Tracking Changes in Corticospinal Excitability During Visuomotor Paired Associative Stimulation to Predict Motor Resonance Rewriting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
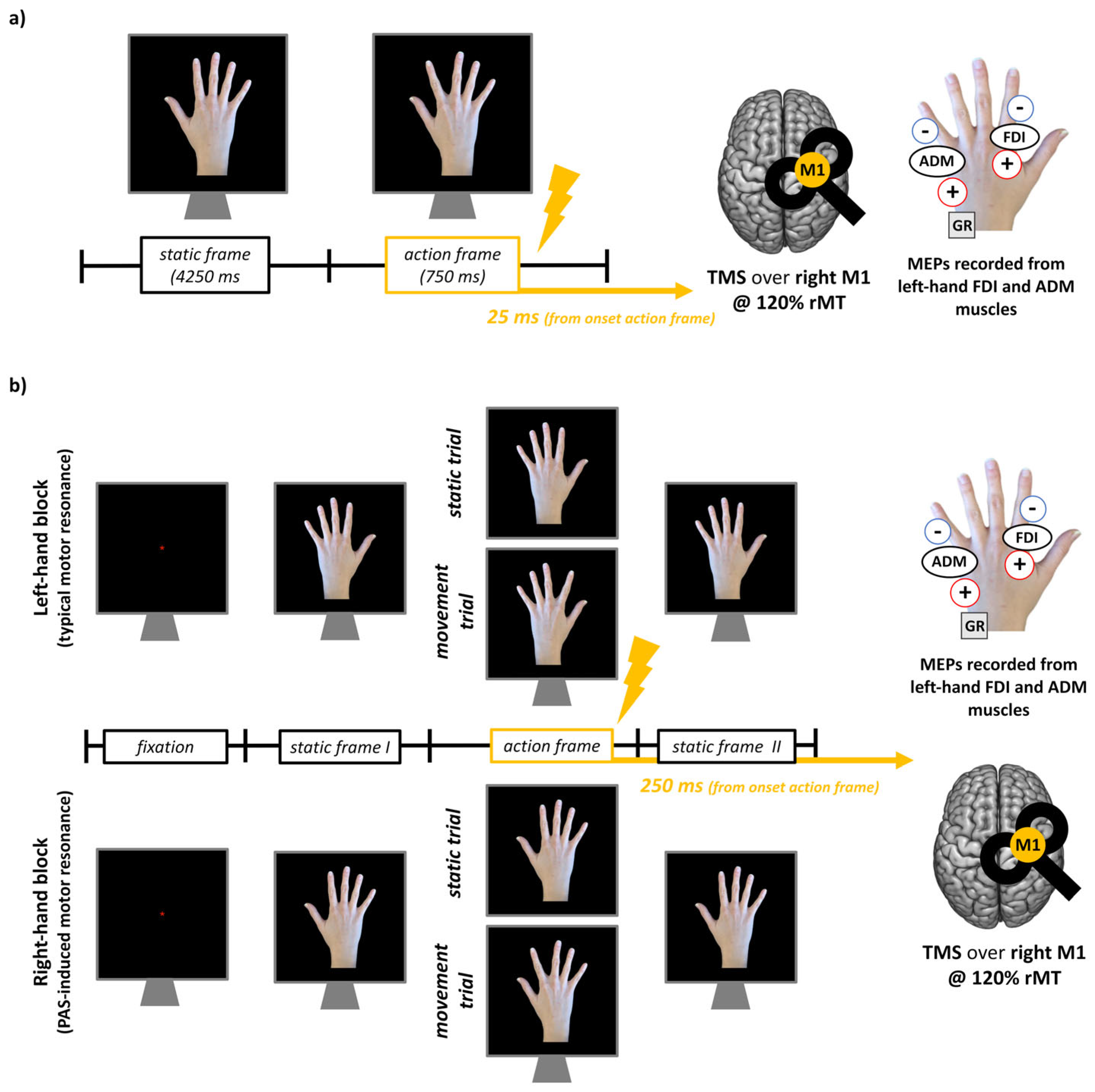
2.2. m-PAS and Action Observation Task
2.3. TMS
2.4. Electromyographic (EMG) Recording and Preprocessing
2.5. Statistical Analyses
3. Results
3.1. Motor Resonance Patterns Before and After m-PAS Administration
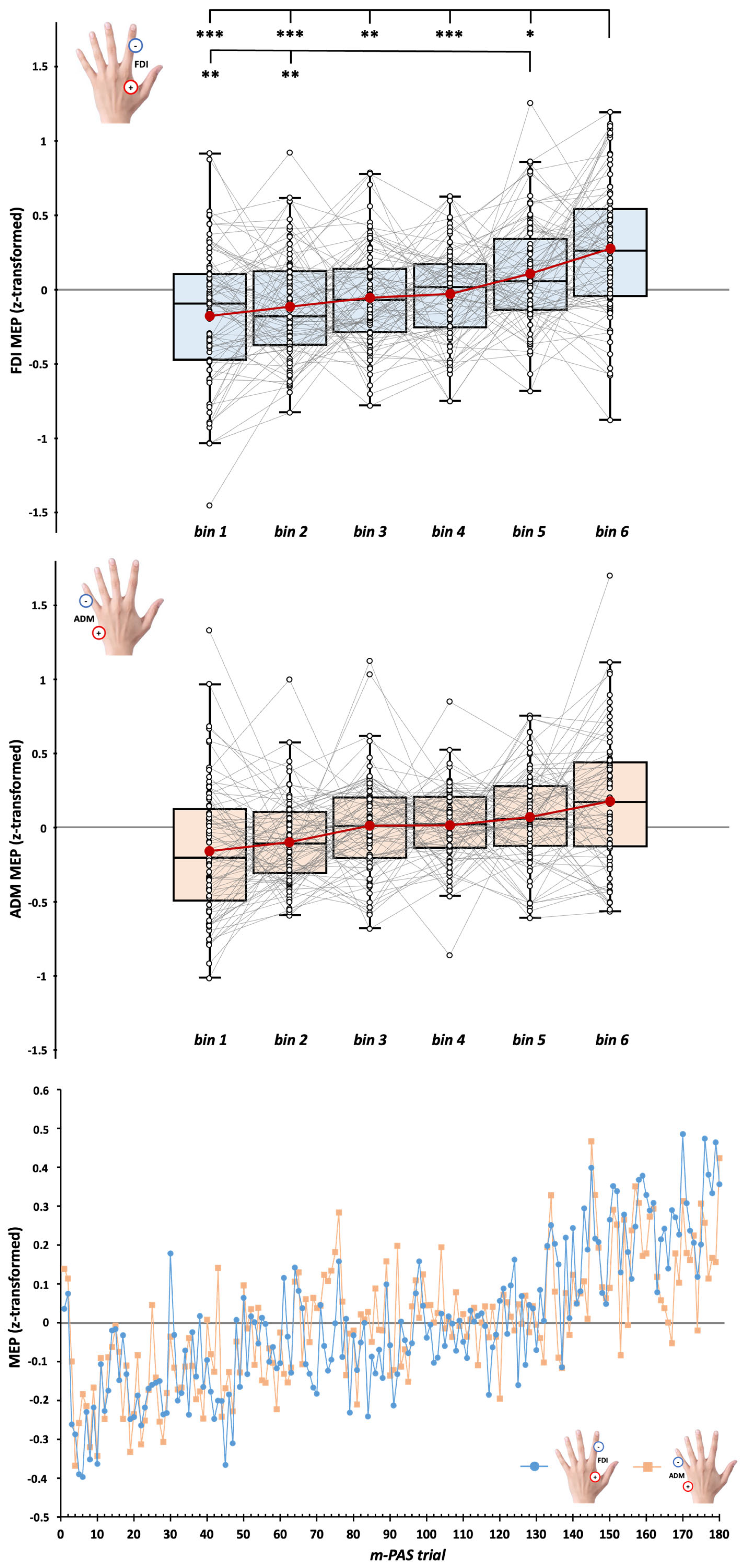
3.2. CSE During m-PAS Protocol
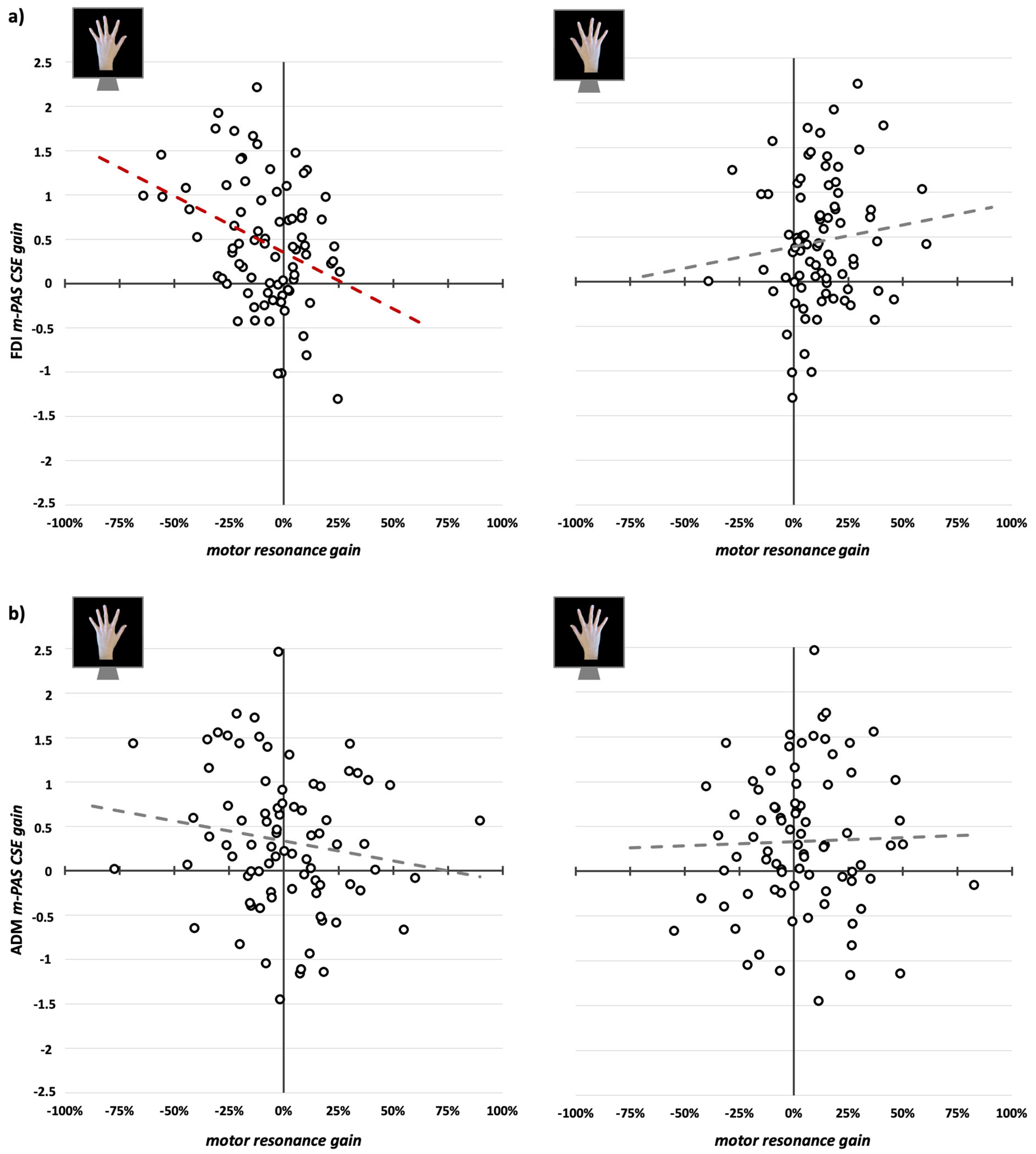
3.3. CSE Changes During m-PAS and Their Relation with Motor Resonance Modulations
4. Discussion
4.1. Motor Resonance Responses Are Reshaped After the m-PAS
4.2. Gradual Online Enhancement of CSE During the m-PAS
4.3. CSE Increase During m-PAS Predicts Only the Rewriting of Typical Motor Resonance
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADM | Abductor digiti minimi |
| AON | Action observation network |
| CSE | Corticospinal excitability |
| FDI | First dorsal interosseus |
| m-PAS | Mirror-paired associative stimulation |
| M1 | Primary motor cortex |
| MEP | Motor-evoked potential |
| PAS | Paired associative stimulation |
| PMv | Ventral premotor cortex |
| rmANOVA | Repeated measures analysis of variance |
| rMT | Resting motor threshold |
| SD | Standard deviation |
| TMS | Transcranial magnetic stimulation |
References
- Heyes, C.; Catmur, C. What Happened to Mirror Neurons? Perspect. Psychol. Sci. 2022, 17, 153–168. [Google Scholar] [CrossRef]
- Cook, R.; Bird, G.; Catmur, C.; Press, C.; Heyes, C. Mirror Neurons: From Origin to Function. Behav. Brain Sci. 2014, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Keysers, C.; Gazzola, V. Hebbian Learning and Predictive Mirror Neurons for Actions, Sensations and Emotions. Philos. Trans. R. Soc. B 2014, 369, 20130175. [Google Scholar] [CrossRef] [PubMed]
- Bardi, L.; Bundt, C.; Notebaert, W.; Brass, M. Eliminating Mirror Responses by Instructions. Cortex 2015, 70, 128–136. [Google Scholar] [CrossRef]
- Brunsdon, V.E.A.; Bradford, E.E.F.; Smith, L.; Ferguson, H.J. Short-Term Physical Training Enhances Mirror System Activation to Action Observation. Soc. Neurosci. 2020, 15, 98–107. [Google Scholar] [CrossRef]
- De Klerk, C.C.J.M.; Johnson, M.H.; Heyes, C.M.; Southgate, V. Baby Steps: Investigating the Development of Perceptual-Motor Couplings in Infancy. Dev. Sci. 2015, 18, 270–280. [Google Scholar] [CrossRef]
- Fitzgibbon, B.M.; Kirkovski, M.; Fornito, A.; Paton, B.; Fitzgerald, P.B.; Enticott, P.G. Emotion Processing Fails to Modulate Putative Mirror Neuron Response to Trained Visuomotor Associations. Neuropsychologia 2016, 84, 7–13. [Google Scholar] [CrossRef]
- Catmur, C.; Walsh, V.; Heyes, C. Sensorimotor Learning Configures the Human Mirror System. Curr. Biol. 2007, 17, 1527–1531. [Google Scholar] [CrossRef]
- Catmur, C.; Heyes, C. Mirroring ‘Meaningful’ Actions: Sensorimotor Learning Modulates Imitation of Goal-Directed Actions. Q. J. Exp. Psychol. 2019, 72, 322–334. [Google Scholar] [CrossRef]
- Zazio, A.; Guidali, G.; Maddaluno, O.; Miniussi, C.; Bolognini, N. Hebbian Associative Plasticity in the Visuo-Tactile Domain: A Cross-Modal Paired Associative Stimulation Protocol. Neuroimage 2019, 201, 116025. [Google Scholar] [CrossRef]
- Maddaluno, O.; Guidali, G.; Zazio, A.; Miniussi, C.; Bolognini, N. Touch Anticipation Mediates Cross-Modal Hebbian Plasticity in the Primary Somatosensory Cortex. Cortex 2020, 126, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Guidali, G.; Carneiro, M.I.S.; Bolognini, N. Paired Associative Stimulation Drives the Emergence of Motor Resonance. Brain Stimul. 2020, 13, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, E.; Turrini, S.; Zanon, M.; Marangon, M.; Borgomaneri, S.; Avenanti, A. Driving Hebbian Plasticity over Ventral Premotor-Motor Projections Transiently Enhances Motor Resonance. Brain Stimul. 2024, 17, 211–220. [Google Scholar] [CrossRef]
- Turrini, S.; Fiori, F.; Bevacqua, N.; Saracini, C.; Lucero, B.; Candidi, M.; Avenanti, A. Spike-Timing-Dependent Plasticity Induction Reveals Dissociable Supplementary- and Premotor-Motor Pathways to Automatic Imitation. Proc. Natl. Acad. Sci. USA 2024, 121, e2404925121. [Google Scholar] [CrossRef] [PubMed]
- Guidali, G.; Picardi, M.; Gramegna, C.; Bolognini, N. Modulating Motor Resonance with Paired Associative Stimulation: Neurophysiological and Behavioral Outcomes. Cortex 2023, 163, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Guidali, G.; Arrigoni, E.; Bolognini, N.; Pisoni, A. M1 Large-Scale Network Dynamics Support Human Motor Resonance and Its Plastic Reshaping. Neuroimage 2025, 308, 121082. [Google Scholar] [CrossRef]
- Craighero, L. Motor Resonance: Neurophysiological Origin, Functional Role, and Contribution of the Motivational, Moral, and Social Aspects of Action. In The Routledge Handbook of Embodied Cognition; Routledge: London, UK, 2024; pp. 442–451. ISBN 9781003322511. [Google Scholar]
- Fadiga, L.; Fogassi, L.; Pavesi, G.; Rizzolatti, G. Motor Facilitation During Action Observation: A Magnetic Stimulation Study. J. Neurophysiol. 1995, 73, 2608–2611. [Google Scholar] [CrossRef]
- Naish, K.R.; Houston-Price, C.; Bremner, A.J.; Holmes, N.P. Effects of Action Observation on Corticospinal Excitability: Muscle Specificity, Direction, and Timing of the Mirror Response. Neuropsychologia 2014, 64, 331–348. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Cattaneo, L.; Fabbri-Destro, M.; Rozzi, S. Cortical Mechanisms Underlying the Organization of Goal-Directed Actions and Mirror Neuron-Based Action Understanding. Physiol. Rev. 2014, 94, 655–706. [Google Scholar] [CrossRef]
- De Beukelaar, T.T.; Alaerts, K.; Swinnen, S.P.; Wenderoth, N. Motor Facilitation during Action Observation: The Role of M1 and PMv in Grasp Predictions. Cortex 2016, 75, 180–192. [Google Scholar] [CrossRef]
- Koch, G.; Versace, V.; Bonnì, S.; Lupo, F.; Gerfo, E.L.; Oliveri, M.; Caltagirone, C. Resonance of Cortico-Cortical Connections of the Motor System with the Observation of Goal Directed Grasping Movements. Neuropsychologia 2010, 48, 3513–3520. [Google Scholar] [CrossRef] [PubMed]
- Lago, A.; Koch, G.; Cheeran, B.; Márquez, G.; Sánchez, J.A.; Ezquerro, M.; Giraldez, M.; Fernández-del-Olmo, M. Ventral Premotor to Primary Motor Cortical Interactions during Noxious and Naturalistic Action Observation. Neuropsychologia 2010, 48, 1802–1806. [Google Scholar] [CrossRef] [PubMed]
- Avenanti, A.; Bolognini, N.; Maravita, A.; Aglioti, S.M. Somatic and Motor Components of Action Simulation. Curr. Biol. 2007, 17, 2129–2135. [Google Scholar] [CrossRef]
- Catmur, C.; Mars, R.B.; Rushworth, M.F.; Heyes, C. Making Mirrors: Premotor Cortex Stimulation Enhances Mirror and Counter-Mirror Motor Facilitation. J. Cogn. Neurosci. 2011, 23, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Cantarero, G.; Galea, J.M.; Ajagbe, L.; Salas, R.; Willis, J.; Celnik, P. Disrupting the Ventral Premotor Cortex Interferes with the Contribution of Action Observation to Use-Dependent Plasticity. J. Cogn. Neurosci. 2011, 23, 3757–3766. [Google Scholar] [CrossRef]
- Suppa, A.; Quartarone, A.; Siebner, H.; Chen, R.; Di Lazzaro, V.; Del Giudice, P.; Paulus, W.; Rothwell, J.C.; Ziemann, U.; Classen, J. The Associative Brain at Work: Evidence from Paired Associative Stimulation Studies in Humans. Clin. Neurophysiol. 2017, 128, 2140–2164. [Google Scholar] [CrossRef]
- Guidali, G.; Roncoroni, C.; Bolognini, N. Modulating Frontal Networks’ Timing-Dependent-Like Plasticity With Paired Associative Stimulation Protocols: Recent Advances and Future Perspectives. Front Hum Neurosci 2021, 15, 658723. [Google Scholar] [CrossRef]
- Guidali, G.; Roncoroni, C.; Bolognini, N. Paired Associative Stimulations: Novel Tools for Interacting with Sensory and Motor Cortical Plasticity. Behav. Brain Res. 2021, 414, 113484. [Google Scholar] [CrossRef]
- Aziz-Zadeh, L.; Maeda, F.; Zaidel, E.; Mazziotta, J.; Iacoboni, M. Lateralization in Motor Facilitation during Action Observation: A TMS Study. Exp. Brain Res. 2002, 144, 127–131. [Google Scholar] [CrossRef]
- Fadiga, L.; Craighero, L.; Olivier, E. Human Motor Cortex Excitability during the Perception of Others’ Action. Curr. Opin. Neurobiol. 2005, 15, 213–218. [Google Scholar] [CrossRef]
- Turrini, S.; Fiori, F.; Chiappini, E.; Santarnecchi, E.; Romei, V.; Avenanti, A. Gradual Enhancement of Corticomotor Excitability during Cortico-Cortical Paired Associative Stimulation. Sci. Rep. 2022, 12, 14670. [Google Scholar] [CrossRef] [PubMed]
- Turrini, S.; Fiori, F.; Chiappini, E.; Lucero, B.; Santarnecchi, E.; Avenanti, A. Cortico-Cortical Paired Associative Stimulation (CcPAS) over Premotor-Motor Areas Affects Local Circuitries in the Human Motor Cortex via Hebbian Plasticity. Neuroimage 2023, 271, 120027. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Cohen Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 2013; ISBN 9780203774441.
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Safety, Ethical Considerations, and Application Guidelines for the Use of Transcranial Magnetic Stimulation in Clinical Practice and Research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef] [PubMed]
- Awiszus, F. TMS and Threshold Hunting. Suppl. Clin. Neurophysiol. 2003, 56, 13–23. [Google Scholar] [CrossRef]
- Guidali, G.; Picardi, M.; Franca, M.; Caronni, A.; Bolognini, N. The Social Relevance and the Temporal Constraints of Motor Resonance in Humans. Sci. Rep. 2023, 13, 15933. [Google Scholar] [CrossRef]
- The Jamovi Project. Jamovi. Version 2.6. The Jamovi Project: Sydney, Australia, 2025. Available online: https://www.jamovi.org (accessed on 25 February 2025).
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]
- Richardson, J.T.E. Eta Squared and Partial Eta Squared as Measures of Effect Size in Educational Research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- George, D.; Mallery, P. IBM SPSS Statistics 26 Step by Step: A Simple Guide and Reference; Routledge: New York, NY, USA, 2019; ISBN 9780134320250. [Google Scholar]
- Kobayashi, M.; Théoret, H.; Pascual-Leone, A. Suppression of Ipsilateral Motor Cortex Facilitates Motor Skill Learning. Eur. J. Neurosci. 2009, 29, 833–836. [Google Scholar] [CrossRef]
- Hamel, R.; Waltzing, B.M.; Hinder, M.R.; McAllister, C.J.; Jenkinson, N.; Galea, J.M. Bilateral Intracortical Inhibition during Unilateral Motor Preparation and Sequence Learning. Brain Stimul. 2024, 17, 349–361. [Google Scholar] [CrossRef]
- Takeuchi, N.; Oouchida, Y.; Izumi, S.I. Motor Control and Neural Plasticity through Interhemispheric Interactions. Neural Plast. 2012, 2012, 823285. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, A.; Heyes, C.; Becchio, C.; Bird, G.; Catmur, C. Timecourse of Mirror and Counter-Mirror Effects Measured with Transcranial Magnetic Stimulation. Soc. Cogn. Affect Neurosci. 2014, 9, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Kilner, J.; Friston, K.J.; Frith, C.D. Predictive Coding: An Account of the Mirror Neuron System. Cogn. Process 2007, 8, 159–166. [Google Scholar] [CrossRef]
- Aglioti, S.M.; Cesari, P.; Romani, M.; Urgesi, C. Action Anticipation and Motor Resonance in Elite Basketball Players. Nat. Neurosci. 2008, 11, 1109–1116. [Google Scholar] [CrossRef]
- Urgesi, C.; Maieron, M.; Avenanti, A.; Tidoni, E.; Fabbro, F.; Aglioti, S.M. Simulating the Future of Actions in the Human Corticospinal System. Cereb. Cortex 2010, 20, 2511–2521. [Google Scholar] [CrossRef]
- Qin, C.; Michon, F.; Onuki, Y.; Ishishita, Y.; Otani, K.; Kawai, K.; Fries, P.; Gazzola, V.; Keysers, C. Predictability Alters Information Flow during Action Observation in Human Electrocorticographic Activity. Cell Rep. 2023, 42, 113432. [Google Scholar] [CrossRef]
- Maranesi, M.; Livi, A.; Fogassi, L.; Rizzolatti, G.; Bonini, L. Mirror Neuron Activation Prior to Action Observation in a Predictable Context. J. Neurosci. 2014, 34, 14827–14832. [Google Scholar] [CrossRef]
- Southgate, V.; Johnson, M.H.; Osborne, T.; Csibra, G. Predictive Motor Activation during Action Observation in Human Infants. Biol. Lett. 2009, 5, 769–772. [Google Scholar] [CrossRef]
- Hernandez-Pavon, J.C.; San Agustín, A.; Wang, M.C.; Veniero, D.; Pons, J.L. Can We Manipulate Brain Connectivity? A Systematic Review of Cortico-Cortical Paired Associative Stimulation Effects. Clin. Neurophysiol. 2023, 154, 169–193. [Google Scholar] [CrossRef]
- Michielsen, M.E.; Selles, R.W.; Van Der Geest, J.N.; Eckhardt, M.; Yavuzer, G.; Stam, H.J.; Smits, M.; Ribbers, G.M.; Bussmann, J.B.J. Motor Recovery and Cortical Reorganization after Mirror Therapy in Chronic Stroke Patients: A Phase II Randomized Controlled Trial. Neurorehabil Neural Repair 2011, 25, 223–233. [Google Scholar] [CrossRef]
- Novaes, M.M.; Palhano-Fontes, F.; Peres, A.; Mazzetto-Betti, K.; Pelicioni, M.; Andrade, K.C.; Dos Santos, A.; Pontes-Neto, O.; Araujo, D. Neurofunctional Changes after a Single Mirror Therapy Intervention in Chronic Ischemic Stroke. Int. J. Neurosci. 2018, 128, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Mekbib, D.B.; Zhao, Z.; Wang, J.; Xu, B.; Zhang, L.; Cheng, R.; Fang, S.; Shao, Y.; Yang, W.; Han, J.; et al. Proactive Motor Functional Recovery Following Immersive Virtual Reality–Based Limb Mirroring Therapy in Patients with Subacute Stroke. Neurotherapeutics 2020, 17, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ding, L.; Wang, X.; Zhuang, J.; Tong, S.; Jia, J.; Guo, X. Evidence of Mirror Therapy for Recruitment of Ipsilateral Motor Pathways in Stroke Recovery: A Resting FMRI Study. Neurotherapeutics 2024, 21, e00320. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Fabbri-Destro, M.; Nuara, A.; Gatti, R.; Avanzini, P. The Role of Mirror Mechanism in the Recovery, Maintenance, and Acquisition of Motor Abilities. Neurosci. Biobehav. Rev. 2021, 127, 404–423. [Google Scholar] [CrossRef]
- Aziz-Zadeh, L.; Koski, L.; Zaidel, E.; Mazziotta, J.; Iacoboni, M. Lateralization of the Human Mirror Neuron System. J. Neurosci. 2006, 26, 2964–2970. [Google Scholar] [CrossRef]
- Molenberghs, P.; Cunnington, R.; Mattingley, J.B. Brain Regions with Mirror Properties: A Meta-Analysis of 125 Human FMRI Studies. Neurosci. Biobehav. Rev. 2012, 36, 341–349. [Google Scholar] [CrossRef]
- Picardi, M.; Guidali, G.; Caronni, A.; Rota, V.; Corbo, M.; Bolognini, N. Visuomotor Paired Associative Stimulation for Post-Stroke Hand Motor Impairments. medRxiv 2024. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guidali, G.; Bolognini, N. Tracking Changes in Corticospinal Excitability During Visuomotor Paired Associative Stimulation to Predict Motor Resonance Rewriting. Brain Sci. 2025, 15, 257. https://doi.org/10.3390/brainsci15030257
Guidali G, Bolognini N. Tracking Changes in Corticospinal Excitability During Visuomotor Paired Associative Stimulation to Predict Motor Resonance Rewriting. Brain Sciences. 2025; 15(3):257. https://doi.org/10.3390/brainsci15030257
Chicago/Turabian StyleGuidali, Giacomo, and Nadia Bolognini. 2025. "Tracking Changes in Corticospinal Excitability During Visuomotor Paired Associative Stimulation to Predict Motor Resonance Rewriting" Brain Sciences 15, no. 3: 257. https://doi.org/10.3390/brainsci15030257
APA StyleGuidali, G., & Bolognini, N. (2025). Tracking Changes in Corticospinal Excitability During Visuomotor Paired Associative Stimulation to Predict Motor Resonance Rewriting. Brain Sciences, 15(3), 257. https://doi.org/10.3390/brainsci15030257






