The Role of Physical Activity on Spatial and Temporal Cognitive Processing in Young Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials
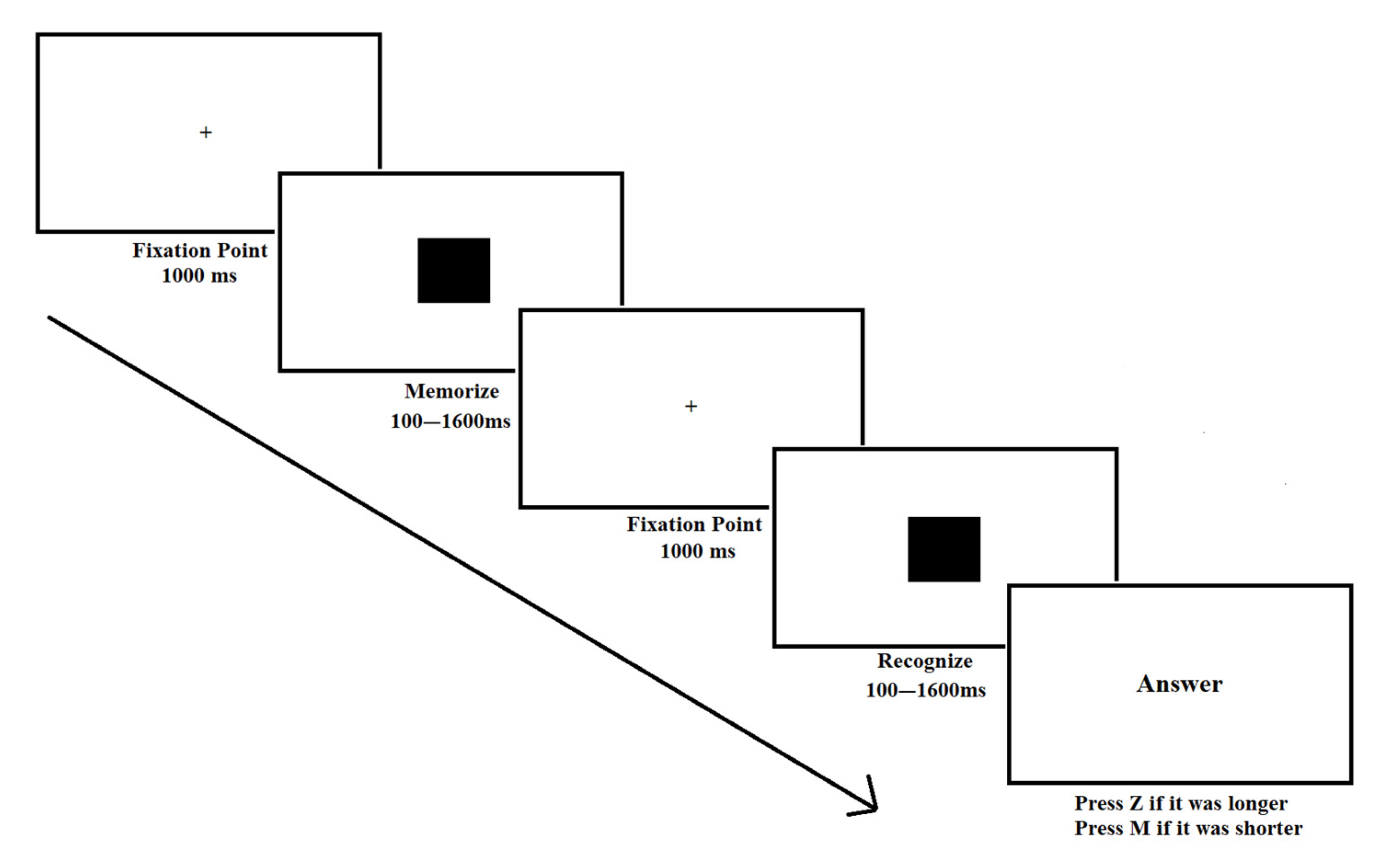
2.2.1. Time Comparison Task [42]
2.2.2. The Boxes Room Task [41]
2.3. Procedure
2.4. Data Analysis
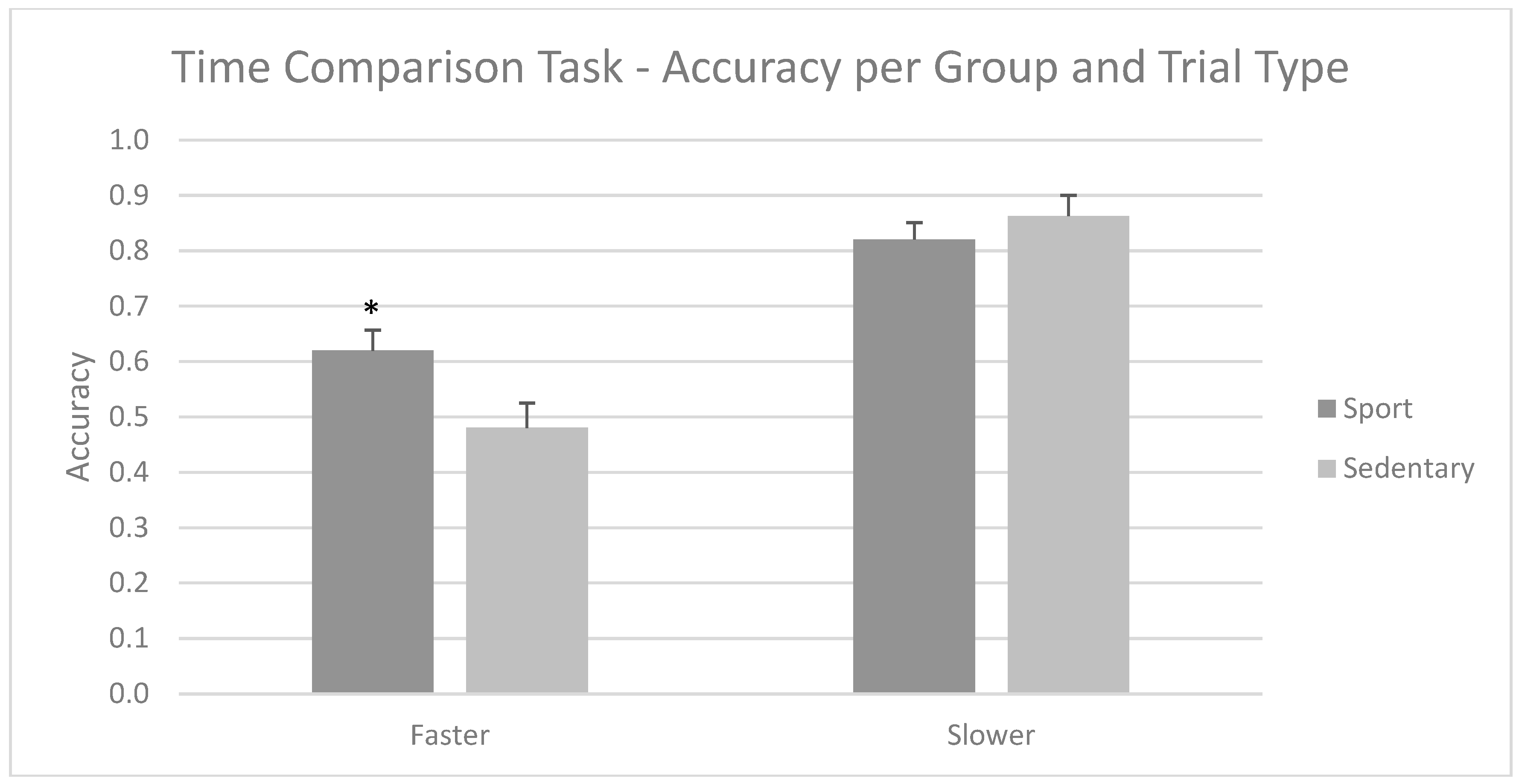
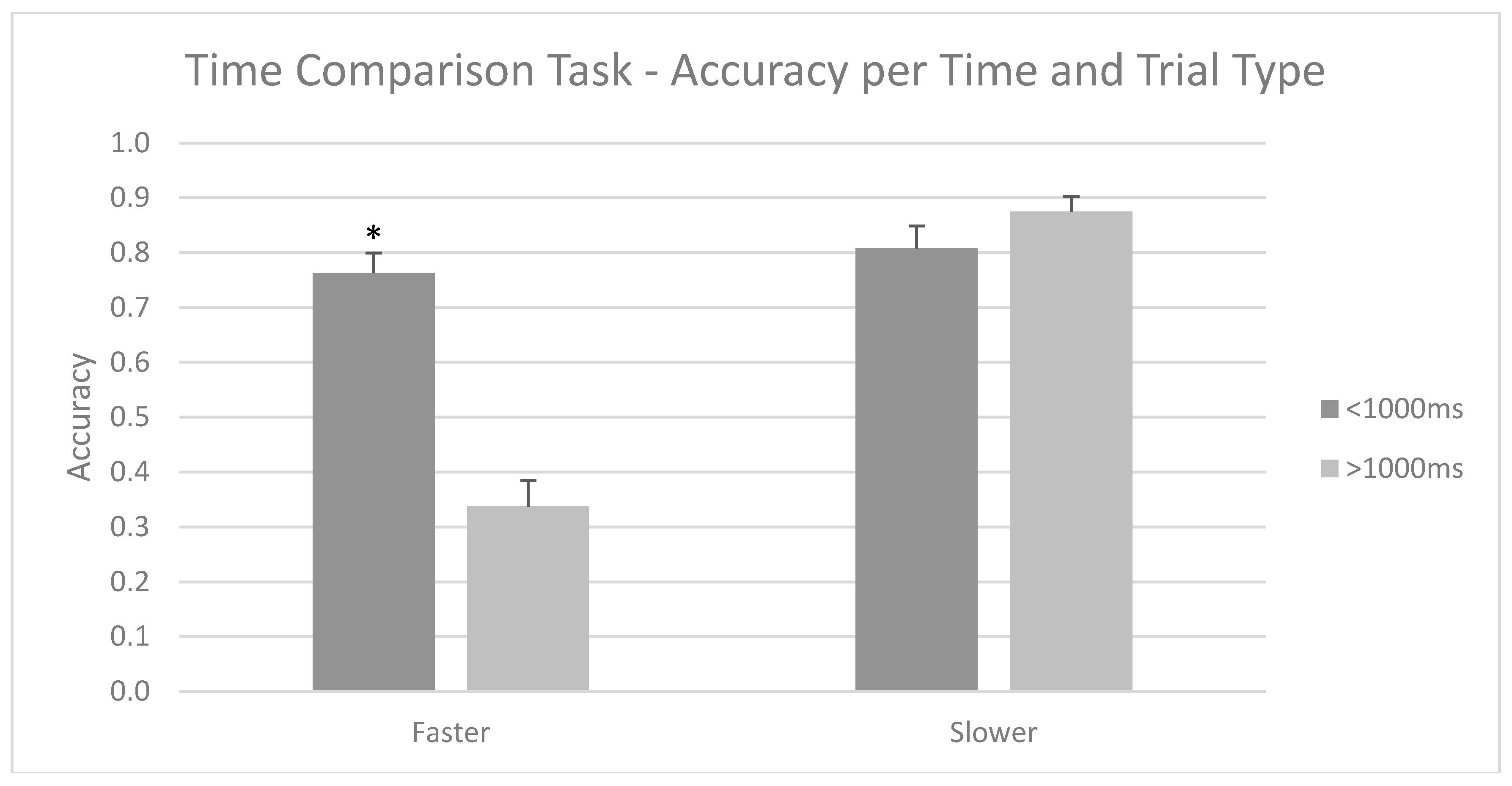
- First, for the Time Comparison Task, two repeated measures ANOVAs (group (athlete/sedentary) × type (faster/slower) × time (above/below 1000 ms)) were performed; the first was based on precision scores, while the second used response times as dependent variables.
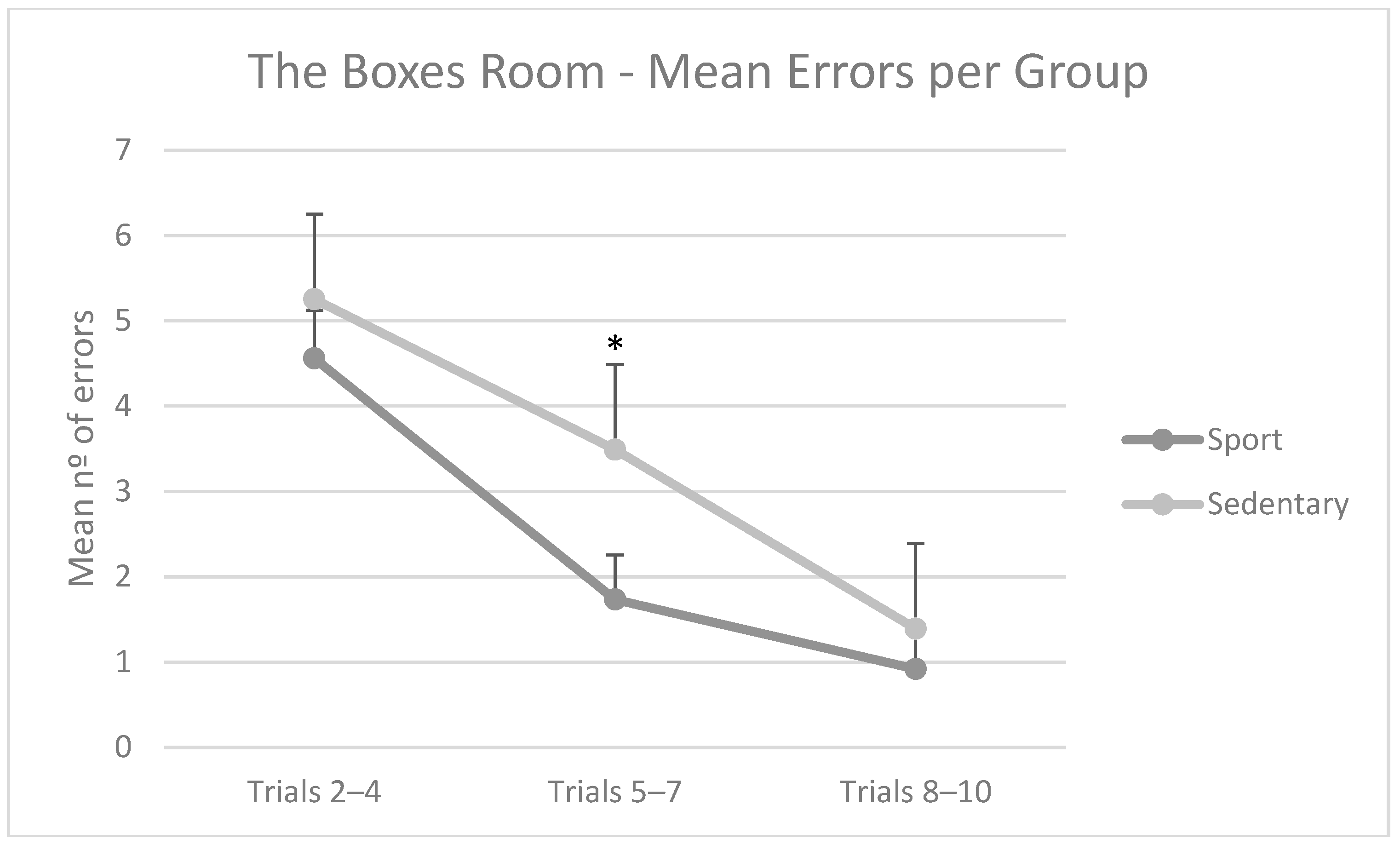
- Then, for The Boxes Room Task, two separate repeated measures ANOVAs (group (athlete/sedentary) × block (2–4/5–7/8–9)) were performed. In this case, the mean errors per block were used in the first ANOVA, while the mean latency per block was used in the second.
- Lastly, Pearson correlations were conducted for all combinations of the variables detailed in the prior paragraph to check internal validity in both tasks alongside the relationship between spatial and temporal measures. Additionally, relevant correlations between tasks were further checked using separate linear regression analyses.
3. Results
3.1. Time Comparison Task—Accuracy and Response Times per Group
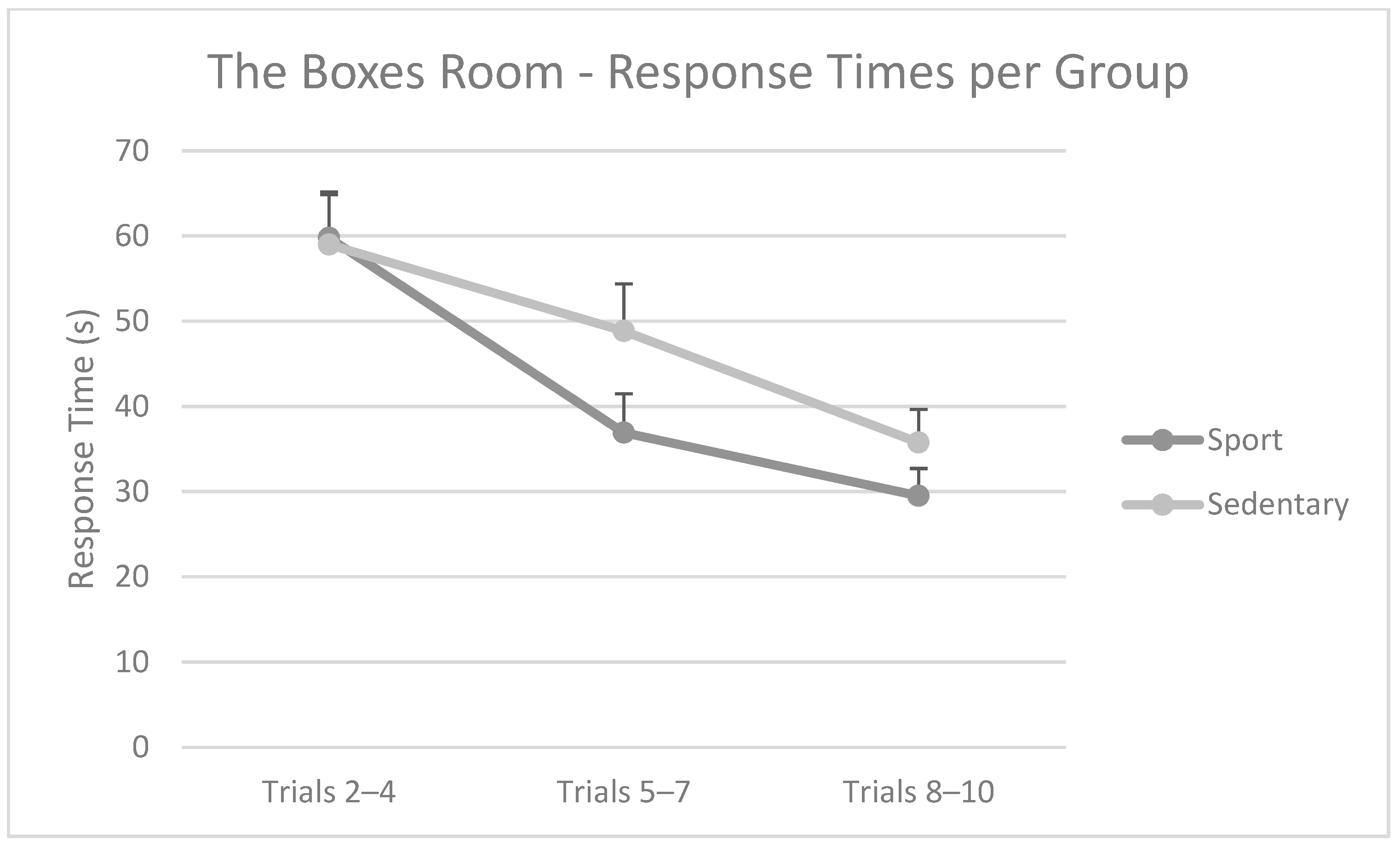
3.2. The Boxes Room Task—Errors and Response Times per Group
3.3. Correlations and Linear Regressions (Full Sample; n = 42)
- Faster trials of the Time Comparison Task compared with the latency of trials 5–7 of The Boxes Room Task: The results showed a statistically significant model (F(1,41) = 6.36; p = 0.016) with an adjusted R2 of 0.116. This means that faster trial accuracy accounted for 11.6% of the variance of the latency in the second block of The Boxes Room Task.
- Faster trials of the Time Comparison Task compared with the latency of trials 8–10 of The Boxes Room Task: The results showed a statistically significant model (F(1,41) = 4.43; p = 0.042) with an adjusted R2 of 0.077. This means that faster trial accuracy accounted for 7.7% of the variance of the latency in the third block of The Boxes Room Task.
- Above 1000 ms trials of the Time Comparison Task compared with the mean errors of trials 5–7 of The Boxes Room Task: The results showed a statistically significant model (F(1,41) = 4.57; p = 0.039) with an adjusted R2 of 0.080. This means above 1000 ms trials accuracy accounted for 8.0% of the variance of the mean errors in the second block of The Boxes Room Task.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| TCT-Global | TCT-Faster | TCT-Slower | TCT- < 1 s | TCT- > 1 s | RT-TCT-Global | RT-TCT-Faster | RT-TCT-Slower | RT-TCT- < 1 s | RT-TCT > 1 s | BOX-B1 | BOX-B2 | BOX-B3 | RT-BOX-B1 | RT-BOX-B2 | RT-BOX-B3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCT-Global | 1 | 0.766 ** | 0.582 ** | 0.682 ** | 0.736 ** | 0.035 | 0.066 | −0.103 | −0.134 | 0.174 | −0.119 | −0.259 | −0.160 | −0.100 | −0.204 | −0.215 |
| TCT-Faster | 1 | −0.076 | 0.357 * | 0.717 ** | −0.018 | −0.067 | −0.078 | −0.110 | 0.069 | −0.055 | −0.294 | −0.242 | −0.144 | −0.370 * | −0.316 * | |
| TCT-Slower | 1 | 0.607 ** | 0.235 | 0.076 | 0.187 | −0.062 | −0.068 | 0.183 | −0.114 | −0.030 | 0.059 | 0.027 | 0.152 | 0.065 | ||
| TCT- < 1 s | 1 | 0.007 | 0.021 | 0.118 | −0.045 | −0.055 | 0.082 | 0.028 | −0.037 | −0.074 | −0.121 | −0.063 | −0.165 | |||
| TCT- > 1 s | 1 | 0.028 | −0.019 | −0.100 | −0.132 | 0.163 | −0.188 | −0.320 * | −0.150 | −0.024 | −0.221 | −0.142 | ||||
| RT-TCT-Global | 1 | 0.940 ** | 0.919 ** | 0.841 ** | 0.878 ** | −0.013 | 0.028 | 0.221 | 0.014 | 0.069 | 0.066 | |||||
| RT-TCT-Faster | 1 | 0.804 ** | 0.772 ** | 0.842 ** | 0.022 | 0.010 | 0.224 | −0.045 | 0.038 | 0.035 | ||||||
| RT-TCT-Slower | 1 | 0.874 ** | 0.717 ** | 0.042 | 0.089 | 0.237 | 0.086 | 0.128 | 0.093 | |||||||
| RT-TCT- < 1 s | 1 | 0.479 ** | 0.193 | 0.148 | 0.311 * | 0.127 | 0.186 | 0.177 | ||||||||
| RT-TCT > 1 s | 1 | −0.191 | −0.085 | 0.084 | −0.090 | −0.053 | −0.050 | |||||||||
| BOX-B1 | 1 | 0.655 ** | 0.365 * | 0.617 ** | 0.643 ** | 0.457 ** | ||||||||||
| BOX-B2 | 1 | 0.706 ** | 0.402 ** | 0.677 ** | 0.632 ** | |||||||||||
| BOX-B3 | 1 | 0.115 | 0.301 | 0.764 ** | ||||||||||||
| RT-BOX-B1 | 1 | 0.782 ** | 0.364 * | |||||||||||||
| RT-BOX-B2 | 1 | 0.535 ** | ||||||||||||||
| RT-BOX-B3 | 1 |
References
- Wegner, M.; Helmich, I.; Machado, S.; Nardi, A.; Arias-Carrion, O.; Budde, H. Effects of Exercise on Anxiety and Depression Disorders: Review of Meta- Analyses and Neurobiological Mechanisms. CNS Neurol. Disord. Drug Targets 2014, 13, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Breuer, C.; Pawlowski, T. Socioeconomic Perspectives on Physical Activity and Aging. Eur. Rev. Aging Phys. Act. 2011, 8, 53–56. [Google Scholar] [CrossRef]
- Bonow, R.O.; Smaha, L.A.; Smith, S.C.; Mensah, G.A.; Lenfant, C. World Heart Day 2002: The International Burden of Cardiovascular Disease: Responding to the Emerging Global Epidemic. Circulation 2002, 106, 1602–1605. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Saltin, B. Exercise as Medicine—Evidence for Prescribing Exercise as Therapy in 26 Different Chronic Diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef]
- ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Riebe, D., Ehrman, J.K., Liguori, G., Magal, M., Eds.; Wolters Kluwer: Alphen aan den Rhein, The Netherlands, 2018. [Google Scholar]
- Angevaren, M.; Aufdemkampe, G.; Verhaar, H.; Aleman, A.; Vanhees, L. Physical Activity and Enhanced Fitness to Improve Cognitive Function in Older People without Known Cognitive Impairment. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef]
- Colcombe, S.; Kramer, A.F. Fitness Effects on the Cognitive Function of Older Adults: A Meta-Analytic Study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef]
- Chieffi, S.; Messina, G.; Villano, I.; Messina, A.; Valenzano, A.; Moscatelli, F.; Salerno, M.; Sullo, A.; Avola, R.; Monda, V.; et al. Neuroprotective Effects of Physical Activity: Evidence from Human and Animal Studies. Front. Neurol. 2017, 8, 188. [Google Scholar] [CrossRef]
- Verburgh, L.; Scherder, E.J.A.; Van Lange, P.A.M.; Oosterlaan, J. Executive Functioning in Highly Talented Soccer Players. PLoS ONE 2014, 9, e91254. [Google Scholar] [CrossRef]
- Van Praag, H.; Christie, B.R.; Sejnowski, T.J.; Gage, F.H. Running Enhances Neurogenesis, Learning, and Long-Term Potentiation in Mice. Proc. Natl. Acad. Sci. USA 1999, 96, 13427–13431. [Google Scholar] [CrossRef]
- Ang, E.-T.; Dawe, G.S.; Wong, P.T.H.; Moochhala, S.; Ng, Y.-K. Alterations in Spatial Learning and Memory after Forced Exercise. Brain Res. 2006, 1113, 186–193. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Lee, K.S.; Fernandes, J.; Oliveira, M.G.M.; Tufik, S.; Meeusen, R.; De Mello, M.T. Spatial Memory Is Improved by Aerobic and Resistance Exercise through Divergent Molecular Mechanisms. Neuroscience 2012, 202, 309–317. [Google Scholar] [CrossRef]
- Sánchez-Horcajo, R.; Llamas-Alonso, J.; Cimadevilla, J.M. Practice of Aerobic Sports Is Associated with Better Spatial Memory in Adults and Older Men. Exp. Aging Res. 2015, 41, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Noguera, C.; Sánchez-Horcajo, R.; Álvarez-Cazorla, D.; Cimadevilla, J.M. Ten Years Younger: Practice of Chronic Aerobic Exercise Improves Attention and Spatial Memory Functions in Ageing. Exp. Gerontol. 2019, 117, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ramirez Butavand, D.; Rodriguez, M.F.; Cifuentes, M.V.; Miranda, M.; Bauza, C.G.; Bekinschtein, P.; Ballarini, F. Acute and Chronic Physical Activity Improves Spatial Memory in an Immersive Virtual Reality Task. iScience 2023, 26, 106176. [Google Scholar] [CrossRef] [PubMed]
- Morawietz, C.; Muehlbauer, T. Effects of Physical Exercise Interventions on Spatial Orientation in Children and Adolescents: A Systematic Scoping Review. Front. Sports Act. Living 2021, 3, 664640. [Google Scholar] [CrossRef]
- Matheis, R.J.; Schultheis, M.T.; Tiersky, L.A.; DeLuca, J.; Millis, S.R.; Rizzo, A. Is Learning and Memory Different in a Virtual Environment? Clin. Neuropsychol. 2007, 21, 146–161. [Google Scholar] [CrossRef]
- Allman, M.J.; Teki, S.; Griffiths, T.D.; Meck, W.H. Properties of the Internal Clock: First- and Second-Order Principles of Subjective Time. Annu. Rev. Psychol. 2014, 65, 743–771. [Google Scholar] [CrossRef]
- Buonomano, D.V.; Maass, W. State-Dependent Computations: Spatiotemporal Processing in Cortical Networks. Nat. Rev. Neurosci. 2009, 10, 113–125. [Google Scholar] [CrossRef]
- Paton, J.J.; Buonomano, D.V. The Neural Basis of Timing: Distributed Mechanisms for Diverse Functions. Neuron 2018, 98, 687–705. [Google Scholar] [CrossRef]
- Fontes, R.; Ribeiro, J.; Gupta, D.S.; Machado, D.; Lopes-Júnior, F.; Magalhães, F.; Bastos, V.H.; Rocha, K.; Marinho, V.; Lima, G.; et al. Time Perception Mechanisms at Central Nervous System. Neurol. Int. 2016, 8, 5939. [Google Scholar] [CrossRef]
- Schwartz, G.; Howard, M.W.; Jing, B.; Kahana, M.J. Shadows of the Past. Psychol. Sci. 2005, 16, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Bicanski, A.; Burgess, N. A Neural-Level Model of Spatial Memory and Imagery. eLife 2018, 7, e33752. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J. Place Units in the Hippocampus of the Freely Moving Rat. Exp. Neurol. 1976, 51, 78–109. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.; Nadel, L. The Hippocampus as a Cognitive Map; Oxford University Press: Oxford, UK, 1978. [Google Scholar]
- Naya, Y.; Suzuki, W.A. Integrating What and When Across the Primate Medial Temporal Lobe. Science 2011, 333, 773–776. [Google Scholar] [CrossRef]
- Castillo Escamilla, J.; León Estrada, I.; Alcaraz-Iborra, M.; Cimadevilla Redondo, J.M. Aging: Working Memory Capacity and Spatial Strategies in a Virtual Orientation Task. GeroScience 2023, 45, 159–175. [Google Scholar] [CrossRef]
- Dormal, V.; Heeren, A.; Pesenti, M.; Maurage, P. Time Perception Is Not for the Faint-Hearted? Physiological Arousal Does Not Influence Duration Categorisation. Cogn. Process. 2018, 19, 399–409. [Google Scholar] [CrossRef]
- Jones, C.R.G.; Jahanshahi, M. Motor and Perceptual Timing in Parkinson’s Disease. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 265–290. [Google Scholar]
- Penney, T.; Vaitilingam, L. Imaging Time. Psychol. Time 2008, 261–294. [Google Scholar]
- Hellström, Å.; Rammsayer, T.H. Effects of Time-Order, Interstimulus Interval, and Feedback in Duration Discrimination of Noise Bursts in the 50- and 1000-Ms Ranges. Acta Psychol. 2004, 116, 1–20. [Google Scholar] [CrossRef]
- Hoopen, G.; Miyauchi, R.; Nakajima, Y. Time-Based Illusions in the Auditory Mode. In Psychology of Time; Emerald Group: Bigley, UK, 2008; pp. 139–188. [Google Scholar]
- Brown, S.W. Time, Change, and Motion: The Effects of Stimulus Movement on Temporal Perception. Percept. Psychophys. 1995, 57, 105–116. [Google Scholar] [CrossRef]
- Kaneko, S.; Murakami, I. Perceived Duration of Visual Motion Increases with Speed. J. Vis. 2009, 9, 14. [Google Scholar] [CrossRef]
- Harris, R.J. Answering Questions Containing Marked and Unmarked Adjectives and Adverbs. J. Exp. Psychol. 1973, 97, 399–401. [Google Scholar] [CrossRef]
- Loftus, E.F.; Palmer, J.C. Reconstruction of Automobile Destruction: An Example of the Interaction between Language and Memory. J. Verbal Learn. Verbal Behav. 1974, 13, 585–589. [Google Scholar] [CrossRef]
- Pedri, S.; Hesketh, B. Time Perception: Effects of Task Speed and Delay. Percept. Mot. Skills 1993, 76, 599–608. [Google Scholar] [CrossRef]
- Allingham, E.; Hammerschmidt, D.; Wöllner, C. Time Perception in Human Movement: Effects of Speed and Agency on Duration Estimation. Q. J. Exp. Psychol. 2021, 74, 559–572. [Google Scholar] [CrossRef]
- Lin, H.-M.; Kuo, S.-H.; Mai, T.P. Slower Tempo Makes Worse Performance? The Effect of Musical Tempo on Cognitive Processing Speed. Front. Psychol. 2023, 14, 998460. [Google Scholar] [CrossRef]
- Gros, A.; Giroud, M.; Bejot, Y.; Rouaud, O.; Guillemin, S.; Aboa Eboulé, C.; Manera, V.; Daumas, A.; Lemesle Martin, M. A Time Estimation Task as a Possible Measure of Emotions: Difference Depending on the Nature of the Stimulus Used. Front. Behav. Neurosci. 2015, 9, 143. [Google Scholar] [CrossRef]
- Cánovas, R.; Espínola, M.; Iribarne, L.; Cimadevilla, J.M. A New Virtual Task to Evaluate Human Place Learning. Behav. Brain Res. 2008, 190, 112–118. [Google Scholar] [CrossRef]
- Castillo Escamilla, J.; Salvador-Viñas, M.d.M.; Cimadevilla, J.M. Sex Based Divergences in Time Processing Were Found in Newly-Developed Tasks. Manuscript under review.
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; ISBN 978-0-203-77158-7. [Google Scholar]
- McGurk, S.R.; Green, M.F.; Wirshing, W.C.; Wirshing, D.A.; Marder, S.R.; Mintz, J.; Kern, R. Antipsychotic and Anticholinergic Effects on Two Types of Spatial Memory in Schizophrenia. Schizophr. Res. 2004, 68, 225–233. [Google Scholar] [CrossRef]
- Cimadevilla, J.M.; López, F.; Nieto, L.; Aguirre, M.J.; Fernández, R. Lidocaine, Tetrodotoxin and Their Effect on Consolidation of Spatial Memory. Psicothema 2009, 21, 471–474. [Google Scholar] [PubMed]
- Miyake, K.; Yagi, S.; Aoki, Y.; Shikano, Y.; Ikegaya, Y.; Sasaki, T. Acute Effects of Ethanol on Hippocampal Spatial Representation and Offline Reactivation. Front. Cell. Neurosci. 2020, 14, 571175. [Google Scholar] [CrossRef] [PubMed]
- Kleen, J.K.; Sitomer, M.T.; Killeen, P.R.; Conrad, C.D. Chronic Stress Impairs Spatial Memory and Motivation for Reward Without Disrupting Motor Ability and Motivation to Explore. Behav. Neurosci. 2006, 120, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Knudson, D.; Kluka, D.A. The Impact of Vision and Vision Training on Sport Performance. J. Phys. Educ. Recreat. Dance 1997, 68, 17–24. [Google Scholar] [CrossRef]
- Miller, J. How Many Participants? How Many Trials? Maximizing the Power of Reaction Time Studies. Behav. Res. Methods 2024, 56, 2398–2421. [Google Scholar] [CrossRef]
- Ortells, J.J.; De Fockert, J.W.; Romera, N.; Fernández, S. Expectancy-Based Strategic Processes Are Influenced by Spatial Working Memory Load and Individual Differences in Working Memory Capacity. Front. Psychol. 2018, 9, 1239. [Google Scholar] [CrossRef]
- Eichenbaum, H.; Fortin, N. Episodic Memory and the Hippocampus: It’s About Time. Curr. Dir. Psychol. Sci. 2003, 12, 53–57. [Google Scholar] [CrossRef]
- Tulving, E. Episodic Memory: From Mind to Brain. Annu. Rev. Psychol. 2002, 53, 1–25. [Google Scholar] [CrossRef]
- Simons, J.S.; Spiers, H.J. Prefrontal and Medial Temporal Lobe Interactions in Long-Term Memory. Nat. Rev. Neurosci. 2003, 4, 637–648. [Google Scholar] [CrossRef]
- Howard, M.W.; Eichenbaum, H. The Hippocampus, Time, and Memory across Scales. J. Exp. Psychol. Gen. 2013, 142, 1211–1230. [Google Scholar] [CrossRef]
- Palombo, D.J.; Keane, M.M.; Verfaellie, M. Does the Hippocampus Keep Track of Time? Hippocampus 2016, 26, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.A.; Nannery, R.; Spiers, H.J. Navigation around London by a Taxi Driver with Bilateral Hippocampal Lesions. Brain 2006, 129, 2894–2907. [Google Scholar] [CrossRef] [PubMed]
- Nedelska, Z.; Andel, R.; Laczó, J.; Vlcek, K.; Horinek, D.; Lisy, J.; Sheardova, K.; Bureš, J.; Hort, J. Spatial Navigation Impairment Is Proportional to Right Hippocampal Volume. Proc. Natl. Acad. Sci. USA 2012, 109, 2590–2594. [Google Scholar] [CrossRef]
- Tonelli, A.; Lunghi, C.; Gori, M. Moderate Physical Activity Alters the Estimation of Time, but Not Space. Front. Psychol. 2022, 13, 1004504. [Google Scholar] [CrossRef]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Hu, L.; Morris, K.S.; White, S.M.; Wójcicki, T.R.; McAuley, E.; Kramer, A.F. Aerobic Fitness Is Associated with Hippocampal Volume in Elderly Humans. Hippocampus 2009, 19, 1030–1039. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise Training Increases Size of Hippocampus and Improves Memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Erickson, K.I.; Leckie, R.L.; Weinstein, A.M. Physical Activity, Fitness, and Gray Matter Volume. Neurobiol. Aging 2014, 35, S20–S28. [Google Scholar] [CrossRef]
- Vargha-Khadem, F.; Gadian, D.G.; Watkins, K.E.; Connelly, A.; Van Paesschen, W.; Mishkin, M. Differential Effects of Early Hippocampal Pathology on Episodic and Semantic Memory. Science 1997, 277, 376–380. [Google Scholar] [CrossRef]
- Burgess, N.; King, J.A. Navigation in Virtual Space: Psychological and Neural Aspects. In International Encyclopedia of the Social & Behavioral Sciences; Elsevier: Amsterdam, The Netherlands, 2001; pp. 10417–10422. ISBN 978-0-08-043076-8. [Google Scholar]
- Danjo, T. Allocentric Representations of Space in the Hippocampus. Neurosci. Res. 2020, 153, 1–7. [Google Scholar] [CrossRef]
- Eriksson, G.; Svenson, O.; Eriksson, L. The Time-Saving Bias: Judgements, Cognition and Perception. Judgm. Decis. Mak. 2013, 8, 492–497. [Google Scholar] [CrossRef]
- Svenson, O. Decisions among Time Saving Options: When Intuition Is Strong and Wrong. Acta Psychol. 2008, 127, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Crisp, Z.C.; Grant, J.E. Impulsivity across Psychiatric Disorders in Young Adults. Compr. Psychiatry 2024, 130, 152449. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.-S.; Hung, T.-M.; Chu, L.-H.; Chou, W.-C.; Fang, C.-L. Working Memory Performance Differentiated by Physical Functional Capacity in Late-Adulthood. Rev. Psicol. Deporte 2017, 26, 61–69. [Google Scholar]
- Russo, G.; Ottoboni, G.; Tessari, A.; Ceciliani, A. The Positive Impact of Physical Activity on Working Memory Abilities: Evidence from a Large Italian Pre-Adolescent Sample. In Proceedings of the Journal of Human Sport and Exercise—2021—Autumn Conferences of Sports Science; Universidad de Alicante: Alicante, Spain, 2021. [Google Scholar]
- Wang, C.-H.; Chang, C.-C.; Liang, Y.-M.; Shih, C.-M.; Chiu, W.-S.; Tseng, P.; Hung, D.L.; Tzeng, O.J.L.; Muggleton, N.G.; Juan, C.-H. Open vs. Closed Skill Sports and the Modulation of Inhibitory Control. PLoS ONE 2013, 8, e55773. [Google Scholar] [CrossRef]
- Murray, S.O.; Schallmo, M.-P.; Kolodny, T.; Millin, R.; Kale, A.; Thomas, P.; Rammsayer, T.H.; Troche, S.J.; Bernier, R.A.; Tadin, D. Sex Differences in Visual Motion Processing. Curr. Biol. 2018, 28, 2794–2799.e3. [Google Scholar] [CrossRef]
- Yagi, S.; Galea, L.A.M. Sex Differences in Hippocampal Cognition and Neurogenesis. Neuropsychopharmacology 2019, 44, 200–213. [Google Scholar] [CrossRef]
- Hanson, N.J.; Buckworth, J. Sex Differences in Time Perception during Self-Paced Running. Int. J. Exerc. Sci. 2016, 9, 514–523. [Google Scholar] [CrossRef]
- Jacobson, J.; Matthaeus, L. Athletics and Executive Functioning: How Athletic Participation and Sport Type Correlate with Cognitive Performance. Psychol. Sport Exerc. 2014, 15, 521–527. [Google Scholar] [CrossRef]
- Edwards, A.M.; McCormick, A. Time Perception, Pacing and Exercise Intensity: Maximal Exercise Distorts the Perception of Time. Physiol. Behav. 2017, 180, 98–102. [Google Scholar] [CrossRef]
- Tobin, S.; Grondin, S. Time Perception Is Enhanced by Task Duration Knowledge: Evidence from Experienced Swimmers. Mem. Cognit. 2012, 40, 1339–1351. [Google Scholar] [CrossRef]
- Droit-Volet, S. Time Perception, Emotions and Mood Disorders. J. Physiol.-Paris 2013, 107, 255–264. [Google Scholar] [CrossRef] [PubMed]
- AlWhaibi, R.M.; Aldhowayan, A.M.; Alshahrani, S.M.; Almadi, B.S.; Alamer, R.A.; Albaqami, F.M.; Mortada, E.M. Exploring the Influence of Video Games on Self-Reported Spatial Abilities Among University Students. Brain Sci. 2024, 14, 1269. [Google Scholar] [CrossRef] [PubMed]
- Zhan, N.; Fan, X.; Shen, F.; Song, L.; Zhou, C.; Xiao, J.; Wu, X.; Li, L.J.; Xi, J.; Jiayi Li, S.; et al. Effects of Grade, Academic Performance, and Sex on Spatial Working Memory and Attention in Primary School Children: A Cross-Sectional Observational Study. J. Bio-X Res. 2022, 5, 90–96. [Google Scholar] [CrossRef]
- Oyanadel, C.; Buela-Casal, G. Time Perception and Psychopathology: Influence of Time Perspective on Quality of Life of Severe Mental Illness. Actas Esp. Psiquiatr. 2014, 42, 99–107. [Google Scholar]
- Wittmann, M.; Lehnhoff, S. Age Effects in Perception of Time. Psychol. Rep. 2005, 97, 921–935. [Google Scholar] [CrossRef]
- Chueh, T.-Y.; Huang, C.-J.; Hsieh, S.-S.; Chen, K.-F.; Chang, Y.-K.; Hung, T.-M. Sports Training Enhances Visuo-Spatial Cognition Regardless of Open-Closed Typology. PeerJ 2017, 5, e3336. [Google Scholar] [CrossRef]


| Square Presentation Times (1st and 2nd in ms) | Time Category (Below/Above 1000 ms) | Trial Type (Faster/Slower). Second Presentation vs. First |
|---|---|---|
| 200–400 ms | Below | Slower |
| 400–200 ms | Below | Faster |
| 400–600 ms | Below | Slower |
| 600–400 ms | Below | Faster |
| 600–800 ms | Below | Slower |
| 800–600 ms | Below | Faster |
| 1000–1200 ms | Above | Slower |
| 1200–1000 ms | Above | Faster |
| 1200–1400 ms | Above | Slower |
| 1400–1200 ms | Above | Faster |
| 1400–1600 ms | Above | Slower |
| 1600–1400 ms | Above | Faster |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Escamilla, J.; Salvador-Viñas, M.d.M.; Cimadevilla, J.M. The Role of Physical Activity on Spatial and Temporal Cognitive Processing in Young Women. Brain Sci. 2025, 15, 431. https://doi.org/10.3390/brainsci15050431
Castillo-Escamilla J, Salvador-Viñas MdM, Cimadevilla JM. The Role of Physical Activity on Spatial and Temporal Cognitive Processing in Young Women. Brain Sciences. 2025; 15(5):431. https://doi.org/10.3390/brainsci15050431
Chicago/Turabian StyleCastillo-Escamilla, Joaquín, María del Mar Salvador-Viñas, and José Manuel Cimadevilla. 2025. "The Role of Physical Activity on Spatial and Temporal Cognitive Processing in Young Women" Brain Sciences 15, no. 5: 431. https://doi.org/10.3390/brainsci15050431
APA StyleCastillo-Escamilla, J., Salvador-Viñas, M. d. M., & Cimadevilla, J. M. (2025). The Role of Physical Activity on Spatial and Temporal Cognitive Processing in Young Women. Brain Sciences, 15(5), 431. https://doi.org/10.3390/brainsci15050431








