Lactylation and Central Nervous System Diseases
Abstract
1. Introduction
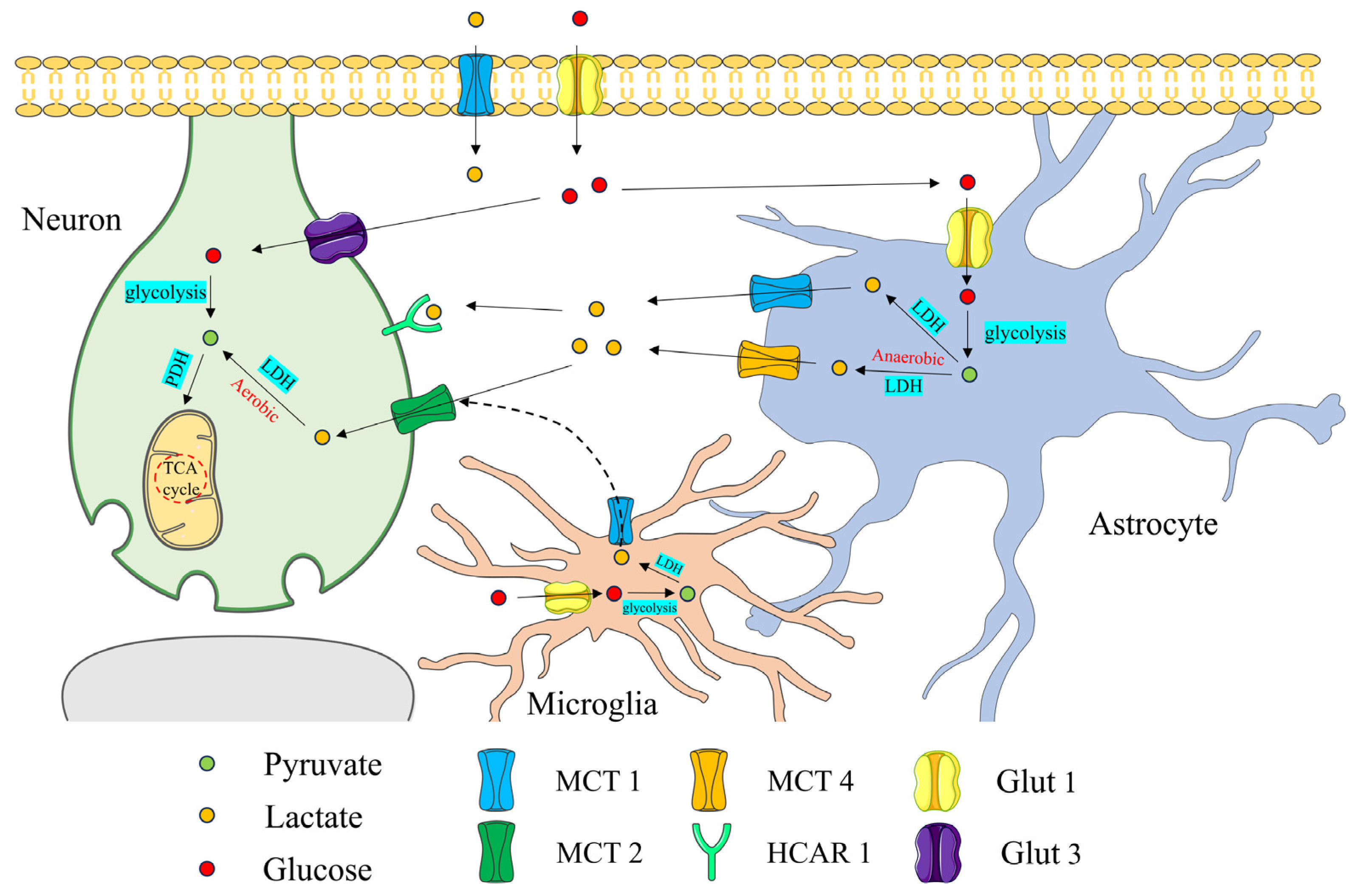
2. Role of Lactate in the Central Nervous System
2.1. Lactate Promotes Basic Nerve Function and Development
2.2. Lactate Regulates Brain pH
2.3. Lactate Participates as a Signaling Molecule in the Physiological Regulation of the CNS
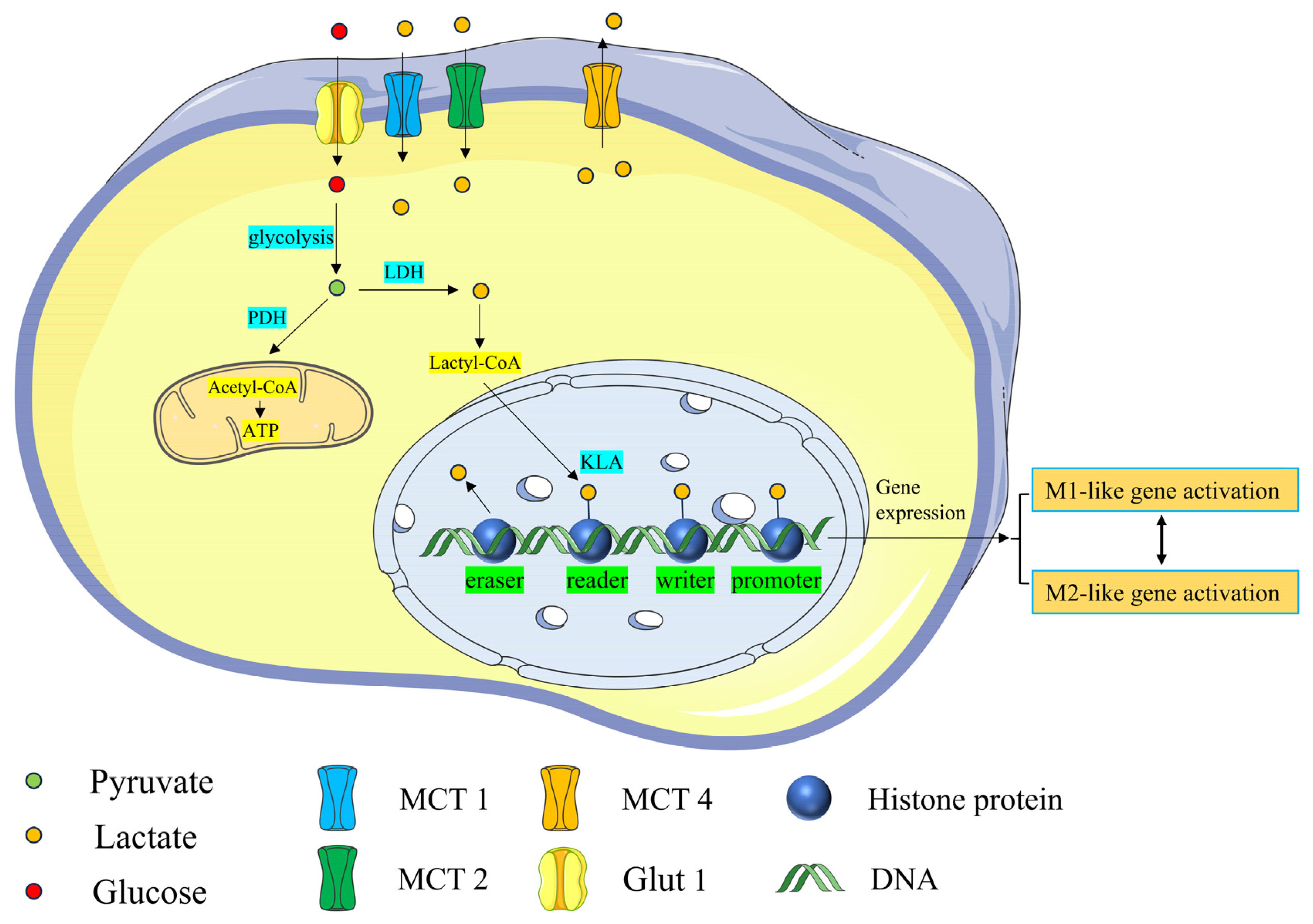
3. Lactylation
3.1. Histone Lactylation
3.2. Nonhistone Lactylation
4. Kla and CNS Diseases
4.1. Traumatic Brain Injury (TBI)
4.1.1. Kla Regulates the Macrophage Phenotype
4.1.2. GPR81 and Brain-Derived Neurotrophic Factor (BDNF)
4.2. Alzheimer’s Disease (AD)
4.2.1. Glycolysis/H4K12 La/PKM2
4.2.2. H3K18
4.2.3. Isocitrate Dehydrogenase 3β (IDH3)
4.2.4. Exercise
4.3. Acute Ischemic Stroke (AIS)
4.3.1. HDAC6
4.3.2. Lymphocyte Cytoplasmic Protein 1 (LCP1)
4.3.3. Low-Density Lipoprotein Receptor-Related Protein-1 (LRP1)
4.4. Schizophrenia (SCZ)
5. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PK/PYK | Pyruvate kinase |
| OXPHOS | Phosphorylation |
| LDH | Lactate dehydrogenase |
| CNS | Central nervous system |
| MCT | Monocarboxylate transporter |
| GPCR | G protein-coupled receptor |
| Glut1 | Glucose transporter 1 |
| PTMs | Post-translational modifications |
| Kla | Lysine lactylation |
| HDAC | Histone deacetylase |
| PKM2 | M2 isoform of PK |
| TBI | Traumatic brain injury |
| BDNF | Brain-derived neurotrophic factor |
| AD | Alzheimer’s disease |
| Aβ | Amyloid beta |
| H4K12la | H4k12 lactylation |
| H3K18la | H3k18 lactylation |
| SASP | Senescence-associated secretory phenotype |
| TCA | Tricarboxylic acid |
| IDH3 | Isocitrate dehydrogenase 3 |
| D-gal | D-galactose |
| AIS | Acute ischemic stroke |
| LCP1 | Lymphocyte cytoplasmic protein 1 |
| MCAO | Middle cerebral artery occlusion |
| LRP1 | Lipoprotein receptor-related protein-1 |
| ARF1 | ADP-ribosylation factor 1 |
| SCZ | Schizophrenia |
| HMGB1 | High mobility group box 1 |
References
- Oesper, R.E. The Collected Papers of Carl Wilhelm Scheele. J. Chem. Educ. 1931, 8, 2304. [Google Scholar] [CrossRef][Green Version]
- Gladden, L.B. 200th anniversary of lactate research in muscle. Exerc. Sport Sci. Rev. 2008, 36, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Certo, M.; Tsai, C.H.; Pucino, V.; Ho, P.C.; Mauro, C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat. Rev. Immunol. 2021, 21, 151–161. [Google Scholar] [CrossRef]
- Rivadeneira, D.B.; Delgoffe, G.M. Antitumor T-cell Reconditioning: Improving Metabolic Fitness for Optimal Cancer Immunotherapy. Clin. Cancer Res. 2018, 24, 2473–2481. [Google Scholar] [CrossRef] [PubMed]
- Proia, P.; Di Liegro, C.M.; Schiera, G.; Fricano, A.; Di Liegro, I. Lactate as a Metabolite and a Regulator in the Central Nervous System. Int. J. Mol. Sci. 2016, 17, 1450. [Google Scholar] [CrossRef] [PubMed]
- Certo, M.; Llibre, A.; Lee, W.; Mauro, C. Understanding lactate sensing and signalling. Trends Endocrinol. Metab. 2022, 33, 722–735. [Google Scholar] [CrossRef]
- Bonvento, G.; Bolaños, J.P. Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab. 2021, 33, 1546–1564. [Google Scholar] [CrossRef]
- Dienel, G.A. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol. Rev. 2019, 99, 949–1045. [Google Scholar] [CrossRef]
- Muraleedharan, R.; Gawali, M.V.; Tiwari, D.; Sukumaran, A.; Oatman, N.; Anderson, J.; Nardini, D.; Bhuiyan, M.A.N.; Tkáč, I.; Ward, A.L.; et al. AMPK-Regulated Astrocytic Lactate Shuttle Plays a Non-Cell-Autonomous Role in Neuronal Survival. Cell Rep. 2020, 32, 108092. [Google Scholar] [CrossRef]
- González-Gutiérrez, A.; Ibacache, A.; Esparza, A.; Barros, L.F.; Sierralta, J. Neuronal lactate levels depend on glia-derived lactate during high brain activity in Drosophila. Glia 2020, 68, 1213–1227. [Google Scholar] [CrossRef]
- Falkowska, A.; Gutowska, I.; Goschorska, M.; Nowacki, P.; Chlubek, D.; Baranowska-Bosiacka, I. Energy Metabolism of the Brain, Including the Cooperation between Astrocytes and Neurons, Especially in the Context of Glycogen Metabolism. Int. J. Mol. Sci. 2015, 16, 25959–25981. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ruchti, E.; Petit, J.M.; Jourdain, P.; Grenningloh, G.; Allaman, I.; Magistretti, P.J. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl. Acad. Sci. USA 2014, 111, 12228–12233. [Google Scholar] [CrossRef]
- Späte, E.; Zhou, B.; Sun, T.; Kusch, K.; Asadollahi, E.; Siems, S.B.; Depp, C.; Werner, H.B.; Saher, G.; Hirrlinger, J.; et al. Downregulated expression of lactate dehydrogenase in adult oligodendrocytes and its implication for the transfer of glycolysis products to axons. Glia 2024, 72, 1374–1391. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic lateral sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef]
- Rinholm, J.E.; Hamilton, N.B.; Kessaris, N.; Richardson, W.D.; Bergersen, L.H.; Attwell, D. Regulation of oligodendrocyte development and myelination by glucose and lactate. J. Neurosci. 2011, 31, 538–548. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; Enerbäck, S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2020, 2, 566–571. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef]
- Gibbs, M.E.; Hertz, L. Inhibition of astrocytic energy metabolism by D-lactate exposure impairs memory. Neurochem. Int. 2008, 52, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.A.; Korol, D.L.; Gold, P.E. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS ONE 2011, 6, e28427. [Google Scholar] [CrossRef]
- Brandebura, A.N.; Paumier, A.; Onur, T.S.; Allen, N.J. Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders. Nat. Rev. Neurosci. 2023, 24, 23–39. [Google Scholar] [CrossRef]
- Ruffin, V.A.; Salameh, A.I.; Boron, W.F.; Parker, M.D. Intracellular pH regulation by acid-base transporters in mammalian neurons. Front. Physiol. 2014, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Nohesara, S.; Abdolmaleky, H.M.; Thiagalingam, S. Potential for New Therapeutic Approaches by Targeting Lactate and pH Mediated Epigenetic Dysregulation in Major Mental Diseases. Biomedicines 2024, 12, 457. [Google Scholar] [CrossRef]
- Pruett, B.S.; Meador-Woodruff, J.H. Evidence for altered energy metabolism, increased lactate, and decreased pH in schizophrenia brain: A focused review and meta-analysis of human postmortem and magnetic resonance spectroscopy studies. Schizophr. Res. 2020, 223, 29–42. [Google Scholar] [CrossRef]
- Hagihara, H.; Murano, T.; Miyakawa, T. The gene expression patterns as surrogate indices of pH in the brain. Front. Psychiatry 2023, 14, 1151480. [Google Scholar] [CrossRef]
- Deitmer, J.W.; Theparambil, S.M.; Ruminot, I.; Noor, S.I.; Becker, H.M. Energy Dynamics in the Brain: Contributions of Astrocytes to Metabolism and pH Homeostasis. Front. Neurosci. 2019, 13, 1301. [Google Scholar] [CrossRef]
- Pucino, V.; Cucchi, D.; Mauro, C. Lactate transporters as therapeutic targets in cancer and inflammatory diseases. Expert Opin. Ther. Targets 2018, 22, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Lactate as a fulcrum of metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef] [PubMed]
- Philips, T.; Mironova, Y.A.; Jouroukhin, Y.; Chew, J.; Vidensky, S.; Farah, M.H.; Pletnikov, M.V.; Bergles, D.E.; Morrison, B.M.; Rothstein, J.D. MCT1 Deletion in Oligodendrocyte Lineage Cells Causes Late-Onset Hypomyelination and Axonal Degeneration. Cell Rep. 2021, 34, 108610. [Google Scholar] [CrossRef]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.W.; et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef]
- Jha, M.K.; Lee, Y.; Russell, K.A.; Yang, F.; Dastgheyb, R.M.; Deme, P.; Ament, X.H.; Chen, W.; Liu, Y.; Guan, Y.; et al. Monocarboxylate transporter 1 in Schwann cells contributes to maintenance of sensory nerve myelination during aging. Glia 2020, 68, 161–177. [Google Scholar] [CrossRef]
- Brooks, G.A.; Curl, C.C.; Leija, R.G.; Osmond, A.D.; Duong, J.J.; Arevalo, J.A. Tracing the lactate shuttle to the mitochondrial reticulum. Exp. Mol. Med. 2022, 54, 1332–1347. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Asenso, J.; Xiao, N.; Gao, J.; Xiao, F.; Kuai, J.; Wei, W.; Wang, C. Lactic Acid Regulation: A Potential Therapeutic Option in Rheumatoid Arthritis. J. Immunol. Res. 2022, 2022, 2280973. [Google Scholar] [CrossRef]
- Medel, V.; Crossley, N.; Gajardo, I.; Muller, E.; Barros, L.F.; Shine, J.M.; Sierralta, J. Whole-brain neuronal MCT2 lactate transporter expression links metabolism to human brain structure and function. Proc. Natl. Acad. Sci. USA 2022, 119, e2204619119. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef] [PubMed]
- Hadzic, A.; Nguyen, T.D.; Hosoyamada, M.; Tomioka, N.H.; Bergersen, L.H.; Storm-Mathisen, J.; Morland, C. The Lactate Receptor HCA1 Is Present in the Choroid Plexus, the Tela Choroidea, and the Neuroepithelial Lining of the Dorsal Part of the Third Ventricle. Int. J. Mol. Sci. 2020, 21, 6457. [Google Scholar] [CrossRef]
- Yang, K.; Fan, M.; Wang, X.; Xu, J.; Wang, Y.; Tu, F.; Gill, P.S.; Ha, T.; Liu, L.; Williams, D.L.; et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 2022, 29, 133–146. [Google Scholar] [CrossRef]
- Lundø, K.; Trauelsen, M.; Pedersen, S.F.; Schwartz, T.W. Why Warburg Works: Lactate Controls Immune Evasion through GPR81. Cell Metab. 2020, 31, 666–668. [Google Scholar] [CrossRef]
- Khatib-Massalha, E.; Bhattacharya, S.; Massalha, H.; Biram, A.; Golan, K.; Kollet, O.; Kumari, A.; Avemaria, F.; Petrovich-Kopitman, E.; Gur-Cohen, S.; et al. Lactate released by inflammatory bone marrow neutrophils induces their mobilization via endothelial GPR81 signaling. Nat. Commun. 2020, 11, 3547. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wang, M.Y.; Pan, Z.Y.; Li, Z.Q.; Wang, Z.F. Chronic microglial inflammation promotes neuronal lactate supply but impairs its utilization in primary rat astrocyte-neuron co-cultures. Biochem. Biophys. Res. Commun. 2022, 607, 28–35. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cai, M.; Shang, Q.; Li, Z.; Feng, Y.; Liu, B.; Xue, X.; Lou, S. Elevated Lactate by High-Intensity Interval Training Regulates the Hippocampal BDNF Expression and the Mitochondrial Quality Control System. Front. Physiol. 2021, 12, 629914. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Lee, D.; Xiong, W.C. Lactate Metabolism, Signaling, and Function in Brain Development, Synaptic Plasticity, Angiogenesis, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 13398. [Google Scholar] [CrossRef]
- Cai, Y.; Guo, H.; Han, T.; Wang, H. Lactate: A prospective target for therapeutic intervention in psychiatric disease. Neural Regen. Res. 2024, 19, 1473–1479. [Google Scholar] [CrossRef]
- Theparambil, S.M.; Kopach, O.; Braga, A.; Nizari, S.; Hosford, P.S.; Sagi-Kiss, V.; Hadjihambi, A.; Konstantinou, C.; Esteras, N.; Gutierrez Del Arroyo, A.; et al. Adenosine signalling to astrocytes coordinates brain metabolism and function. Nature 2024, 632, 139–146. [Google Scholar] [CrossRef]
- Sharma, N.K.; Pal, J.K. Metabolic Ink Lactate Modulates Epigenomic Landscape: A Concerted Role of Pro-tumor Microenvironment and Macroenvironment During Carcinogenesis. Curr. Mol. Med. 2021, 21, 177–181. [Google Scholar] [CrossRef]
- Tessarz, P.; Kouzarides, T. Histone core modifications regulating nucleosome structure and dynamics. Nat. Rev. Mol. Cell Biol. 2014, 15, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.I.; Reinberg, D. Histones: Annotating chromatin. Annu. Rev. Genet. 2009, 43, 559–599. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Fan, H.; Yang, F.; Xiao, Z.; Luo, H.; Chen, H.; Chen, Z.; Liu, Q.; Xiao, Y. Lactylation: Novel epigenetic regulatory and therapeutic opportunities. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E330–E338. [Google Scholar] [CrossRef]
- Moreno-Yruela, C.; Zhang, D.; Wei, W.; Bæk, M.; Liu, W.; Gao, J.; Danková, D.; Nielsen, A.L.; Bolding, J.E.; Yang, L.; et al. Class I histone deacetylases (HDAC1-3) are histone lysine delactylases. Sci. Adv. 2022, 8, eabi6696. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zheng, D.; Wen, X.; Xiao, W.; Han, F.; Lang, H.; Xiong, S.; Jiang, W.; Hu, Y.; He, M.; et al. Proteomic and Morphological Profiling of Mice Ocular Tissue During High-altitude Acclimatization Process: An Animal Study at Lhasa. J. Inflamm. Res. 2022, 15, 2835–2853. [Google Scholar] [CrossRef] [PubMed]
- Varner, E.L.; Trefely, S.; Bartee, D.; von Krusenstiern, E.; Izzo, L.; Bekeova, C.; O’Connor, R.S.; Seifert, E.L.; Wellen, K.E.; Meier, J.L.; et al. Quantification of lactoyl-CoA (lactyl-CoA) by liquid chromatography mass spectrometry in mammalian cells and tissues. Open Biol. 2020, 10, 200187. [Google Scholar] [CrossRef]
- Pan, R.Y.; He, L.; Zhang, J.; Liu, X.; Liao, Y.; Gao, J.; Liao, Y.; Yan, Y.; Li, Q.; Zhou, X.; et al. Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer’s disease. Cell Metab. 2022, 34, 634–648.e6. [Google Scholar] [CrossRef]
- Li, X.; Yang, N.; Wu, Y.; Wang, X.; Sun, J.; Liu, L.; Zhang, F.; Gong, Y.; Zhang, Y.; Li, X.; et al. Hypoxia regulates fibrosis-related genes via histone lactylation in the placentas of patients with preeclampsia. J. Hypertens. 2022, 40, 1189–1198. [Google Scholar] [CrossRef]
- Hagihara, H.; Shoji, H.; Otabi, H.; Toyoda, A.; Katoh, K.; Namihira, M.; Miyakawa, T. Protein lactylation induced by neural excitation. Cell Rep. 2021, 37, 109820. [Google Scholar] [CrossRef]
- Oses, J.P.; Müller, A.P.; Strogulski, N.R.; Moreira, J.D.; Böhmer, A.E.; Hansel, G.; Carteri, R.B.; Busnello, J.V.; Kopczynski, A.; Rodolphi, M.S.; et al. Sustained elevation of cerebrospinal fluid glucose and lactate after a single seizure does not parallel with mitochondria energy production. Epilepsy Res. 2019, 152, 35–41. [Google Scholar] [CrossRef]
- Geng, H.; Chen, H.; Wang, H.; Wang, L. The Histone Modifications of Neuronal Plasticity. Neural Plast. 2021, 2021, 6690523. [Google Scholar] [CrossRef] [PubMed]
- Irizarry-Caro, R.A.; McDaniel, M.M.; Overcast, G.R.; Jain, V.G.; Troutman, T.D.; Pasare, C. TLR signaling adapter BCAP regulates inflammatory to reparatory macrophage transition by promoting histone lactylation. Proc. Natl. Acad. Sci. USA 2020, 117, 30628–30638. [Google Scholar] [CrossRef]
- Cui, H.; Xie, N.; Banerjee, S.; Ge, J.; Jiang, D.; Dey, T.; Matthews, Q.L.; Liu, R.M.; Liu, G. Lung Myofibroblasts Promote Macrophage Profibrotic Activity through Lactate-induced Histone Lactylation. Am. J. Respir. Cell Mol. Biol. 2021, 64, 115–125. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, N.; Liang, W. Systematic Analysis of Lysine Lactylation in the Plant Fungal Pathogen Botrytis cinerea. Front. Microbiol. 2020, 11, 594743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Jiang, N.; Yu, L.; Guan, T.; Sang, X.; Feng, Y.; Chen, R.; Chen, Q. Protein Lactylation Critically Regulates Energy Metabolism in the Protozoan Parasite Trypanosoma brucei. Front. Cell Dev. Biol. 2021, 9, 719720. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yu, H.; Liu, X.; Wang, T.; Yao, Y.; Zhou, Q.; Zheng, X.; Tan, F. Systematic identification of the lysine lactylation in the protozoan parasite Toxoplasma gondii. Parasit. Vectors 2022, 15, 180. [Google Scholar] [CrossRef] [PubMed]
- Egger, G.; Liang, G.; Aparicio, A.; Jones, P.A. Epigenetics in human disease and prospects for epigenetic therapy. Nature 2004, 429, 457–463. [Google Scholar] [CrossRef]
- Agbleke, A.A.; Amitai, A.; Buenrostro, J.D.; Chakrabarti, A.; Chu, L.; Hansen, A.S.; Koenig, K.M.; Labade, A.S.; Liu, S.; Nozaki, T.; et al. Advances in Chromatin and Chromosome Research: Perspectives from Multiple Fields. Mol. Cell 2020, 79, 881–901. [Google Scholar] [CrossRef]
- Berson, A.; Nativio, R.; Berger, S.L.; Bonini, N.M. Epigenetic Regulation in Neurodegenerative Diseases. Trends Neurosci. 2018, 41, 587–598. [Google Scholar] [CrossRef]
- Lambertus, M.; Øverberg, L.T.; Andersson, K.A.; Hjelden, M.S.; Hadzic, A.; Haugen, Ø.P.; Storm-Mathisen, J.; Bergersen, L.H.; Geiseler, S.; Morland, C. L-lactate induces neurogenesis in the mouse ventricular-subventricular zone via the lactate receptor HCA1. Acta Physiol. 2021, 231, e13587. [Google Scholar] [CrossRef]
- Abdolmaleky, H.M.; Gower, A.C.; Wong, C.K.; Cox, J.W.; Zhang, X.; Thiagalingam, A.; Shafa, R.; Sivaraman, V.; Zhou, J.R.; Thiagalingam, S. Aberrant transcriptomes and DNA methylomes define pathways that drive pathogenesis and loss of brain laterality/asymmetry in schizophrenia and bipolar disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2019, 180, 138–149. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef]
- Park, H.J.; Choi, I.; Leem, K.H. Decreased Brain pH and Pathophysiology in Schizophrenia. Int. J. Mol. Sci. 2021, 22, 8358. [Google Scholar] [CrossRef]
- Han, H.; Zhao, Y.; Du, J.; Wang, S.; Yang, X.; Li, W.; Song, J.; Zhang, S.; Zhang, Z.; Tan, Y.; et al. Exercise improves cognitive dysfunction and neuroinflammation in mice through Histone H3 lactylation in microglia. Immun. Ageing 2023, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, L.; Gu, Y.; Cang, W.; Sun, P.; Xiang, Y. Lactate-Lactylation Hands between Metabolic Reprogramming and Immunosuppression. Int. J. Mol. Sci. 2022, 23, 11943. [Google Scholar] [CrossRef]
- Morland, C.; Lauritzen, K.H.; Puchades, M.; Holm-Hansen, S.; Andersson, K.; Gjedde, A.; Attramadal, H.; Storm-Mathisen, J.; Bergersen, L.H. The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: Expression and action in brain. J. Neurosci. Res. 2015, 93, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Li, J.; Li, L.; Sun, Y.; Zhang, X.; Xue, Y.; Lv, J.; Gao, Y.; Li, S.; Yan, W.; et al. L-lactate preconditioning promotes plasticity-related proteins expression and reduces neurological deficits by potentiating GPR81 signaling in rat traumatic brain injury model. Brain Res. 2020, 1746, 146945. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Yang, X.; Wang, J.; Wang, Z.; Wang, Q.; Ding, Y.; Yu, A. H3K18 lactylation of senescent microglia potentiates brain aging and Alzheimer’s disease through the NFκB signaling pathway. J. Neuroinflamm. 2023, 20, 208. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Yu, H.T.; Xie, J.Z.; Zhao, J.N.; Fang, Z.T.; Qu, M.; Zhang, Y.; Yang, Y.; Wang, J.Z. A positive feedback inhibition of isocitrate dehydrogenase 3β on paired-box gene 6 promotes Alzheimer-like pathology. Signal Transduct. Target. Ther. 2024, 9, 105. [Google Scholar] [CrossRef]
- Zhang, S.S.; Zhu, L.; Peng, Y.; Zhang, L.; Chao, F.L.; Jiang, L.; Xiao, Q.; Liang, X.; Tang, J.; Yang, H.; et al. Long-term running exercise improves cognitive function and promotes microglial glucose metabolism and morphological plasticity in the hippocampus of APP/PS1 mice. J. Neuroinflamm. 2022, 19, 34. [Google Scholar] [CrossRef]
- Cao, J.; Hong, Z.; Zeng, F.; Qin, Z.; Lin, M.; Wang, H.; Zuo, D.; Tao, T. Lactate-derived HDAC6 Lactylation as a new target for neuronal protection in cerebral ischemic reperfusion injury. bioRxiv 2022. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, L.; Yu, Z.; Zhang, M.; Liu, J.; Zhou, J. Inhibition of the Glycolysis Prevents the Cerebral Infarction Progression Through Decreasing the Lactylation Levels of LCP1. Mol. Biotechnol. 2023, 65, 1336–1345. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Peng, J.; Zhang, X.; Zhang, F.; Wu, Y.; Huang, A.; Du, F.; Liao, Y.; He, Y.; et al. Astrocytic LRP1 enables mitochondria transfer to neurons and mitigates brain ischemic stroke by suppressing ARF1 lactylation. Cell Metab. 2024, 36, 2054–2068.e14. [Google Scholar] [CrossRef]
- Xie, J.; Hong, S.; Zhang, X.; Li, Y.; Xie, R. Inhibition of glycolysis prevents behavioural changes in mice with MK801-induced SCZ model by alleviating lactate accumulation and lactylation. Brain Res. 2023, 1812, 148409. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shao, A.; Yao, Y.; Tu, S.; Deng, Y.; Zhang, J. Dual roles of astrocytes in plasticity and reconstruction after traumatic brain injury. Cell Commun. Signal 2020, 18, 62. [Google Scholar] [CrossRef]
- Killen, M.J.; Giorgi-Coll, S.; Helmy, A.; Hutchinson, P.J.; Carpenter, K.L. Metabolism and inflammation: Implications for traumatic brain injury therapeutics. Expert. Rev. Neurother. 2019, 19, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Certo, M.; Elkafrawy, H.; Pucino, V.; Cucchi, D.; Cheung, K.C.P.; Mauro, C. Endothelial cell and T-cell crosstalk: Targeting metabolism as a therapeutic approach in chronic inflammation. Br. J. Pharmacol. 2021, 178, 2041–2059. [Google Scholar] [CrossRef]
- Holloway, R.; Zhou, Z.; Harvey, H.B.; Levasseur, J.E.; Rice, A.C.; Sun, D.; Hamm, R.J.; Bullock, M.R. Effect of lactate therapy upon cognitive deficits after traumatic brain injury in the rat. Acta Neurochir. 2007, 149, 919–927; discussion 927. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B. The hypoxia-lactate axis tempers inflammation. Nat. Rev. Immunol. 2020, 20, 85–86. [Google Scholar] [CrossRef]
- Murray, P.J. On macrophage diversity and inflammatory metabolic timers. Nat. Rev. Immunol. 2020, 20, 89–90. [Google Scholar] [CrossRef]
- Song, J.; Lee, K.; Park, S.W.; Chung, H.; Jung, D.; Na, Y.R.; Quan, H.; Cho, C.S.; Che, J.H.; Kim, J.H.; et al. Lactic Acid Upregulates VEGF Expression in Macrophages and Facilitates Choroidal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3747–3754. [Google Scholar] [CrossRef]
- Andersson, A.K.; Rönnbäck, L.; Hansson, E. Lactate induces tumour necrosis factor-alpha, interleukin-6 and interleukin-1beta release in microglial- and astroglial-enriched primary cultures. J. Neurochem. 2005, 93, 1327–1333. [Google Scholar] [CrossRef]
- Fischer, K.; Hoffmann, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 2007, 109, 3812–3819. [Google Scholar] [CrossRef]
- Colton, C.A. Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 2009, 4, 399–418. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, S.; Baglietto-Vargas, D.; Caballero, C.; Moreno-Gonzalez, I.; Torres, M.; Sanchez-Varo, R.; Ruano, D.; Vizuete, M.; Gutierrez, A.; Vitorica, J. Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer’s disease: Age-dependent switch in the microglial phenotype from alternative to classic. J. Neurosci. 2008, 28, 11650–11661. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heredero, G.; Gómez de Las Heras, M.M.; Gabandé-Rodríguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A key player in the inflammatory response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef]
- Kuei, C.; Yu, J.; Zhu, J.; Wu, J.; Zhang, L.; Shih, A.; Mirzadegan, T.; Lovenberg, T.; Liu, C. Study of GPR81, the lactate receptor, from distant species identifies residues and motifs critical for GPR81 functions. Mol. Pharmacol. 2011, 80, 848–858. [Google Scholar] [CrossRef]
- Liu, C.; Wu, J.; Zhu, J.; Kuei, C.; Yu, J.; Shelton, J.; Sutton, S.W.; Li, X.; Yun, S.J.; Mirzadegan, T.; et al. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J. Biol. Chem. 2009, 284, 2811–2822. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.J.; Geoghegan, S.; Lawhorne, L.W. An Exploratory Analysis of Potential New Biomarkers of Cognitive Function. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 299–305. [Google Scholar] [CrossRef]
- Ichai, C.; Payen, J.F.; Orban, J.C.; Quintard, H.; Roth, H.; Legrand, R.; Francony, G.; Leverve, X.M. Half-molar sodium lactate infusion to prevent intracranial hypertensive episodes in severe traumatic brain injured patients: A randomized controlled trial. Intensive Care Med. 2013, 39, 1413–1422. [Google Scholar] [CrossRef]
- Bouzat, P.; Sala, N.; Suys, T.; Zerlauth, J.B.; Marques-Vidal, P.; Feihl, F.; Bloch, J.; Messerer, M.; Levivier, M.; Meuli, R.; et al. Cerebral metabolic effects of exogenous lactate supplementation on the injured human brain. Intensive Care Med. 2014, 40, 412–421. [Google Scholar] [CrossRef]
- Zhou, J.; Burns, M.P.; Huynh, L.; Villapol, S.; Taub, D.D.; Saavedra, J.M.; Blackman, M.R. Temporal Changes in Cortical and Hippocampal Expression of Genes Important for Brain Glucose Metabolism Following Controlled Cortical Impact Injury in Mice. Front. Endocrinol. 2017, 8, 231. [Google Scholar] [CrossRef]
- Müller, P.; Duderstadt, Y.; Lessmann, V.; Müller, N.G. Lactate and BDNF: Key Mediators of Exercise Induced Neuroplasticity? J. Clin. Med. 2020, 9, 1136. [Google Scholar] [CrossRef]
- Huang, Y.; Mucke, L. Alzheimer mechanisms and therapeutic strategies. Cell 2012, 148, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Sharma, V.; Deshmukh, R. Activation of microglia and astrocytes: A roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology 2019, 27, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Perea, J.R.; Bolós, M.; Avila, J. Microglia in Alzheimer’s Disease in the Context of Tau Pathology. Biomolecules 2020, 10, 1439. [Google Scholar] [CrossRef]
- Sankowski, R.; Böttcher, C.; Masuda, T.; Geirsdottir, L.; Sagar; Sindram, E.; Seredenina, T.; Muhs, A.; Scheiwe, C.; Shah, M.J.; et al. Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nat. Neurosci. 2019, 22, 2098–2110. [Google Scholar] [CrossRef]
- Hu, Y.; Mai, W.; Chen, L.; Cao, K.; Zhang, B.; Zhang, Z.; Liu, Y.; Lou, H.; Duan, S.; Gao, Z. mTOR-mediated metabolic reprogramming shapes distinct microglia functions in response to lipopolysaccharide and ATP. Glia 2020, 68, 1031–1045. [Google Scholar] [CrossRef]
- McIntosh, A.; Mela, V.; Harty, C.; Minogue, A.M.; Costello, D.A.; Kerskens, C.; Lynch, M.A. Iron accumulation in microglia triggers a cascade of events that leads to altered metabolism and compromised function in APP/PS1 mice. Brain Pathol. 2019, 29, 606–621. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, H. Microglial lactate metabolism as a potential therapeutic target for Alzheimer’s disease. Mol. Neurodegener. 2022, 17, 36. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Yin, F.; Sancheti, H.; Patil, I.; Cadenas, E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic. Biol. Med. 2016, 100, 108–122. [Google Scholar] [CrossRef]
- Johnson, E.C.B.; Dammer, E.B.; Duong, D.M.; Ping, L.; Zhou, M.; Yin, L.; Higginbotham, L.A.; Guajardo, A.; White, B.; Troncoso, J.C.; et al. Large-scale proteomic analysis of Alzheimer’s disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat. Med. 2020, 26, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.H.; Kang, S.; Lee, W.; Choi, H.; Chung, S.; Kim, J.I.; Mook-Jung, I. A Breakdown in Metabolic Reprogramming Causes Microglia Dysfunction in Alzheimer’s Disease. Cell Metab. 2019, 30, 493–507.e496. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Feng, F.; Wu, J.; Fan, S.; Han, J.; Wang, S.; Yang, L.; Liu, W.; Wang, C.; Xu, K. Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacol. Res. 2022, 181, 106270. [Google Scholar] [CrossRef]
- Bernier, L.P.; York, E.M.; Kamyabi, A.; Choi, H.B.; Weilinger, N.L.; MacVicar, B.A. Microglial metabolic flexibility supports immune surveillance of the brain parenchyma. Nat. Commun. 2020, 11, 1559. [Google Scholar] [CrossRef]
- Vercellino, I.; Sazanov, L.A. The assembly, regulation and function of the mitochondrial respiratory chain. Nat. Rev. Mol. Cell Biol. 2022, 23, 141–161. [Google Scholar] [CrossRef]
- Darmanis, S.; Sloan, S.A.; Zhang, Y.; Enge, M.; Caneda, C.; Shuer, L.M.; Hayden Gephart, M.G.; Barres, B.A.; Quake, S.R. A survey of human brain transcriptome diversity at the single cell level. Proc. Natl. Acad. Sci. USA 2015, 112, 7285–7290. [Google Scholar] [CrossRef] [PubMed]
- Bubber, P.; Haroutunian, V.; Fisch, G.; Blass, J.P.; Gibson, G.E. Mitochondrial abnormalities in Alzheimer brain: Mechanistic implications. Ann. Neurol. 2005, 57, 695–703. [Google Scholar] [CrossRef]
- Coco, M. The brain behaves as a muscle? Neurol. Sci. 2017, 38, 1865–1868. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; de la Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res. Rev. 2020, 62, 101108. [Google Scholar] [CrossRef]
- Diskin, C.; Ryan, T.A.J.; O’Neill, L.A.J. Modification of Proteins by Metabolites in Immunity. Immunity 2021, 54, 19–31. [Google Scholar] [CrossRef]
- Luo, L.; Yan, T.; Yang, L.; Zhao, M. Aluminum chloride and D-galactose induced a zebrafish model of Alzheimer’s disease with cognitive deficits and aging. Comput. Struct. Biotechnol. J. 2024, 23, 2230–2239. [Google Scholar] [CrossRef] [PubMed]
- Teter, B.; Morihara, T.; Lim, G.P.; Chu, T.; Jones, M.R.; Zuo, X.; Paul, R.M.; Frautschy, S.A.; Cole, G.M. Curcumin restores innate immune Alzheimer’s disease risk gene expression to ameliorate Alzheimer pathogenesis. Neurobiol. Dis. 2019, 127, 432–448. [Google Scholar] [CrossRef]
- Sun, W.; Li, G.; Zeng, X.; Lai, Z.; Wang, M.; Ouyang, Y.; Zeng, G.; Peng, J.; Zhong, J.; Xiao, D.; et al. Clinical and Imaging Characteristics of Cerebral Infarction in Patients with Nonvalvular Atrial Fibrillation Combined with Cerebral Artery Stenosis. J. Atheroscler. Thromb. 2018, 25, 720–732. [Google Scholar] [CrossRef]
- Tu, W.J.; Wang, L.D. China stroke surveillance report 2021. Mil. Med. Res. 2023, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zeng, L.; Ge, A.; Wang, S.; Zeng, J.; Yuan, X.; Mei, Z.; Wang, G.; Ge, J. A systematic review of the research progress of non-coding RNA in neuroinflammation and immune regulation in cerebral infarction/ischemia-reperfusion injury. Front. Immunol. 2022, 13, 930171. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yuan, W.; Wang, Z. Advances in Glycolysis Metabolism of Atherosclerosis. J. Cardiovasc. Transl. Res. 2023, 16, 476–490. [Google Scholar] [CrossRef]
- Weinstein, P.R.; Hong, S.; Sharp, F.R. Molecular identification of the ischemic penumbra. Stroke 2004, 35, 2666–2670. [Google Scholar] [CrossRef]
- Wan, N.; Wang, N.; Yu, S.; Zhang, H.; Tang, S.; Wang, D.; Lu, W.; Li, H.; Delafield, D.G.; Kong, Y.; et al. Cyclic immonium ion of lactyllysine reveals widespread lactylation in the human proteome. Nat. Methods 2022, 19, 854–864. [Google Scholar] [CrossRef]
- Buscemi, L.; Blochet, C.; Price, M.; Magistretti, P.J.; Lei, H.; Hirt, L. Extended preclinical investigation of lactate for neuroprotection after ischemic stroke. Clin. Transl. Neurosci. 2020, 4, 2514183X20904571. [Google Scholar] [CrossRef]
- Fan, M.; Yang, K.; Wang, X.; Chen, L.; Gill, P.S.; Ha, T.; Liu, L.; Lewis, N.H.; Williams, D.L.; Li, C. Lactate promotes endothelial-to-mesenchymal transition via Snail1 lactylation after myocardial infarction. Sci. Adv. 2023, 9, eadc9465. [Google Scholar] [CrossRef]
- Lopez Krol, A.; Nehring, H.P.; Krause, F.F.; Wempe, A.; Raifer, H.; Nist, A.; Stiewe, T.; Bertrams, W.; Schmeck, B.; Luu, M.; et al. Lactate induces metabolic and epigenetic reprogramming of pro-inflammatory Th17 cells. EMBO Rep. 2022, 23, e54685. [Google Scholar] [CrossRef]
- Annoni, F.; Peluso, L.; Gouvêa Bogossian, E.; Creteur, J.; Zanier, E.R.; Taccone, F.S. Brain Protection after Anoxic Brain Injury: Is Lactate Supplementation Helpful? Cells 2021, 10, 1714. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Leng, Y.; Wang, J.; Liao, H.M.; Bergman, J.; Leeds, P.; Kozikowski, A.; Chuang, D.M. Tubastatin A, an HDAC6 inhibitor, alleviates stroke-induced brain infarction and functional deficits: Potential roles of α-tubulin acetylation and FGF-21 up-regulation. Sci. Rep. 2016, 6, 19626. [Google Scholar] [CrossRef] [PubMed]
- Osseni, A.; Ravel-Chapuis, A.; Thomas, J.L.; Gache, V.; Schaeffer, L.; Jasmin, B.J. HDAC6 regulates microtubule stability and clustering of AChRs at neuromuscular junctions. J. Cell Biol. 2020, 219, e201901099. [Google Scholar] [CrossRef] [PubMed]
- d’Ydewalle, C.; Krishnan, J.; Chiheb, D.M.; Van Damme, P.; Irobi, J.; Kozikowski, A.P.; Vanden Berghe, P.; Timmerman, V.; Robberecht, W.; Van Den Bosch, L. HDAC6 inhibitors reverse axonal loss in a mouse model of mutant HSPB1-induced Charcot-Marie-Tooth disease. Nat. Med. 2011, 17, 968–974. [Google Scholar] [CrossRef]
- Balmik, A.A.; Chidambaram, H.; Dangi, A.; Marelli, U.K.; Chinnathambi, S. HDAC6 ZnF UBP as the Modifier of Tau Structure and Function. Biochemistry 2020, 59, 4546–4562. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, G.; Dong, A. Prediction Model between Serum Vitamin D and Neurological Deficit in Cerebral Infarction Patients Based on Machine Learning. Comput. Math. Methods Med. 2022, 2022, 2914484. [Google Scholar] [CrossRef]
- Yao, Y.; Bade, R.; Li, G.; Zhang, A.; Zhao, H.; Fan, L.; Zhu, R.; Yuan, J. Global-Scale Profiling of Differential Expressed Lysine-Lactylated Proteins in the Cerebral Endothelium of Cerebral Ischemia-Reperfusion Injury Rats. Cell Mol. Neurobiol. 2023, 43, 1989–2004. [Google Scholar] [CrossRef]
- Mahat, U.; Garg, B.; Yang, C.Y.; Mehta, H.; Hanna, R.; Rogers, H.J.; Flagg, A.; Ivanov, A.I.; Corey, S.J. Lymphocyte cytosolic protein 1 (L-plastin) I232F mutation impairs granulocytic proliferation and causes neutropenia. Blood Adv. 2022, 6, 2581–2594. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Mo, Y.; Li, R.; Huang, S.; Zhang, A.; Ni, X.; Dai, Q.; Wang, J. DCA Protects against Oxidation Injury Attributed to Cerebral Ischemia-Reperfusion by Regulating Glycolysis through PDK2-PDH-Nrf2 Axis. Oxid. Med. Cell. Longev. 2021, 2021, 5173035. [Google Scholar] [CrossRef]
- Zhang, X.C.; Gu, A.P.; Zheng, C.Y.; Li, Y.B.; Liang, H.F.; Wang, H.J.; Tang, X.L.; Bai, X.X.; Cai, J. YY1/LncRNA GAS5 complex aggravates cerebral ischemia/reperfusion injury through enhancing neuronal glycolysis. Neuropharmacology 2019, 158, 107682. [Google Scholar] [CrossRef]
- Chen, K.; Martens, Y.A.; Meneses, A.; Ryu, D.H.; Lu, W.; Raulin, A.C.; Li, F.; Zhao, J.; Chen, Y.; Jin, Y.; et al. LRP1 is a neuronal receptor for α-synuclein uptake and spread. Mol. Neurodegener. 2022, 17, 57. [Google Scholar] [CrossRef]
- Stępnicki, P.; Kondej, M.; Kaczor, A.A. Current Concepts and Treatments of Schizophrenia. Molecules 2018, 23, 2087. [Google Scholar] [CrossRef]
- Friedman, N.P.; Robbins, T.W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef]
- Klengel, T.; Binder, E.B. Epigenetics of Stress-Related Psychiatric Disorders and Gene × Environment Interactions. Neuron 2015, 86, 1343–1357. [Google Scholar] [CrossRef]
- Chen, Q.; Li, D.; Jin, W.; Shi, Y.; Li, Z.; Ma, P.; Sun, J.; Chen, S.; Li, P.; Lin, P. Research Progress on the Correlation Between Epigenetics and Schizophrenia. Front. Neurosci. 2021, 15, 688727. [Google Scholar] [CrossRef]
- Richetto, J.; Meyer, U. Epigenetic Modifications in Schizophrenia and Related Disorders: Molecular Scars of Environmental Exposures and Source of Phenotypic Variability. Biol. Psychiatry 2021, 89, 215–226. [Google Scholar] [CrossRef]
- Orešič, M.; Tang, J.; Seppänen-Laakso, T.; Mattila, I.; Saarni, S.E.; Saarni, S.I.; Lönnqvist, J.; Sysi-Aho, M.; Hyötyläinen, T.; Perälä, J.; et al. Metabolome in schizophrenia and other psychotic disorders: A general population-based study. Genome Med. 2011, 3, 19. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- Long, J.; Huang, G.; Liang, W.; Liang, B.; Chen, Q.; Xie, J.; Jiang, J.; Su, L. The prevalence of schizophrenia in mainland China: Evidence from epidemiological surveys. Acta Psychiatr. Scand. 2014, 130, 244–256. [Google Scholar] [CrossRef]
- Al-Dujaili, A.H.; Mousa, R.F.; Al-Hakeim, H.K.; Maes, M. High Mobility Group Protein 1 and Dickkopf-Related Protein 1 in Schizophrenia and Treatment-Resistant Schizophrenia: Associations With Interleukin-6, Symptom Domains, and Neurocognitive Impairments. Schizophr. Bull. 2021, 47, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Nan, K.; Han, Y.; Fang, Q.; Huang, C.; Yu, L.; Ge, W.; Xiang, F.; Tao, Y.X.; Cao, H.; Li, J. HMGB1 gene silencing inhibits neuroinflammation via down-regulation of NF-κB signaling in primary hippocampal neurons induced by Aβ25–35. Int. Immunopharmacol. 2019, 67, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Seo, J.H.; Baek, S.B.; Kim, C.J.; Kim, T.W. Treadmill exercise inhibits hippocampal apoptosis through enhancing N-methyl-D-aspartate receptor expression in the MK-801-induced schizophrenic mice. J. Exerc. Rehabil. 2014, 10, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Białoń, M.; Wąsik, A. Advantages and Limitations of Animal Schizophrenia Models. Int. J. Mol. Sci. 2022, 23, 5968. [Google Scholar] [CrossRef]
- Xu, B.; Zang, S.C.; Li, S.Z.; Guo, J.R.; Wang, J.F.; Wang, D.; Zhang, L.P.; Yang, H.M.; Lian, S. HMGB1-mediated differential response on hippocampal neurotransmitter disorder and neuroinflammation in adolescent male and female mice following cold exposure. Brain Behav. Immun. 2019, 76, 223–235. [Google Scholar] [CrossRef]
| Diseases | Target | Mechanisms | References |
|---|---|---|---|
| Traumatic brain injury | GPR81 | Lactate activates GPR81, improving synaptic function and promoting neuronal protection. | [73,74] |
| Alzheimer’s disease | H4K12la | glycolysis/H4K12la/PKM2 feedback loop exacerbates microglial dysfunction. | [54] |
| H3K18la | The H3K18la/NFκB axis promotes AD pathology by regulating SASP components IL-6 and IL-8. | [75] | |
| IDH3β | Lactate enhances the IDH3β-lactate-PAX6 loop, worsening AD pathology and cognition. | [76] | |
| exercise | Increased brain lactate after exercise promotes microglial transformation to the M2 repair phenotype via histone Kla, reducing neuroinflammation in AD. | [77] | |
| Acute ischemic stroke | HDAC6 | By binding to the immunoglobulin heavy chain binding protein, HDAC6 Kla alters calcium homeostasis, controlling dendritic architecture and promoting neuronal survival after AIS. | [78] |
| LCP1 | LCP1 modulates immune cells to worsen neuroinflammation and drive AIS progression. | [79] | |
| LRP1 | LRP1 reduces lactate and ARF1 Kla, boosts mitochondrial transport to neurons, and improves AIS. | [80] | |
| Schizophrenia | H3K9la | Lactate accumulation and elevated H3K9la/H3K18la levels increase hippocampal HMGB1 and induce behavioral changes in SCZ mice, yet 2-DG treatment reduces these alterations by lowering H3K9la levels. | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Xiao, D.; Li, X. Lactylation and Central Nervous System Diseases. Brain Sci. 2025, 15, 294. https://doi.org/10.3390/brainsci15030294
Chen Y, Xiao D, Li X. Lactylation and Central Nervous System Diseases. Brain Sciences. 2025; 15(3):294. https://doi.org/10.3390/brainsci15030294
Chicago/Turabian StyleChen, Ye, Dongqiong Xiao, and Xihong Li. 2025. "Lactylation and Central Nervous System Diseases" Brain Sciences 15, no. 3: 294. https://doi.org/10.3390/brainsci15030294
APA StyleChen, Y., Xiao, D., & Li, X. (2025). Lactylation and Central Nervous System Diseases. Brain Sciences, 15(3), 294. https://doi.org/10.3390/brainsci15030294





