I Got Rhythm and Executive Function, Memory, and More: The Automated Test of Embodied Cognition (ATEC)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures: Automated Test of Embodied Cognition
2.2.1. ATEC Novel Tasks and Rhythm
2.2.2. Neurocognitive Tests
2.3. Procedures
2.4. Analysis
3. Results
3.1. Factor Analysis (Table 2; N = 104)
| Component | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| BALANCE | 0.730 | −0.067 | 0.408 |
| WORKING MEMORY | 0.710 | 0.475 | −0.063 |
| RESPONSE INHIBITION | 0.831 | 0.151 | 0.096 |
| SELF REGULATION | 0.899 | 0.191 | 0.070 |
| RHYTHM | 0.810 | 0.159 | 0.054 |
| ATTENTION | 0.563 | 0.547 | −0.168 |
| EMBODIED MEMORY RECALL | 0.083 | 0.881 | 0.166 |
| MOTOR SPEED | 0.093 | 0.096 | 0.937 |
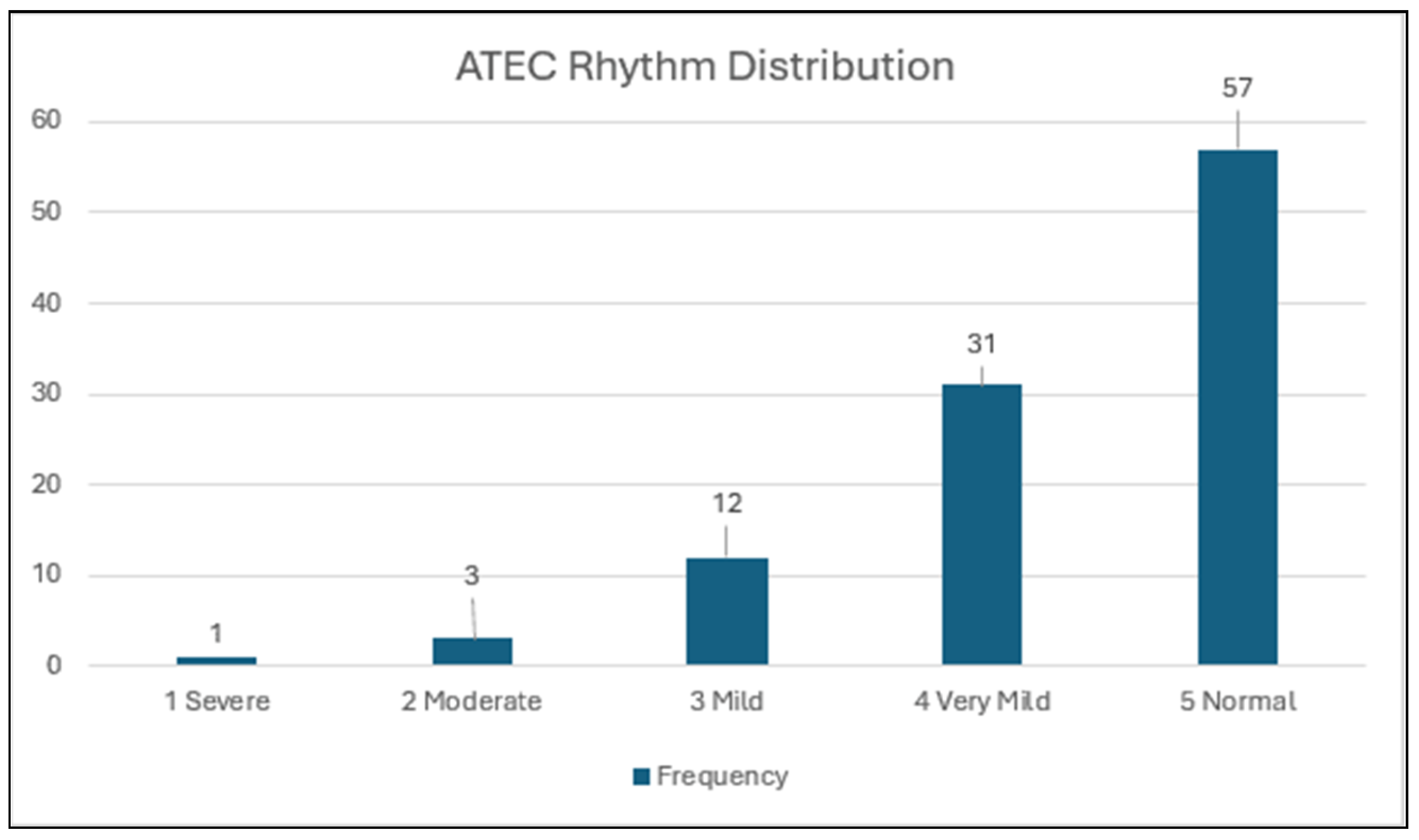
3.2. Distribution of Rhythm Scores (Figure 1; N = 104)
3.3. Comparison of AUD/At-Risk Sample with Community Controls (Table 3; N = 90)
| Impaired | Unimpaired | Total | ||
|---|---|---|---|---|
| AUD/At-Risk | Count | 29 | 33 | 62 |
| % within AUD/At-Risk | 46.8% | 53.2% | 100% | |
| Cty Control | Count | 6 | 22 | 28 |
| % within Cty Controls | 21.4% | 78.6% | 100% |
3.4. Correlations with Neurocognitive Assessments
3.4.1. Executive Function Measures and ATEC Rhythm Scores
3.4.2. Memory Measures and ATEC Rhythm Scores
3.4.3. Cognitive Reserve and ATEC Rhythm
3.5. Age, Gender, and Rhythm
4. Discussion
Limitations
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATEC | Automated Test of Embodied Cognition |
| AUD | Alcohol Use Disorder |
| EF | Executive Function |
| SUD | Substance Use Disorder |
| MoCA | Montreal Cognitive Assessment |
| NIAAA | National Institute on Alcohol Abuse and Alcoholism |
| M.I.N.I. | Mini International Neuropsychiatric Interview |
| WTAR | Wechsler Test of Adult Reading |
| MDS-UPDRS | Movement Disorder Society-Unified Parkinson’s Disease Rating Scale |
| AI | Artificial Intelligence |
| ML | Machine Learning |
| IVA | Integrated Visual and Auditory-2 Continuous Performance test |
| NAB | Neuropsychological Assessment Battery |
| WCST | Wisconsin Card Sorting Test |
| BVMT | Brief Visual Memory Test |
| HVLT | Hopkins Verbal Learning Test |
| VACHS | VA Connecticut Healthcare System |
References
- Bell, M.D.; Hauser, A.J.; Weinstein, A.J. The Automated Test of Embodied Cognition: Concept, Development, and Preliminary Findings. Brain Sci. 2023, 13, 856. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.D.; Weinstein, A.J.; Pittman, B.; Gorman, R.M.; Abujelala, M. The Activate Test of Embodied Cognition (ATEC): Reliability, concurrent validity and discriminant validity in a community sample of children using cognitively demanding physical tasks related to executive functioning. Child Neuropsychol. 2021, 27, 973–983. [Google Scholar] [CrossRef]
- Thaut, M.H.; Trimarchi, P.D.; Parsons, L.M. Human Brain Basis of Musical Rhythm Perception: Common and Distinct Neural Substrates for Meter, Tempo, and Pattern. Brain Sci. 2014, 4, 428–452. [Google Scholar] [CrossRef]
- Betancourt, A.; Pérez, O.; Gámez, J.; Mendoza, G.; Merchant, H. Premotor population dynamics as neural substrate for auditory and visual rhythmic entrainment. bioRxiv 2022. [Google Scholar] [CrossRef]
- Grahn, J.A.; Watson, S.L. Perspectives on Rhythm Processing In Motor Regions of the Brain. Music Ther. Perspect. 2013, 31, 25–30. [Google Scholar] [CrossRef]
- Grahn, J.A.; Brett, M. Rhythm and Beat Perception in Motor Areas of the Brain. J. Cogn. Neurosci. 2007, 19, 893–906. [Google Scholar] [CrossRef]
- Ivry, R.B.; Spencer, R.M. The neural representation of time. Curr. Opin. Neurobiol. 2004, 14, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ramnani, N. The primate cortico-cerebellar system: Anatomy and function. Nat. Rev. Neurosci. 2006, 7, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Zentner, M.; Eerola, T. Rhythmic engagement with music in infancy. Proc. Natl. Acad. Sci. USA 2010, 107, 5768–5773. [Google Scholar] [CrossRef]
- Woodruff Carr, K.; White-Schwoch, T.; Tierney, A.T.; Strait, D.L.; Kraus, N. Beat synchronization predicts neural speech encoding and reading readiness in preschoolers. Proc. Natl. Acad. Sci. USA 2014, 111, 14559–14564. [Google Scholar] [CrossRef] [PubMed]
- Knott, D.; Thaut, M.H. Musical Mnemonics Enhance Verbal Memory in Typically Developing Children. Front. Educ. 2018, 3, 31. [Google Scholar] [CrossRef]
- Oh, S.-y.; Chong, H.J. Correlation Between Rhythm Reproduction Task Performance and Cognitive Function in School-Aged Children. J. Music Hum. Behav. 2016, 13, 1–18. [Google Scholar] [CrossRef]
- Tierney, A.T.; Kraus, N. The ability to tap to a beat relates to cognitive, linguistic, and perceptual skills. Brain Lang. 2013, 124, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.M.; Goswami, U. Rhythmic processing in children with developmental dyslexia: Auditory and motor rhythms link to reading and spelling. J. Physiol. Paris 2008, 102, 120–129. [Google Scholar] [CrossRef]
- Wolff, P.H. Timing precision and rhythm in developmental dyslexia. Read. Writ. 2002, 15, 179–206. [Google Scholar] [CrossRef]
- Moreno, S.; Bialystok, E.; Barac, R.; Schellenberg, E.G.; Cepeda, N.J.; Chau, T. Short-Term Music Training Enhances Verbal Intelligence and Executive Function. Psychol. Sci. 2011, 22, 1425–1433. [Google Scholar] [CrossRef]
- Jentschke, S.; Koelsch, S. Musical training modulates the development of syntax processing in children. Neuroimage 2009, 47, 735–744. [Google Scholar] [CrossRef]
- Zanto, T.P.; Giannakopoulou, A.; Gallen, C.L.; Ostrand, A.E.; Younger, J.W.; Anguera-Singla, R.; Anguera, J.A.; Gazzaley, A. Digital rhythm training improves reading fluency in children. Dev. Sci. 2024, 27, e13473. [Google Scholar] [CrossRef]
- Daniel, S.; Wimpory, D.; Delafield-Butt, J.T.; Malloch, S.; Holck, U.; Geretsegger, M.; Tortora, S.; Osborne, N.; Schögler, B.; Koch, S.; et al. Rhythmic Relating: Bidirectional Support for Social Timing in Autism Therapies. Front. Psychol. 2022, 13, 793258. [Google Scholar] [CrossRef]
- Finnigan, E.; Starr, E. Increasing social responsiveness in a child with autism: A comparison of music and non-music interventions. Autism 2010, 14, 321–348. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Wigram, T.; Gold, C. The Effects of Improvisational Music Therapy on Joint Attention Behaviors in Autistic Children: A Randomized Controlled Study. J. Autism Dev. Disord. 2008, 38, 1758–1766. [Google Scholar] [CrossRef]
- Lim, H.A. Effect of “Developmental Speech and Language Training Through Music” on Speech Production in Children with Autism Spectrum Disorders. J. Music Ther. 2010, 47, 2–26. [Google Scholar] [CrossRef]
- Mostofsky, S.H.; Dubey, P.; Jerath, V.K.; Jansiewicz, E.M.; Goldberg, M.C.; Denckla, M.B. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. J. Int. Neuropsychol. Soc. 2006, 12, 314–326. [Google Scholar] [CrossRef]
- LaGasse, A.B.; Hardy, M.W. Rhythm, movement, and autism: Using rhythmic rehabilitation research as a model for autism. Front. Integr. Neurosci. 2013, 7, 41687. [Google Scholar]
- Ding, X.; Wu, J.; Li, D.; Liu, Z. The benefit of rhythm-based interventions for individuals with autism spectrum disorder: A systematic review and meta-analysis with random controlled trials. Front. Psychiatry 2024, 15, 1436170. [Google Scholar] [CrossRef] [PubMed]
- Roesch, A.D.; Gschwandtner, U.; Handabaka, I.; Meyer, A.; Taub, E.; Fuhr, P. Effects of Rhythmic Interventions on Cognitive Abilities in Parkinson’s Disease. Dement. Geriatr. Cogn. Disord. 2021, 50, 372–386. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive reserve. Neuropsychologia 2009, 47, 2015–2028. [Google Scholar] [CrossRef]
- Finkbeiner, S. Huntington’s Disease. Cold Spring Harb Perspect. Biol. 2011, 3, a007476. [Google Scholar] [CrossRef]
- Thaut, M.H.; Miltner, R.; Lange, H.W.; Hurt, C.P.; Hoemberg, V. Velocity modulation and rhythmic synchronization of gait in Huntington’s disease. Mov. Disord. 1999, 14, 808–819. [Google Scholar] [CrossRef]
- Schwartz, A.E.; van Walsem, M.R.; Brean, A.; Frich, J.C. Therapeutic Use of Music, Dance, and Rhythmic Auditory Cueing for Patients with Huntington’s Disease: A Systematic Review. J. Huntington’s Dis. 2019, 8, 393–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Ni Wen, X.; Chen, Y.; Xu, N.; Zhang, J.H.; Hou, X.; Liu, J.P.; Li, P.; Chen, J.Y.; Wang, J.H.; et al. Effects of movement training based on rhythmic auditory stimulation in cognitive impairment: A meta-analysis of randomized controlled clinical trial. Front. Neurosci. 2024, 18, 1360935. [Google Scholar] [CrossRef] [PubMed]
- Thaut, M.H.; Gardiner, J.C.; Holmberg, D.; Horwitz, J.; Kent, L.; Andrews, G.; Donelan, B.; McIntosh, G.R. Neurologic Music Therapy Improves Executive Function and Emotional Adjustment in Traumatic Brain Injury Rehabilitation. Ann. New York Acad. Sci. 2009, 1169, 406–416. [Google Scholar] [CrossRef]
- Patterson, K.K.; Wong, J.S.; Prout, E.C.; Brooks, D. Dance for the rehabilitation of balance and gait in adults with neurological conditions other than Parkinson’s disease: A systematic review. Heliyon 2018, 4, e00584. [Google Scholar] [CrossRef]
- Seashore, C.E.; Lewis, D.; Saetveit, J.G. Seashore Measures of Musical Talents. Seashore Measures of Musical Talents; Psychological Corp: Oxford, UK, 1956; p. 11. [Google Scholar]
- Seashore, R.H. Studies in motor rhythm. Psychol. Monogr. 1926, 36, 142–189. [Google Scholar] [CrossRef]
- Reitan, R.M.; Wolfson, D. The seashore rhythm test and brain functions. Clin. Neuropsychol. 1989, 3, 70–78. [Google Scholar] [CrossRef]
- Sherer, M.; Parsons, O.A.; Nixon, S.J.; Sherer, M.; Adams, R.L. Clinical validity of the speech-sounds perception test and the seashore rhythm test. J. Clin. Exp. Neuropsychol. 1991, 13, 741–751. [Google Scholar] [CrossRef]
- Yoon, G.; Sofuoglu, M.; Petrakis, I.L.; Pittman, B.; Bell, M.D. The combination of donepezil and cognitive training for improving treatment outcomes for alcohol use disorder: Design of a randomized controlled trial. Contemp. Clin. Trials 2024, 145, 107657. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Balker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 22–33. [Google Scholar]
- Wechsler, D. Wechsler Test of Adult Reading Wtar; Psychological Corporation: Oxford, UK, 2001. [Google Scholar]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Conners, C.K. Conners’ Continuous Performance Test II: Computer Program for Windows Technical Guide and Software Manual; Staff, M., Ed.; Mutli-Health Systems: North Tonwanda, NY, USA, 2000. [Google Scholar]
- Stern, R.A.; White, T. NAB, Neuropsychological Assessment Battery: Administration, Scoring, and Interpretation Manual; Psychological Assessment Resources: Lutz, FL, USA, 2003. [Google Scholar]
- Heaton, R.K. Wisconsin Card Sorting Test Manual: Revised and Expanded; Psychological Assessment Resources: Lutz, FL, USA, 1993. [Google Scholar]
- Benedict, R. Brief Visuospatial Memory Test-Revised Professional Manual; Psychological Assessment Resources, Inc.: Odessa, FL, USA, 1997. [Google Scholar]
- Brandt, J. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. Clin. Neuropsychol. 1991, 5, 125–142. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Memory Scale Revised; The psychological corporation: New York, NY, USA, 1987. [Google Scholar]
- Strick, P.L.; Dum, R.P.; Fiez, J.A. Cerebellum and nonmotor function. Annu. Rev. Neurosci. 2009, 32, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Kovar, J.; Bajwa, J.S.; Mian, Y.; Ahmad, A.; Moreno, M.M.; Price, T.J.; Lee, Y.S. Rhythmic motor behavior explains individual differences in grammar skills in adults. Sci. Rep. 2024, 14, 3710. [Google Scholar] [CrossRef] [PubMed]
- Frischen, U.; Degé, F.; Schwarzer, G. The relation between rhythm processing and cognitive abilities during child development: The role of prediction. Front. Psychol. 2022, 13, 920513. [Google Scholar] [CrossRef]
- Alhamdan, A.A.; Murphy, M.J.; Pickering, H.E.; Crewther, S.G. The Contribution of Visual and Auditory Working Memory and Non-Verbal IQ to Motor Multisensory Processing in Elementary School Children. Brain Sci. 2023, 13, 270. [Google Scholar] [CrossRef]
- Singh, T.; Fridriksson, J.; Perry, C.M.; Tryon, S.C.; Ross, A.; Fritz, S.; Herter, T.M. A novel computational model to probe visual search deficits during motor performance. J. Neurophysiol. 2017, 117, 79–92. [Google Scholar] [CrossRef]
- Martin, N.; Saffran, E.M. Effects of Word Processing and Short-term Memory Deficits on Verbal Learning: Evidence from Aphasia. Int. J. Psychol. 1999, 34, 339–346. [Google Scholar] [CrossRef]
- Zheng, R.Z. Influence of Multimedia and Cognitive Strategies in Deep and Surface Verbal Processing; IGI Global: Hershey, PA, USA, 2020. [Google Scholar]
- Carlson, M. The Haunted Stage: The Theatre as Memory Machine. In Bibliovault OAI Repository; The University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar]
- Moll, B.; Sykes, E. Optimized virtual reality-based Method of Loci memorization techniques through increased immersion and effective memory palace designs: A feasibility study. Virtual Real. 2023, 27, 941–966. [Google Scholar] [CrossRef]
- Varilias, L. Imagining a Memory Palace: Method of Loci and the Effect of Object and Spatial Imagery Skill. Ph.D. Thesis, Seton Hall University, South Orange Village, NJ, USA, 2019. [Google Scholar]
| Type | Tasks | Domains |
|---|---|---|
| Gross Motor and Gait, Proprioception, Vestibular 1 | Timed Up and Go, Dual Attention, Tandem Gait, Romberg, Standing on One Foot (L, R) | Balance and Gait |
| Rhythmic Movement 2 | Marching Slow and Fast | Rhythm |
| Embodied Memory 2 | 3-, 4-, 5-step maps | Working Memory/Rhythm |
| Embodied Delayed Recall 2 | 20-min Delayed Recall | Delayed Recall |
| Bilateral Coordination 1 | Ball Pass to the Beat Slow and Fast | Coordination/Rhythm |
| Response Inhibition 2 | Red Light/Green Light/Yellow Light Slow and Fast/Auditory and Visual | Response Inhibition, Rhythm, Attention |
| Bilateral Coordination and Self-regulation 2 | Cross Your Body | Self-Regulation, Rhythm, Working Memory |
| Rapid Sequential Movements 1 | Foot Tap, Foot Stomp, Fist Open and Close, Hand Pronate/Supinate, Finger Tap (L, R) | Motor Speed, Fluidity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bell, M.D.; Gonzalez, Y.; Weinstein, A.J.; Ciosek, D.; Wang, Y.; Yoon, G. I Got Rhythm and Executive Function, Memory, and More: The Automated Test of Embodied Cognition (ATEC). Brain Sci. 2025, 15, 299. https://doi.org/10.3390/brainsci15030299
Bell MD, Gonzalez Y, Weinstein AJ, Ciosek D, Wang Y, Yoon G. I Got Rhythm and Executive Function, Memory, and More: The Automated Test of Embodied Cognition (ATEC). Brain Sciences. 2025; 15(3):299. https://doi.org/10.3390/brainsci15030299
Chicago/Turabian StyleBell, Morris D., Yarani Gonzalez, Andrea J. Weinstein, David Ciosek, Yan Wang, and Gihyun Yoon. 2025. "I Got Rhythm and Executive Function, Memory, and More: The Automated Test of Embodied Cognition (ATEC)" Brain Sciences 15, no. 3: 299. https://doi.org/10.3390/brainsci15030299
APA StyleBell, M. D., Gonzalez, Y., Weinstein, A. J., Ciosek, D., Wang, Y., & Yoon, G. (2025). I Got Rhythm and Executive Function, Memory, and More: The Automated Test of Embodied Cognition (ATEC). Brain Sciences, 15(3), 299. https://doi.org/10.3390/brainsci15030299







