Identification of Brain Activation Areas in Response to Active Tactile Stimulation by Gripping a Stress Ball
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimuli Task
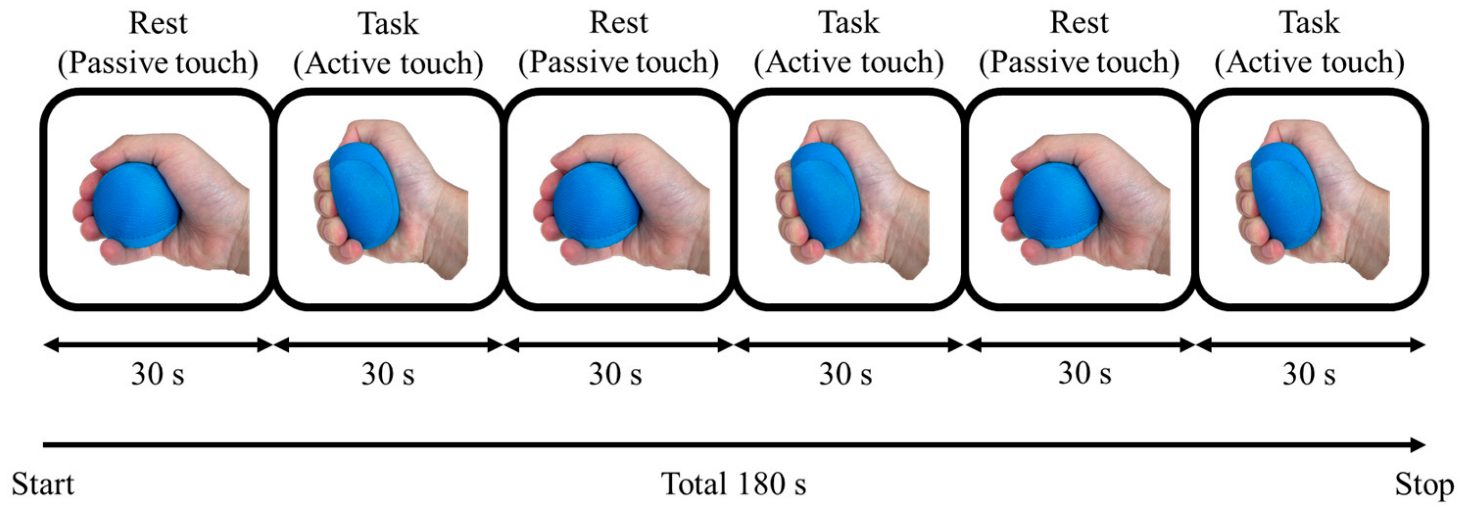
2.3. Block Design
2.4. Apparatus
2.5. MRI Acquisition
2.6. fMRI Data Analyses
2.7. Questionnaire
3. Results
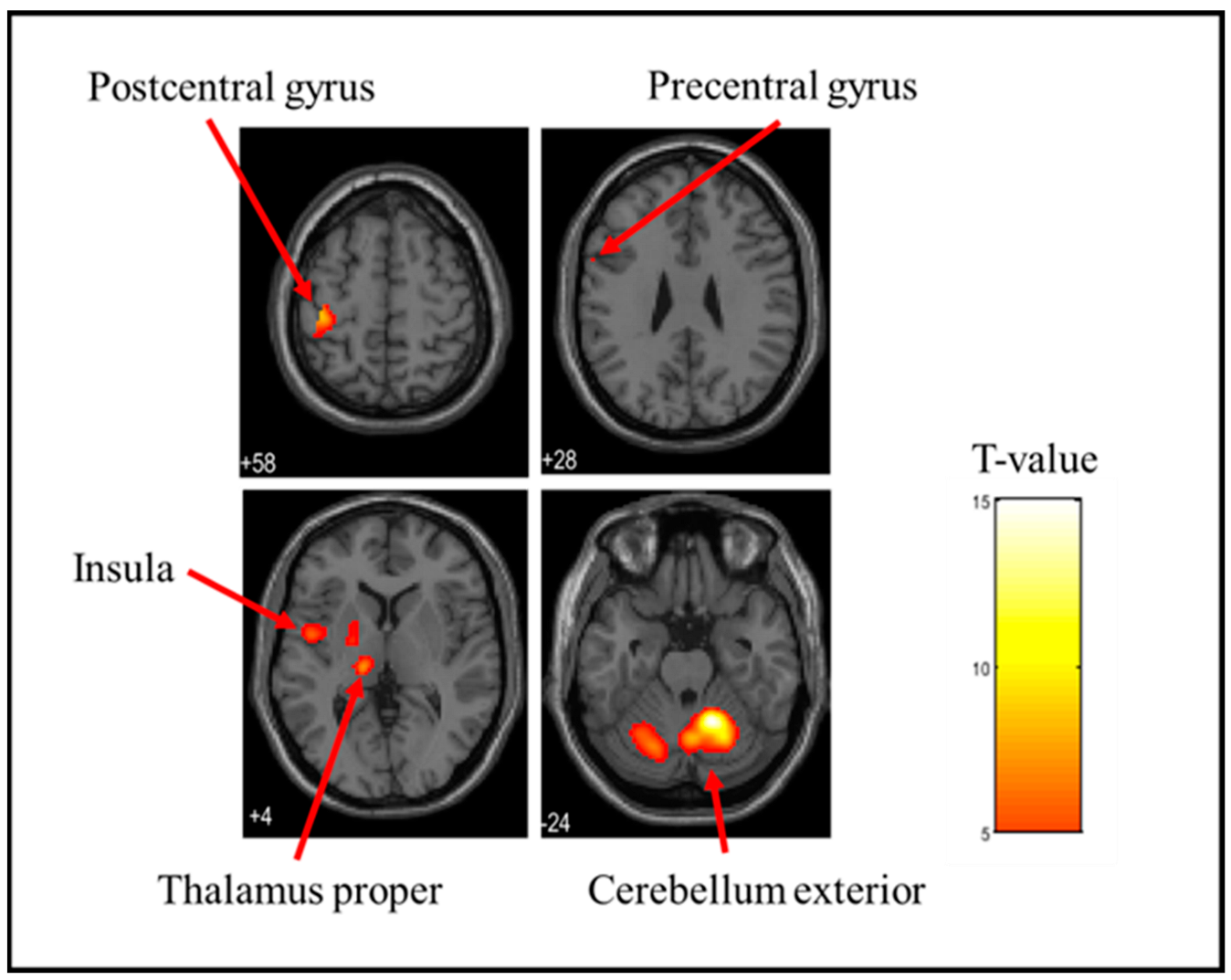
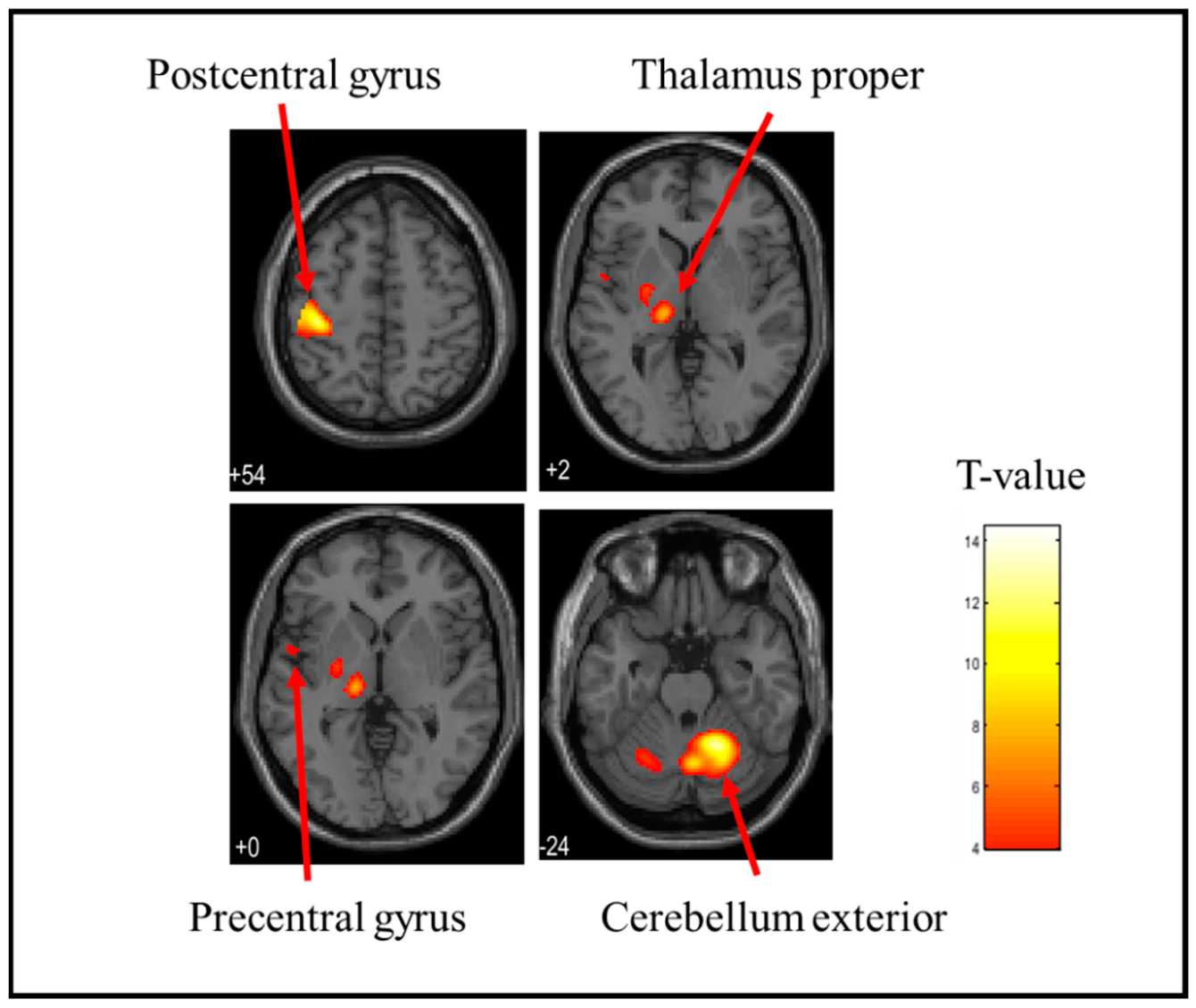
3.1. Brain Activation Sites When Holding a Stress Ball
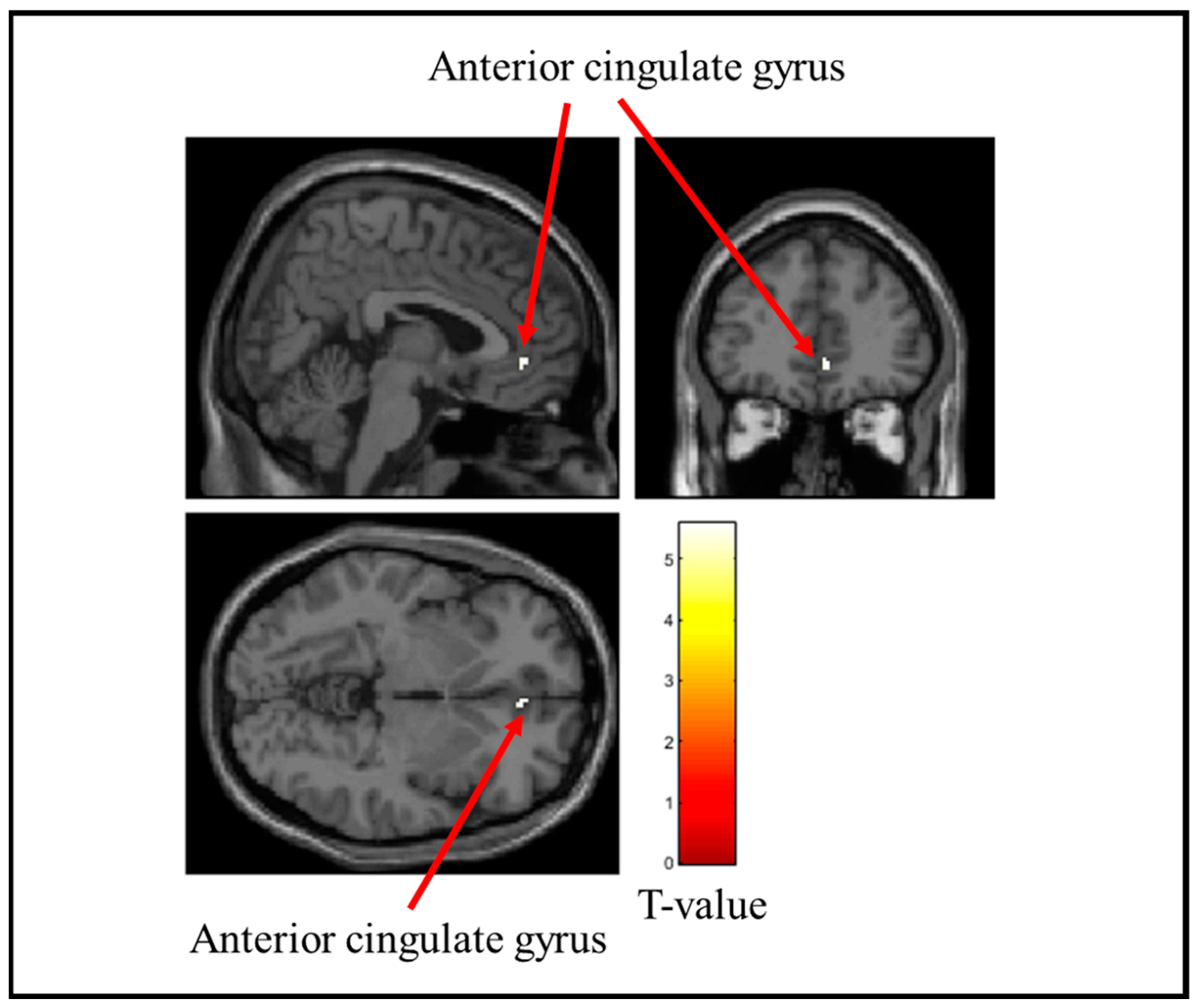
3.2. Brain Regions with Reduced Activity During Stress Ball Gripping
3.3. Results of Survey Score Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Löken, L.S.; Wessberg, J.; Morrison, I.; McGlone, F.; Olausson, H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 2009, 12, 547–548. [Google Scholar] [CrossRef]
- Olausson, H.W.; Cole, J.; Vallbo, A.; McGlone, F.; Elam, M.; Krämer, H.H.; Rylander, K.; Wessberg, J.; Bushnell, M.C. Unmyelinated tactile afferents have opposite effects on insular and somatosensory cortical processing. Neurosci. Lett. 2008, 436, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Olausson, H.; Lamarre, Y.; Backlund, H.; Morin, C.; Wallin, B.G.; Starck, G.; Ekholm, S.; Strigo, I.; Worsley, K.; Vallbo, A.B.; et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci. 2002, 5, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Olausson, H.; Wessberg, J.; Morrison, I.; McGlone, F.; Vallbo, A. The neurophysiology of unmyelinated tactile afferents. Neurosci. Biobehav. Rev. 2010, 34, 185–191. [Google Scholar] [CrossRef]
- Watkins, R.H.; Dione, M.; Ackerley, R.; Backlund Wasling, H.; Wessberg, J.; Löken, L.S. Evidence for sparse C-tactile afferent innervation of glabrous human hand skin. J. Neurophysiol. 2021, 125, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Morrison, I.; Löken, L.S.; Olausson, H. The skin as a social organ. Exp. Brain Res. 2010, 204, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Vallbo, A.B.; Olausson, H.; Wessberg, J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J. Neurophysiol. 1999, 81, 2753–2763. [Google Scholar] [CrossRef]
- Yoon, D.-E.; Lee, S.; Kim, J.; Kim, K.; Park, H.-J.; Napadow, V.; Lee, I.-S.; Chae, Y. Graded brain fMRI response to somatic and visual acupuncture stimulation. Cereb. Cortex 2023, 33, 11269–11278. [Google Scholar] [CrossRef]
- Schaefer, M.; Kühnel, A.; Gärtner, M. Sensory processing sensitivity and somatosensory brain activation when feeling touch. Sci. Rep. 2022, 12, 12024. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef] [PubMed]
- Abagnale, S.; Panico, F.; Sagliano, L.; Gosseries, O.; Trojano, L. Pleasant touch: Behavioural and hemodynamic responses to a protocol for systematic assessment of tactile stimulation. Cortex 2025, 184, 236–249, ISSN 0010-9452. [Google Scholar] [CrossRef]
- Swash, M. Henry Head’s lifelong studies of cutaneous sensation. J. Med. Biogr. 2022, 30, 57–63. [Google Scholar] [CrossRef]
- Rolls, E.T.; O’Doherty, J.; Kringelbach, M.L.; Francis, S.; Bowtell, R.; McGlone, F. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb. Cortex 2003, 13, 308–317. [Google Scholar] [CrossRef]
- Francis, S.; Rolls, E.T.; Bowtell, R.; McGlone, F.; O’Doherty, J.; Browning, A.; Clare, S.; Smith, E. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. NeuroReport 1999, 10, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.D.; Reiman, E.M.; Bradley, M.M.; Lang, P.J.; Ahern, G.L.; Davidson, R.J.; Schwartz, G.E. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia 1997, 35, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. Taste and smell processing in the brain. Handb. Clin. Neurol. 2019, 164, 97–118. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Ottaviani, C.; Spitoni, G.F. Affective touch: A meta-analysis on sex differences. Neurosci. Biobehav. Rev. 2020, 108, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Field, T.; Diego, M.; Hernandez-Reif, M. Massage therapy research. Dev. Rev. 2007, 27, 75–89. [Google Scholar] [CrossRef]
- Perlman, A.I.; Ali, A.; Njike, V.Y.; Hom, D.; Davidi, A.; Gould-Fogerite, S.; Milak, C.; Katz, D.L. Massage therapy for osteoarthritis of the knee: A randomized dose-finding trial. PLoS ONE 2012, 7, e30248. [Google Scholar] [CrossRef]
- Krauss, K.E. The effects of deep pressure touch on anxiety. Am. J. Occup. Ther. 1987, 41, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Grandin, T. Calming Effects of deep touch pressure in patients with autistic disorder, college students, and animals. J. Child Adolesc. Psychopharmacol. 1992, 2, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Field, T.; Diego, M.; Hernandez-Reif, M. Moderate pressure is essential for massage therapy effects. Int. J. Neurosci. 2010, 120, 381–385. [Google Scholar] [CrossRef]
- Diego, M.A.; Field, T.; Sanders, C.; Hernandez-Reif, M. Massage therapy of moderate and light pressure and vibrator effects on EEG and heart rate. Int. J. Neurosci. 2004, 114, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Case, L.K.; Liljencrantz, J.; McCall, M.V.; Bradson, M.; Necaise, A.; Tubbs, J.; Olausson, H.; Wang, B.; Catherine Bushnell, M. Pleasant deep pressure: Expanding the social touch hypothesis. Neuroscience 2021, 464, 3–11, ISSN 0306-4522. [Google Scholar] [CrossRef]
- Karatas, T.C.; Gezginci, E. The effect of using a stress ball during endoscopy on pain, anxiety, and satisfaction: A randomized controlled trial. Gastroenterol. Nurs. 2023, 46, 309–317. [Google Scholar] [CrossRef]
- Ozen, N.; Berse, S.; Tosun, B. Effects of using a stress ball on anxiety and depression in patients undergoing hemodialysis: A prospective, balanced, single-blind, crossover study. Hemodial. Int. 2023, 27, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Sakurai, N.; Yuguchi, Y.; Kasai, S.; Kodama, N. Identification of areas of the brain activated by active stimulation in hairless skin. Behav. Brain Res. 2024, 458, 114758. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Qiao, L.; Liu, K.; Liu, J.; Piccinni-Ash, T.J.; Chen, Z.F. Molecular and neural basis of pleasant touch sensation. Science 2022, 376, 483–491. [Google Scholar] [CrossRef]
- Pasqualotto, A.; Ng, M.; Tan, Z.Y.; Kitada, R. Tactile perception of pleasantness in relation to perceived softness. Sci. Rep. 2020, 10, 11189. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, J.A.; Santistevan, A.C.; Raven, E.P.; Bennett, J.L.; Russ, B.E.; Bliss-Moreau, E. Evolutionarily conserved neural responses to affective touch in monkeys transcend consciousness and change with age. Proc. Natl. Acad. Sci. USA 2024, 121, e2322157121. [Google Scholar] [CrossRef]
- Savallampi, M.; Maallo, A.M.S.; Shaikh, S.; McGlone, F.; Bariguian-Revel, F.J.; Olausson, H.; Boehme, R. Social touch reduces pain perception—An fMRI study of cortical mechanisms. Brain Sci. 2023, 13, 393. [Google Scholar] [CrossRef]
- Baciero, A.; Perea, M.; Duñabeitia, J.A.; Gómez, P. TouchScope: A passive-haptic device to investigate tactile perception using a refreshable braille display. J. Cogn. 2023, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.J. Observations on active touch. Psychol. Rev. 1962, 69, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Livianos, L.; González-Valls, P.I.; García-Blanco, A.C.; Tobella, H.; Díaz-Alonso, I.; Alberola, N.; García-Aznar, S.; Bellot, R.; Saiz, V.; Ros, L. Hypoesthesia of the malleolus as a soft sign in depression. J. Affect. Disord. 2015, 171, 128–131, ISSN 0165-0327. [Google Scholar] [CrossRef]
- Phelan, K.; McDermid, H.E. The 22q13.3 deletion syndrome (Phelan-McDermid Syndrome). Mol. Syndromol. 2012, 2, 186–201. [Google Scholar] [CrossRef]
- Habas, C.; Kamdar, N.; Nguyen, D.; Prater, K.; Beckmann, C.F.; Menon, V.; Greicius, M.D. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 2009, 29, 8586–8594. [Google Scholar] [CrossRef]
- Krienen, F.M.; Buckner, R.L. Segregated Fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb. Cortex 2009, 19, 2485–2497. [Google Scholar] [CrossRef]
- O’Reilly, J.X.; Beckmann, C.F.; Tomassini, V.; Ramnani, N.; Johansen-Berg, H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb. Cortex 2010, 20, 953–965. [Google Scholar] [CrossRef]
- De Zeeuw, C.I.; Ten Brinke, M.M. Motor learning and the cerebellum. Cold Spring Harb. Perspect. Biol. 2015, 7, a021683. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Davis, C.; Thomas, A.M.; Economo, M.N.; Abrego, A.M.; Svoboda, K.; De Zeeuw, C.I.; Li, N. A cortico-cerebellar loop for motor planning. Nature 2018, 563, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, J.; Yeon, J.; Park, J.Y.; Chung, D.; Kim, S.P. Neural correlates of tactile hardness intensity perception during active grasping. PeerJ 2021, 9, e11760. [Google Scholar] [CrossRef] [PubMed]
- Haase, L.; Thom, N.J.; Shukla, A.; Davenport, P.W.; Simmons, A.N.; Stanley, E.A.; Paulus, M.P.; Johnson, D.C. Mindfulness-based training attenuates insula response to an aversive interoceptive challenge. Soc. Cogn. Affect. Neurosci. 2016, 11, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.-C.; Chien, J.-H.; Greenspan, J.D.; Garonzik, I.; Weiss, N.; Ohara, S.; Lenz, F.A. Neuronal responses to tactile stimuli and tactile sensations evoked by microstimulation in the human thalamic principal somatic sensory nucleus (Ventral Caudal). J. Neurophysiol. 2016, 115, 2421–2433. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, L. A Network Model of the emotional brain. Trends Cogn. Sci. 2017, 21, 357–371, ISSN 1364-6613. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.; Whalen, P.J. The amygdala: Vigilance and emotion. Mol. Psychiatry 2001, 6, 13–34. [Google Scholar] [CrossRef]
- Ledoux, J. The emotional brain, fear, and the amygdala. Cell. Mol. Neurobiol. 2003, 23, 727–738. [Google Scholar] [CrossRef]
- Garavan, H.; Pendergrass, J.C.; Ross, T.J.; Stein, E.A.; Risinger, R.C. Amygdala response to both positively and negatively valenced stimuli. NeuroReport 2001, 12, 2779–2783. [Google Scholar] [CrossRef]
- Hamann, S.; Mao, H. Positive and negative emotional verbal stimuli elicit activity in the left amygdala. NeuroReport 2002, 13, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Lanteaume, L.; Khalfa, S.; Régis, J.; Marquis, P.; Chauvel, P.; Bartolomei, F. Emotion induction after direct intracerebral stimulations of human amygdala. Cereb. Cortex 2007, 17, 1307–1313. [Google Scholar] [CrossRef]
- Dai, Q.; Wei, J.; Shu, X.; Feng, Z. Negativity bias for sad faces in depression: An event-related potential study. Clin. Neurophysiol. 2016, 127, 3552–3560. [Google Scholar] [CrossRef]
- Stevens, F.L.; Hurley, R.A.; Taber, K.H. Anterior cingulate cortex: Unique role in cognition and emotion. J. Neuropsychiatry Clin. Neurosci. 2011, 23, 121–125. [Google Scholar] [CrossRef]
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000, 4, 215–222. [Google Scholar] [CrossRef] [PubMed]




| Hemisphere | Locations | Cluster p-Value (FWE) | Cluster Size (Voxels) | T-Value | X [mm] | Y [mm] | Z [mm] | |
|---|---|---|---|---|---|---|---|---|
| Soft | Right | Cerebellum Exterior | <0.001 | 2378 | 14.98 | 16 | −54 | −24 |
| Left | Postcentral gyrus | <0.001 | 420 | 9.88 | −40 | −26 | 58 | |
| Left | Thalamus Proper | <0.001 | 339 | 8.88 | −14 | −22 | 4 | |
| Left | Anterior insula | <0.001 | 219 | 7.73 | −48 | 0 | 2 | |
| Left | Parietal operculum | <0.001 | 166 | 7.54 | −50 | −24 | 16 | |
| Left | Putamen | <0.001 | 128 | 6.95 | −22 | −6 | 2 | |
| Left | Precentral gyrus | 0.028 | 3 | 5.84 | −58 | 8 | 28 | |
| Medium | Left | Postcentral gyrus | <0.001 | 659 | 14.41 | −38 | −26 | 58 |
| Right | Cerebellum Exterior | <0.001 | 1713 | 13.14 | 18 | −54 | −24 | |
| Left | Thalamus Proper | <0.001 | 515 | 9.47 | −14 | −22 | 4 | |
| Left | Parietal operculum | <0.001 | 189 | 9.09 | −48 | −24 | 14 | |
| Left | Cerebellum Exterior | <0.001 | 206 | 8.73 | −28 | −62 | −28 | |
| Right | Caudate | <0.001 | 65 | 7.59 | 16 | −16 | 20 | |
| Left | Putamen | <0.001 | 72 | 7.10 | −24 | −8 | 2 | |
| Left | Precentral gyrus | 0.012 | 12 | 5.47 | −52 | 2 | 0 | |
| Hard | Right | Cerebellum Exterior | <0.001 | 2479 | 15.09 | 18 | −56 | −22 |
| Left | Postcentral gyrus | <0.001 | 454 | 10.12 | −40 | −26 | 56 | |
| Left | Thalamus Proper | <0.001 | 363 | 9.66 | −14 | −20 | −2 | |
| Left | Anterior insula | <0.001 | 275 | 7.33 | −48 | 0 | 4 | |
| Right | parietal operculum | <0.001 | 173 | 7.07 | 66 | −24 | 18 | |
| Right | Middle frontal gyrus | 0.003 | 39 | 6.78 | 54 | 4 | 40 | |
| Left | Central operculum | <0.001 | 143 | 6.53 | −52 | −22 | 16 | |
| Left | Amygdala | 0.005 | 28 | 6.00 | −22 | −10 | −4 | |
| Right | Anterior insula | 0.001 | 51 | 5.73 | 48 | 2 | 10 | |
| Left | Precentral gyrus | 0.039 | 1 | 5.34 | −58 | 6 | 28 | |
| Right | Opercular part of the inferior frontal gyrus | 0.039 | 1 | 5.23 | 60 | 10 | 16 | |
| Right | Supramarginal gyrus | 0.039 | 1 | 5.23 | 60 | −26 | 46 |
| Hemisphere | Locations | Cluster p-Value (FWE) | Cluster Size(Voxels) | T-Value | X [mm] | Y [mm] | Z [mm] | |
|---|---|---|---|---|---|---|---|---|
| Soft | Right | Insula | 0.001 | 35 | 6.88 | 34 | −20 | 16 |
| Right | Middle temporal gyrus | 0.006 | 23 | 6.09 | 52 | −28 | 4 | |
| Medium | Right | Insula | 0.034 | 3 | 5.39 | 36 | −18 | 18 |
| Hard | Right | Anterior cingulate gyrus | 0.02 | 8 | 5.57 | 4 | 42 | −4 |
| Likert Scale Point | Unpleasant | Pleasant | Ave | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| Soft | 1 | 0 | 1 | 4 | 2 | 7 | 10 | 9 | 6 | 6.7 ± 3.7 |
| Medium | 0 | 1 | 4 | 3 | 5 | 11 | 10 | 5 | 1 | 5.9 ± 3.9 |
| Hard | 2 | 4 | 8 | 6 | 5 | 10 | 2 | 2 | 1 | 4.6 ± 3.1 |
| Likert Scale Point | Soft | Hard | Ave | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| Soft | 6 | 8 | 16 | 3 | 3 | 1 | 2 | 1 | 0 | 3.1 ± 5.0 |
| Medium | 0 | 0 | 2 | 12 | 9 | 11 | 5 | 1 | 0 | 5.2 ± 5.0 |
| Hard | 0 | 1 | 1 | 1 | 0 | 9 | 10 | 12 | 6 | 7.1 ± 4.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, K.; Sakurai, N.; Yoshida, N.; Oishi, M.; Kasai, S.; Kodama, N. Identification of Brain Activation Areas in Response to Active Tactile Stimulation by Gripping a Stress Ball. Brain Sci. 2025, 15, 264. https://doi.org/10.3390/brainsci15030264
Sasaki K, Sakurai N, Yoshida N, Oishi M, Kasai S, Kodama N. Identification of Brain Activation Areas in Response to Active Tactile Stimulation by Gripping a Stress Ball. Brain Sciences. 2025; 15(3):264. https://doi.org/10.3390/brainsci15030264
Chicago/Turabian StyleSasaki, Kei, Noriko Sakurai, Nobukiyo Yoshida, Misuzu Oishi, Satoshi Kasai, and Naoki Kodama. 2025. "Identification of Brain Activation Areas in Response to Active Tactile Stimulation by Gripping a Stress Ball" Brain Sciences 15, no. 3: 264. https://doi.org/10.3390/brainsci15030264
APA StyleSasaki, K., Sakurai, N., Yoshida, N., Oishi, M., Kasai, S., & Kodama, N. (2025). Identification of Brain Activation Areas in Response to Active Tactile Stimulation by Gripping a Stress Ball. Brain Sciences, 15(3), 264. https://doi.org/10.3390/brainsci15030264








