. Ablation of LatVglut2+ Neurons Impairs Locomotor Activity and Motor Coordination
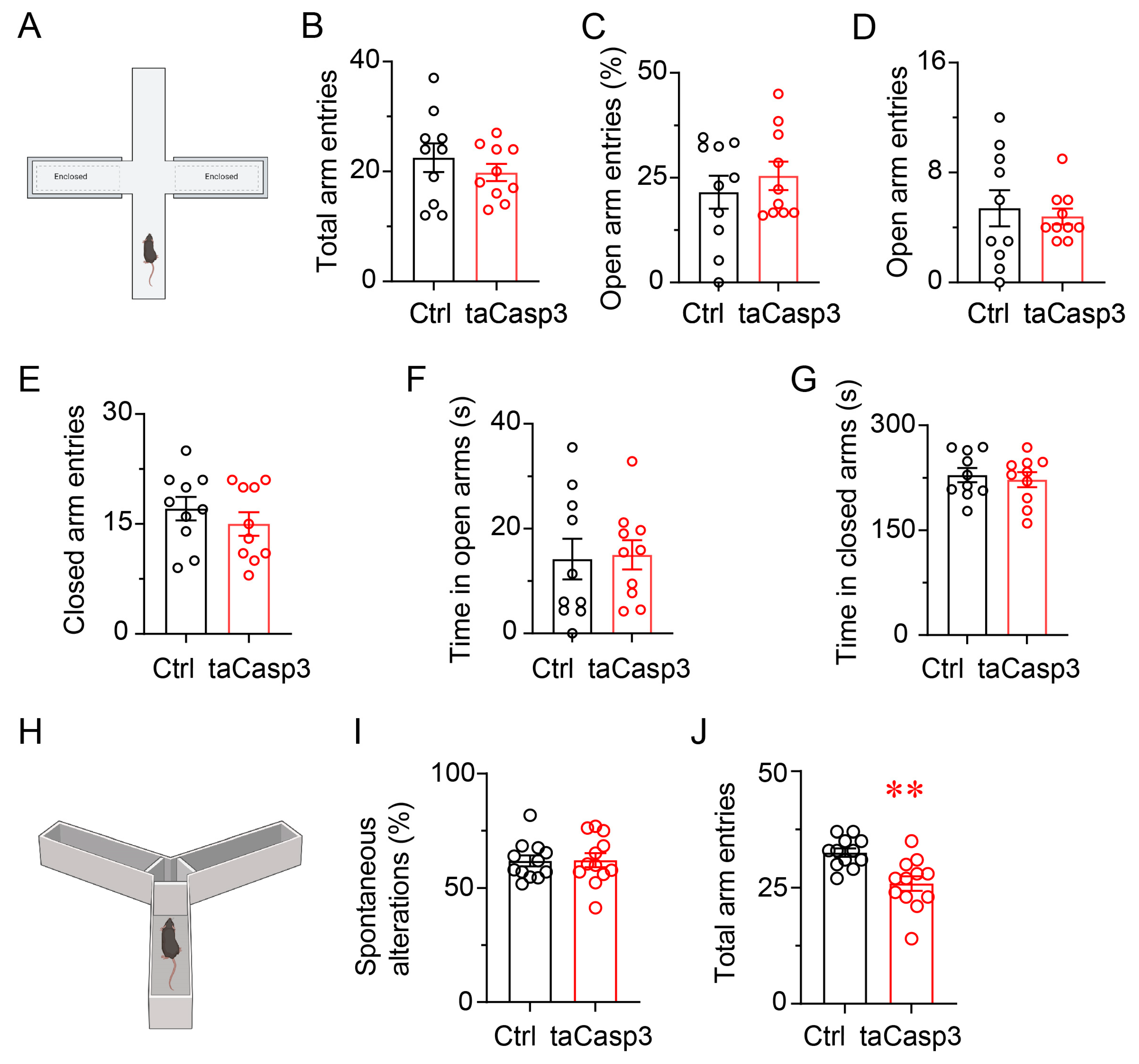
3.6. Ablation of LatVglut2+ Neurons Does Not Induce Anxiety-like Behavior or Impair Spatial Working Memory
3.7. Chemogenetic Inhibition of LatVglut2+ Neurons Attenuated CSD-Induced Impairments in Locomotor Activity and Motor Coordination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| CSD | chronic sleep disruption |
| DCN | deep cerebellar nuclei |
| EEG | electroencephalogram |
| EMG | electromyogram |
| IntA | interposed anterior nucleus |
| IntDL | dorsolateral interposed nucleus |
| IntP | posterior interposed nucleus |
| Lat | lateral nucleus |
| Med | medial nucleus |
| MedDL | dorsolateral medial nucleus |
| NREM | non-rapid eye movement |
| REM | rapid eye movement |
References
- Garbarino, S.; Lanteri, P.; Bragazzi, N.L.; Magnavita, N.; Scoditti, E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun. Biol. 2021, 4, 1304. [Google Scholar] [CrossRef]
- Diekelmann, S.; Born, J. The memory function of sleep. Nat. Rev. Neurosci. 2010, 11, 114–126. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Medic, G.; Wille, M.; Hemels, M.E. Short- and long-term health consequences of sleep disruption. Nat. Sci. Sleep 2017, 9, 151–161. [Google Scholar] [CrossRef]
- Bailey, G.A.; Hubbard, E.K.; Fasano, A.; Tijssen, M.A.; Lynch, T.; Anderson, K.N.; Peall, K.J. Sleep disturbance in movement disorders: Insights, treatments and challenges. J. Neurol. Neurosurg. Psychiatry 2021, 92, 723–736. [Google Scholar] [CrossRef]
- Fischer, S.; Hallschmid, M.; Elsner, A.L.; Born, J. Sleep forms memory for finger skills. Proc. Natl. Acad. Sci. USA 2002, 99, 11987–11991. [Google Scholar] [CrossRef]
- Csabi, E.; Varszegi-Schulz, M.; Janacsek, K.; Malecek, N.; Nemeth, D. The consolidation of implicit sequence memory in obstructive sleep apnea. PLoS ONE 2014, 9, e109010. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, J.; He, Y.; Qu, W.; Huang, Z.; Ding, F. Sleep fragmentation reduces explorative behaviors and impairs motor coordination in male mice. J. Neurosci. Res. 2024, 102, e25268. [Google Scholar] [CrossRef]
- Koziol, L.F.; Budding, D.; Andreasen, N.; D’Arrigo, S.; Bulgheroni, S.; Imamizu, H.; Ito, M.; Manto, M.; Marvel, C.; Parker, K.; et al. Consensus paper: The cerebellum’s role in movement and cognition. Cerebellum 2014, 13, 151–177. [Google Scholar] [CrossRef]
- Canto, C.B.; Onuki, Y.; Bruinsma, B.; van der Werf, Y.D.; De Zeeuw, C.I. The Sleeping Cerebellum. Trends Neurosci. 2017, 40, 309–323. [Google Scholar] [CrossRef]
- Kelly, R.M.; Strick, P.L. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J. Neurosci. 2003, 23, 8432–8444. [Google Scholar] [CrossRef] [PubMed]
- Dum, R.P.; Strick, P.L. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J. Neurophysiol. 2003, 89, 634–639. [Google Scholar] [CrossRef]
- Kebschull, J.M.; Casoni, F.; Consalez, G.G.; Goldowitz, D.; Hawkes, R.; Ruigrok, T.J.H.; Schilling, K.; Wingate, R.; Wu, J.; Yeung, J.; et al. Cerebellum Lecture: The Cerebellar Nuclei-Core of the Cerebellum. Cerebellum 2024, 23, 620–677. [Google Scholar] [CrossRef]
- Rios-Zermeno, J.; Ballesteros-Herrera, D.; Dominguez-Vizcayno, P.; Carrillo-Ruiz, J.D.; Moreno-Jimenez, S. Dentate nucleus: A review and implications for dentatotomy. Acta Neurochir. 2024, 166, 219. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E. What Is the Role of the Dentate Nucleus in Normal and Abnormal Cerebellar Function? Neurology 2024, 103, e209636. [Google Scholar] [CrossRef]
- Uusisaari, M.; Knöpfel, T. Functional classification of neurons in the mouse lateral cerebellar nuclei. Cerebellum 2011, 10, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, E.; Balbo, I.; Miniaci, M.C.; Tempia, F. Purkinje Cell Signaling Deficits in Animal Models of Ataxia. Front. Synaptic Neurosci. 2018, 10, 6. [Google Scholar] [CrossRef]
- Chopra, R.; Shakkottai, V.G. Translating cerebellar Purkinje neuron physiology to progress in dominantly inherited ataxia. Future Neurol. 2014, 9, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.F.; Beevis, J.C.; Empson, R.M. Essential Tremor-A Cerebellar Driven Disorder? Neuroscience 2021, 462, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.T.; Chen, H.; Samaco, R.C.; Xue, M.; Chahrour, M.; Yoo, J.; Neul, J.L.; Gong, S.; Lu, H.C.; Heintz, N.; et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 2010, 468, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ba, L.; Wang, M.; Deng, S.Y.; Chen, S.M.; Huang, L.F.; Zhang, M.; Wang, W.; Ding, F.F. Chronic sleep fragmentation shares similar pathogenesis with neurodegenerative diseases: Endosome-autophagosome-lysosome pathway dysfunction and microglia-mediated neuroinflammation. CNS Neurosci. Ther. 2020, 26, 215–227. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Z.K.; Guo, H.; Yuan, X.S.; Liu, C.W.; Qu, W.M.; Huang, Z.L. Striatal neurons expressing dopamine D(1) receptor promote wakefulness in mice. Curr. Biol. 2022, 32, 600–613.e4. [Google Scholar] [CrossRef]
- Chen, C.R.; Zhong, Y.H.; Jiang, S.; Xu, W.; Xiao, L.; Wang, Z.; Qu, W.M.; Huang, Z.L. Dysfunctions of the paraventricular hypothalamic nucleus induce hypersomnia in mice. eLife 2021, 10, e69909. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, B.X.; Chen, H.; Jiang, X.W.; Qu, W.M.; Huang, Z.L. Acute or Chronic Exposure to Corticosterone Promotes Wakefulness in Mice. Brain Sci. 2023, 13, 1472. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, J.B.; Xu, W.; Zhang, M.T.; Chen, H.; Shi, H.Y.; Wang, L.; He, M.; Lazarus, M.; Li, S.Q.; et al. Parasubthalamic calretinin neurons modulate wakefulness associated with exploration in male mice. Nat. Commun. 2023, 14, 2346. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, T.X.; Zhou, J.C.; Qu, W.M.; Huang, Z.L. Dopamine D1 and D2 receptors mediate analgesic and hypnotic effects of l-tetrahydropalmatine in a mouse neuropathic pain model. Psychopharmacology 2019, 236, 3169–3182. [Google Scholar] [CrossRef]
- Franklin, K.B.; Paxinos, P.G. Franklin’s The Mouse Brain in Stereotaxic Coordinates, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Zhu, Y.; Zhan, G.; Fenik, P.; Brandes, M.; Bell, P.; Francois, N.; Shulman, K.; Veasey, S. Chronic Sleep Disruption Advances the Temporal Progression of Tauopathy in P301S Mutant Mice. J. Neurosci. 2018, 38, 10255–10270. [Google Scholar] [CrossRef]
- Zhang, L.; Santoni, L.; Ngo, N.A.; Simayi, R.; Ficiará, E.; de Vivo, L.; Bellesi, M. Rocking during sleep reduces motor deficits and beta-amyloid levels in an Alzheimer’s mouse model. iScience 2025, 28, 112036. [Google Scholar] [CrossRef] [PubMed]
- Christova, M.; Aftenberger, H.; Nardone, R.; Gallasch, E. Adult Gross Motor Learning and Sleep: Is There a Mutual Benefit? Neural Plast. 2018, 2018, 3076986. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhu, J.C. A Narrative Review of Cerebellar Malfunctions and Sleep Disturbances. Front. Neurosci. 2021, 15, 590619. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.I.; Park, M.H.; Park, B.; Kim, S.Y.; Kim, Y.O.; Kim, B.N.; Park, S.; Song, C.H. Cerebellar Gray Matter Volume, Executive Function, and Insomnia: Gender Differences in Adolescents. Sci. Rep. 2019, 9, 855. [Google Scholar] [CrossRef]
- Huang, M.; Verbeek, D.S. Why do so many genetic insults lead to Purkinje Cell degeneration and spinocerebellar ataxia? Neurosci. Lett. 2019, 688, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.J.; Watchon, M.; Laird, A.S. Aberrant Cerebellar Circuitry in the Spinocerebellar Ataxias. Front. Neurosci. 2020, 14, 707. [Google Scholar] [CrossRef]
- Zhang, L.B.; Zhang, J.; Sun, M.J.; Chen, H.; Yan, J.; Luo, F.L.; Yao, Z.X.; Wu, Y.M.; Hu, B. Neuronal Activity in the Cerebellum During the Sleep-Wakefulness Transition in Mice. Neurosci. Bull. 2020, 36, 919–931. [Google Scholar] [CrossRef]
- McCarley, R.W.; Hobson, J.A. Simple spike firing patterns of cat cerebellar Purkinje cells in sleep and waking. Electroencephalogr. Clin. Neurophysiol. 1972, 33, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Heiney, S.A.; Kim, J.; Augustine, G.J.; Medina, J.F. Precise control of movement kinematics by optogenetic inhibition of Purkinje cell activity. J. Neurosci. 2014, 34, 2321–2330. [Google Scholar] [CrossRef]
- Shakkottai, V.G.; Chou, C.H.; Oddo, S.; Sailer, C.A.; Knaus, H.G.; Gutman, G.A.; Barish, M.E.; LaFerla, F.M.; Chandy, K.G. Enhanced neuronal excitability in the absence of neurodegeneration induces cerebellar ataxia. J. Clin. Investig. 2004, 113, 582–590. [Google Scholar] [CrossRef]
- Kebschull, J.M.; Richman, E.B.; Ringach, N.; Friedmann, D.; Albarran, E.; Kolluru, S.S.; Jones, R.C.; Allen, W.E.; Wang, Y.; Cho, S.W.; et al. Cerebellar nuclei evolved by repeatedly duplicating a conserved cell-type set. Science 2020, 370, eabd5059. [Google Scholar] [CrossRef] [PubMed]
- Caligiore, D.; Pezzulo, G.; Baldassarre, G.; Bostan, A.C.; Strick, P.L.; Doya, K.; Helmich, R.C.; Dirkx, M.; Houk, J.; Jörntell, H.; et al. Consensus Paper: Towards a Systems-Level View of Cerebellar Function: The Interplay Between Cerebellum, Basal Ganglia, and Cortex. Cerebellum 2017, 16, 203–229. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Qi, W.-Q.; Kong, L.-X.; Lin, Y.-M.; Ding, F.-F.; Huang, Z.-L.; Qu, W.-M. Glutamatergic Neurons in the Cerebellar Lateral Nucleus Contribute to Motor Deficits Induced by Chronic Sleep Disturbance. Brain Sci. 2025, 15, 1185. https://doi.org/10.3390/brainsci15111185
Zhu J, Qi W-Q, Kong L-X, Lin Y-M, Ding F-F, Huang Z-L, Qu W-M. Glutamatergic Neurons in the Cerebellar Lateral Nucleus Contribute to Motor Deficits Induced by Chronic Sleep Disturbance. Brain Sciences. 2025; 15(11):1185. https://doi.org/10.3390/brainsci15111185
Chicago/Turabian StyleZhu, Jian, Wan-Qiao Qi, Ling-Xi Kong, Yan-Mei Lin, Feng-Fei Ding, Zhi-Li Huang, and Wei-Min Qu. 2025. "Glutamatergic Neurons in the Cerebellar Lateral Nucleus Contribute to Motor Deficits Induced by Chronic Sleep Disturbance" Brain Sciences 15, no. 11: 1185. https://doi.org/10.3390/brainsci15111185
APA StyleZhu, J., Qi, W.-Q., Kong, L.-X., Lin, Y.-M., Ding, F.-F., Huang, Z.-L., & Qu, W.-M. (2025). Glutamatergic Neurons in the Cerebellar Lateral Nucleus Contribute to Motor Deficits Induced by Chronic Sleep Disturbance. Brain Sciences, 15(11), 1185. https://doi.org/10.3390/brainsci15111185







